Abstract
Kaolinite is a single 2D layer of kaolin or metakaolin (MK), common clays that can be characterized as layered 3D materials. We show that because of its chemical composition, kaolinite can be converted into an amorphous 3D material by chemical means. This dimensional transformation is possible due to the large surface to volume ratio and chemical reactivity of kaolinite. We investigate the formation and influence of quasi- or nanocrystalline phases in MK-based alkali-activated materials (AAM) that are related to the Si/Al ratio. We analyze the formation of an AAM from a MK precursor, which is a 3D bonded network that preserves the layered structure at the nanometer scale. We also exfoliate the remaining layered phase to examine the effects of the alkali-activation in the final sheet structures embedded within the amorphous network. The final material can be used as a cement with no carbon dioxide produced by the transformation reaction.
Similar content being viewed by others
Introduction
Portland cement is an invention from the early nineteenth century. The first patent from Joseph Aspdin was issued on 21 October 1824. Hence, Portland cement technology will celebrate 200 years in 2024. There is no question that this invention is one of the most important for mankind and possibly one of the oldest and prevalent technologies that are still used to this date. However, looking into a historical context, this technology is a fruit of the Industrial Revolution that started in the eighteenth century1,2. It was created at a time where environmental concerns were not considered important. As such, Portland cement technology is wasteful and polluting as the Industrial Revolution itself. Therefore, Portland cement manufacturing is responsible for an enormous amount of carbon dioxide emissions in the atmosphere (measured in tens of giga metric tons)3. In the last 30 years, research, development, and commercial activities related to alkali-activated concrete have increased due to the growing awareness of the high carbon footprint associated with Portland cement2. However, accounts describing the different morphological stages of green cement production, from a crystalline 2D to an amorphous 3D inorganic material, are limited. We approach kaolinite’s journey through the lenses of 2D materials, to better understand what is left of its original composition and structure in the final cementitious 3D material.
The forefront of industrial applications of 2D materials is usually represented by graphene and its derivatives. Graphene is obtained from a 3D layered material, graphite, in the same way kaolinite is obtained from a layered material, kaolin/metakaolin. Graphene4 and kaolinite5 belong to a large family of 2D materials that includes transition metal dichalcogenides, boron-nitride, among others. However, a number of challenges have hindered the adoption of graphene derivatives in commercial applications, where standardization, quality variation, and high production costs are among the most prominent6. Consequently, graphene-related materials are mainly used as additives because their cost is still higher than other bulk raw materials such as common ceramics, metals and polymers7,8. As a consequence, a search for readily available materials that can be exfoliated down to 2D is an important area of research9,10,11,12.
An important family of layered materials is the clays. Clays have been used for millennia in pottery and construction, and cementitious gels, which have been found in ancient Roman concrete13. The development of alkali-activated materials (AAMs) using clay precursors began in the nineteenth century and accelerated at the beginning of the twentieth century14. But the recent growth of awareness of the high carbon footprint associated with Portland cement has boosted research, development, and commercial activities related to alkali-activated concrete2.
Clays are layered structures made of sheet-like monolayers with one dimension in the nanometer range, which can be classified as another large family of 2D materials. The joint nomenclature committees (JNC) of the Association Internationale pour l’Etude des Argiles (AIPEA), and the Clay Minerals Society define clay minerals as either natural or synthetic, phyllosilicates or non-phyllosilicates. Traditionally classified under silicates, clay minerals have more oxygen than Si, Al, and Mg in their chemical compositions so that they can be regarded as layered hydroxides15. The layered structure of the phyllosilicates consists of sheets made of tetrahedra (T) and octahedra (O) that form TO or TOT layers, which have a mainly negative surface charge. In the layered double hydroxides of metal ions (LDH), the structure consists of an octahedral (O) sheet with a positive surface charge, but the layers can also be uncharged, as in talc and pyrophyllite16. The tetrahedra are formed by a cation coordinated to four oxygen atoms, which are sheared in three corners with adjacent tetrahedra. They form an infinite 2D hexagonal pattern along the a and b crystallographic directions17. In the octahedra, a cation is coordinated to six oxygen atoms and the edges are sheared to neighbor octahedra. The ratio of T/O sheets, along with their charge, results in a large variety of clays16.
Clays have been studied from very different perspectives of geology, mineralogy, chemistry, physics, and biology, and are still the object of intensive research. Abundant, inexpensive, readily suitable for large-scale industrial applications, and environmentally friendly (either in their natural form or after modifications)16, clays have found applications as sustainable functional materials in de-polluting agents, bio-compatible composites, and construction materials. Some clays, particularly those belonging to the kaolinitic branch, are aluminosilicate precursors that result in a three-dimensional (3D) gel when subjected to alkali activation, which can lead to a robust material with high mechanical strength18.
The sheet-like structure of kaolinite (Al2Si2O5(OH)4), the major component in kaolin, has been explored to obtain sheets with thicknesses in the nanometer range. Processes such as intercalation-exfoliation, chemical and physical exfoliation can yield kaolinite with thickness down to 1.3 nm, allowing for the fabrication of membranes made from the 2D sheets19,20. Calcination, acid leaching, and ultrasonic dispersion can be applied to raw kaolin to obtain modified kaolinite nano-layers21, which are reported in applications such as drug delivery for cancer treatment22. The well-known exfoliation methods used to obtain graphene and 2D materials have been derived, in part, from delamination techniques employed in the clay industry23,24. For example, a common technique in nanotechnology for controlling the growth and agglomeration of nanoparticles and 2D materials is using polymer-surfactant complexes of opposite charges25. Kaolinite can be exfoliated to individual 1:1 layers by intercalating organic solvents, urea, and polymeric compounds (such as oppositely charged polyelectrolytes)26, modifying its surface.
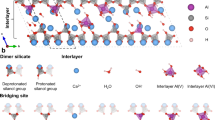
We explore the relationship between the structure and bonding energetics in kaolinite, metakaolin (MK), and alkali-activated derived materials (AAM). We start by revisiting the change of structure in kaolinite/metakaolin transformation. For that, we investigate the presence of quasi- or nanocrystalline phases in MK-based AAM, which has been reported as being directly related to the Si/Al ratio due to the formation of zeolite-like phases27,28,29,30. Subsequently, we analyze the formation of an AAM from a MK precursor, a 3D bonded network that preserves the layered structure at the nanometer scale. Finally, we exfoliate the remaining layered phase to examine the effects of the alkali-activation in the final sheet structures, as shown in Fig. 1.
Schematic representation of the transformations involved in producing a 3D amorphous material from 2D crystalline kaolinite. (a) Kaolin structure followed by (b) its thermal conversion into metakaolin, and (c) alkali-activation into a composite material containing an amorphous 3D matrix and nanometer 2D crystalline domains.
Results and discussion
From kaolin to metakaolin
The structure of kaolinite consists of a single sheet with AlO6 octahedra on one side and SiO4 tetrahedra on the other. The layers are stacked with an offset to form a triclinic symmetry with the space group C131. The vertex-sharing SiO4 tetrahedra (T) form rings of six silicates, and the edge-sharing AlO6 octahedra (O) form rings of four aluminates. This configuration gives rise to its classification as a 1:1 T:O dioctahedral planar hydrous phyllosilicate. The term dioctahedral is due to the valence 2 of the Al2+ cations in the octahedra centers. Strong ionic and covalent bonds join the TO sheets of silicates and aluminates through the apical oxygens, but between TO layers, the connection is through hydrogen bonds32 (Fig. 1a).
Due to the hydrogen bonds, the interlayer interaction is stronger than in graphite. We define the exfoliation energy of the crystal as the energy difference between the per layer energy, E(bulk crystal), of the bulk material and that of a free single layer, E(single layer): Eexf = E(bulk crystal)—E(single layer). Thus, the exfoliation energy of kaolin is − 38 meV/Å2 (Table 1), compared to − 28.7 meV/Å2 for graphite33. Individual kaolinite layers are obtained at room temperature, but with a curved morphology, due to the defects in matching between the tetrahedral and octahedral sheets. For example, exfoliation of kaolinite and dickite in organic solvents has resulted in the formation of folded structures and mineral nanotubes26.
Metakaolin (Al2O3.2SiO2) is the product of the thermal treatment of kaolinite. The complex transformation from kaolin to MK at the atomic level has been studied by least-squares real-space refinement using neutron pair distribution function (PDF) data and energy minimization with density functional modelling34 and molecular dynamics simulations32. The thermal treatment causes a de-hydroxylation process with subsequent structural transformations. The loss of hydroxyl groups on the surface of the inter-layer spacing causes the aluminum to migrate into the vacant sites of the inter-layer spaces inducing a strain in the aluminum sites, so the aluminum atoms reorganize to relieve the strain. Consequently, all atoms shift their positions, which leads to the buckling of both AlO6 and SiO4 layers. The most significant degree of reorganization corresponds to the AlO6 layers. The SiO4 layers tend to retain their configuration and accommodate according to the changes of the AlO6 layers. Additionally, partial transformation from O to T aluminum coordination has been observed34.
The buckling of the layers (Fig. 1b) is responsible for the atomic disorder and the subsequent absence of Bragg peaks in the X-ray diffraction pattern. Thus, MK maintains short-range order with the original layering of kaolin up to around 920°C37. Even so, without diffusion of the cations across the layers, the structure still retains the 2D layers inherent in kaolinite but with corrugation. The remaining micro-crystalline structure of MK has been revealed using XRD and EDX (see Sections "Alkali-activated material (AAM) from metakaolin (MK)" and "Crystalline phase composition of MK and AAM", respectively). Transmission electron diffraction patterns of the kaolin treated at 500–800 °C show 2D pseudo-hexagonal arrays of spots corresponding to the (hk0) reflections, even when the (001) reflection, corresponding to the third dimension, has disappeared38. Figure 2 presents the crystalline and electronic band structure of a single layer of MK, obtained from simulations (see experimental section).
The low bending rigidity of MK leads the system to form crumpled structures even at room temperature. Nevertheless, a two-dimensional order in particles with sizes up to around 100 nm is still present. Small Angle Scattering (SAXS) reveals a scattered intensity dependence, which is caused by particles with sizes of 10 nm to 100nm39.
At the < 1 nm scale, the average lattice parameters of MK reveal that the dehydration of kaolin results in nearly no change of the layer area. The distance between layers is reduced by about 0.2 Å in average, and the exfoliation energy is slightly reduced to -31 meV/Å2 (Table 1), due to the loss of hydrogen bonds. The radial distribution functions (RDF), shown in Fig. 3, reveal a contrast between the crystalline kaolinite structure and the more disordered structure of MK. The aluminum layer shows more disorder than the silicon layer. While the Si–O bond peak at 1.80 Å remains undisturbed, the Al-O peak at 1.99 Å gains a shoulder at shorter lengths due to the change in Al coordination from octahedral to tetrahedral. The Si–Si and Al-Al nearest neighbor distances at 3.01 Å and 3.19 Å, respectively, are maintained due to the rigidity of the Si–O-Si and Al–O–Al units. However, the peaks corresponding to second nearest Si–Si or Al-Al neighbors are strongly reduced (at 5.40 Å and 5.39 Å) due to the disorder in the Si and Al hexagonal rings. In general, the order associated with the shorter bonds below 2.5 Å is preserved, but no order remains above second nearest-neighbor distances.
As shown in Fig. 2, both kaolinite and MK are wide bandgap insulators, with DFT-PBE bandgaps of 5.0 eV and 2.8 eV, respectively. The reduction of the electronic gap implies a reduction of the material’s work function and electron affinity and, hence, a substantial increase in its chemical reactivity. The valence band is predominantly localized on the oxygen 2p orbitals, whereas the conduction band is made up predominantly of Al and Si states. The smaller bandgap of MK and there are flat bands at the gap edges, corresponding to highly localized states. The removal of the hydrogen attached to the surface oxygen atoms leads to the formation of additional Al-O bonds, resulting in penta-coordinated aluminium, which has been argued to be partially responsible for the high reactivity of metakaolin40.
Alkali-activated material (AAM) from metakaolin (MK)
The process of alkali activation of MK usually involves a series of reactions between the MK powder precursor and alkali cations in a high pH aqueous solution (NaOH, KOH, Na or K liquid silicates, or a combination thereof). After mixing MK and alkaline solution41, the reaction mechanism of MK-based AAM comprises of 4 main steps27: (1) MK dissolution and release of Si and Al species into solution, a process in which Al(V) and Al(VI) turn into Al(IV); (2) the reaction of the Si and Al species with various silicate units present in the alkali solution to form aluminosilicates oligomers; (3) formation of a gel-like, amorphous phase through reorganization (polymerization and gelation); (4) a final process of polycondensation that, depending on temperature and composition, results in a structurally stable 3D matrix.
Using the above-mentioned process, we obtained an alkali-activated material (AAM) from MK and applied it to the formation of a mortar (see experimental section). The effect of the GGBFS and slag, minor components containing Ca, is to increase the final compressive strength of the material [Cement and Concrete Composites 137 (2023) 104925].The atomic compositions of product and precursor were investigated using ICP-OES, yielding a Si:Al mass ratio of 0.38 for the MK precursor, and 5.8 for the AAM. However, this does not reflect the compositions at the crystalline phases and is a direct effect of the slag used, as will be discussed in Section "Crystalline phase composition of MK and AAM". The congruent (proportional Si/Al dissolution) or incongruent (excess Al dissolved) nature of MK dissolution is related to the alkali metal present in the activating solution. Sodium favors superficial and intralayer dissolution (congruent), whereas Potassium, which has a larger ionic size, favors a lateral-to-center dissolution favoring dealumination (incongruent)42. Here, we use an activator comprising both (Na/K ~ 7 mol/mol). The alkaline-earth metals found in our AAM (mostly Ca, limited Mg) come from the slag we added. The complete bulk atomic compositions can be found in Supplementary Information (SI, Table S1).
X-ray diffraction (XRD) analysis was carried out considering the potential presence of kaolinite, quartz, muscovite, anatase, microcline, montmorillonite, illite, anatase and phengite phases, which have been reported in the literature39,43 , for the precursor MK and the resulting AAM. Both the MK and AAM contain SiO2 (α-quartz) and muscovite, and their diffractograms and Rietveld refinement are shown in Fig. 4a and b, respectively. The quartz can be due to the presence of sand in the mortar, or nanocrystal formation during alkali activation, as discussed later.
The quantitative phase composition and the final residuals of the Rietveld refinements extracted from Fig. 4 (details in the experimental section) are presented in Table 2. The refined structural and microstructural parameters of muscovite phase (cell parameters, atomic positions and fractions, atomic angles, atomic distances, size and strain) are reported in the Supplementary information, where we provide Crystallographic Information Files (CIF).
After the refinements, the crystalline and amorphous mass fraction of the samples was estimated, considering that the scattering in amorphous material is the main contribution to the background in the diffractograms44. The total area and the area of the refined background were calculated for each diffractogram, resulting in a crystalline/amorphous mass fraction for AAM of around 17 wt%/83wt% and for MK of around 3wt%/97 wt% (Fig. 4 and Table 2). The detailed phase-specific structural and atomic compositions will be discussed below.
Amorphous phase composition of MK and AAM
The small angle X-ray scattering (SAXS) image data of MK and AAM were integrated using the software GSAS–II and adjusted using the Porod model (Fig. 5). The best fittings resulted in particles with the main sizes around 20 nm for MK and 5 nm for AAM, where latter presents a remarkably narrower particle size distribution than MK (Fig. 5a vs. b). Also, wide-angle x-ray scattering (WAXS) results show low-intensity Bragg reflections for AAM in contrast to MK, are presented in SI (Figure S2).
The amorphous phase in AAM is produced by chemical reaction with the alkali and is inhomogeneous and far from equilibrium, likely consisting in a mixture of amorphous NaAlSi2O6 and SiO2 with a ratio [Si]:[Al] 2:1. Previous molecular dynamics simulations indicate that the dissolution of MK occurs layer by layer as the Na ions penetrate the surface, and that the sublayer of Al tetrahedra is more resistant to dissolution than the sublayer of Si tetrahedra45,46. However, this is a debated mechanism that is dependent on composition and on the dominant cationic species (Na or K)47,48. Regardless, this results in heterogeneity, with multilayer structures remaining due to incomplete reaction. According to microscopy imaging, MK undergoes expansion of the layers during dissolution. Since the dissolution has already been covered by previous studies, here we will concentrate on the structure of the amorphous product of the dissolution reaction.
We use models of NaAlSi2O6 obtained by molecular dynamics simulations at constant temperature to understand the local chemical environments in the amorphous fraction of the AAM. The amorphous SiO2 structure has been extensively studied in previous literature49. The models were obtained using the REAX classical potential50 (more details can be found in the methods section). We have benchmarked the accuracy of the potential by calculating the lattice constant of cubic analcime at 0 K, which is 4% overestimated with respect to the experimental value51.
We start by describing the amorphous NaAlSi2O6 phase, which is similar to the model previously proposed for the K-Poly(sialate-siloxo)52, a three-dimensional structure where both Al and Si form tetrahedra sharing the vertices. The Na atoms occupy the empty galleries or channels of the structure. A typical structure and its RDF are shown in Fig. 6a-b. The shortest bonds are Si–O at 1.79 Å, with a shoulder at 2.20 Å which is due to five-fold coordinated Si. There are additional peaks at about 4.17, 6.72 and 9.07 Å indicating that there is a correlation between the silicon tetrahedra. In contrast, in the Al-O RDF shows a peak at 2.28 Å followed by a broad feature centered at about 4.6 Å. This is due to the preference of Al tetrahedra for sharing corners with Si tetrahedra rather than with another Al tetrahedra. For the same reason, the Si–Si RDF shows multiple peaks, the first at 3.21 Å, and subsequently at about 5.7, 8.01 and 10.8 , but the Al-Al RDF shows a single sharp peak for nearest neighbors at 3.50 Å, followed by a broad feature centered at about 6.9 Å. Note that the radial distribution functions are computed for an infinite material, and therefore does not show a long-range extinction due to the particle size, which will be discussed later.
Crystalline phase composition of MK and AAM
The crystalline phases of MK and AAM were observed using SEM, and both present very defined layered structures in their bulk conditions. While MK is constituted of more loosely and disordered stacked sheets with large size distribution (Fig. 7a), AAM presents a more compact layered structure embedded into an amorphous phase (Fig. 7b), akin to a sintered structure53.
For better visualization of the fundamental structure of the layered crystalline phases, MK and AAM were exfoliated using an adaptation of a liquid biphasic exfoliation system (LBFE, details in the experimental section)54, to decrease the in-plane crystal fracturing implied by cavitation. Moreover, LBFE allowed breaking apart the different phases of the mortar and reveal the clean remaining crystalline 2D phase of AAM by stabilizing it at the solvent’s interphase, and isolated collection. Upon exfoliation, the MK clusters become partially unstacked (Fig. 7c) revealing smaller micro-aggregates that are 40-60 nm thick, and few-layer (~ 6 nm thick) platelets with lateral sizes in the hundreds of nanometers (Fig. 7d-e). Differently, AAM aggregates break apart into wider sheet structures, followed mostly by attached amorphous debris (Fig. 7f). The clusters are an order of magnitude thinner than MK’s (Fig. 7g-h), and on top of them very fine 2D particulates can be found presenting thicknesses below 6 nm. In fact, the thinnest observed structures reach 2 nm, which is most likely a monolayer sheet.
Altogether, MK presents an overall more brittle structure and breaks apart into a random sheet size distribution, while AAM presents fewer but better preserved and larger sheets with thinner thickness profiles. In fact, AAM seems to reach close to monolayer structure while preserving (or reconstructing) a large lateral size (even after exfoliation), and its amorphous phase seems to be broken down by the process.
Using SEM/EDX and XPS we evaluate the atomic composition of the exfoliated/isolated crystal phases. The MK composition presents a ratio [Si]:[Al] ~ 1:1 with both EDX and XPS (Fig. 8a and c), and this ratio has only a small variation before and after exfoliation (Figure S3a). On the other hand, although AAM presents also a corroborating ratio between EDX and XPS, it has a largely increased silicon content ([Si]:[Al] ~ 4:1, Fig. 8b and d), which is due to the addition of sand, silica fume and GGBFS, and falls to half after exfoliation ([Si]:[Al] ~ 2:1, Figure S3b). The inset in Fig. 8b shows a large discrepancy in Si content when comparing the sheet area (demonstrated as the red dotted area) and the peripheric regions. This indicates that most of the Si excess is present in the amorphous phase as SiO2 clusters, or quartz from the SLAG and aggregates. During the alkali-activation, the dissolution of MK in the alkaline solution favors the formation of the SiO2 phase due to the separation of the SiO4 tetrahedral sheets and the AlO6 octahedral sheets. This could explain the excess formation of the amorphous silica found in the AAM samples. However, the crystal composition seems to also favor the inclusion of Si in the final composition after alkali activation, as [Si]:[Al] from the crystalline phase of AAM is twice as large as MK’s (see SI, Figure S3).
Atomic distribution of (a–c) MK and (b–d) AAM as obtained by EDX and XPS, respectively. The inset in (b) shows an SEM/EDX image with heterogeneous Al (blue) and Si (yellow) distribution when comparing the crystalline and amorphous phases in AAM, and the red dotted line highlights the edge limits of an AAM crystallite.
The 2D order of the residual nanocrystals affects the formation of the amorphous surrounding of the crystalline phases in AAM, as observed by the SAXS profiles and corresponding particle size distributions. The SAXS intensity follows a power-law decay, in which the high-q tail of scattering is caused by particles with a narrow distribution of sizes between ~ 30 and ~ 50 nm (Fig. 5b), which is consistent with the reported formation of SiO4 and AlO4 tetrahedra in the structure of AAMs43. Furthermore, these sizes correspond to a solidification after a reaction in a liquid solution, which could allow for the nucleated/templated growth of some of this material as a continuous crystalline phase or the direct crosslinking between crystalline and amorphous phases. Such crystal growth behavior during alkali-activation were previously observed55. However, in our systems they seem to be nucleated by the residual crystals, forming a Si-rich and heterogeneous composition in the transition between the crystal and amorphous phases (see Fig. 8b, inset). However, we cannot exclude the possibility that some of the crystals are residual, from the mortar powder fillers.
Conclusion
We have shown that a crystalline 2D material, namely kaolinite, can be converted into a 3D amorphous material with short range 2D order by chemical reaction in an alkaline medium. This dimensional transformation, combined with an order to disorder mechanism, is possible by the consumption of the 2D material due to its large surface chemical reactivity. However, the chemical reaction is self-limiting resulting in a complex structure, an intermediate between a 3D glass and a 2D crystal, where the nanoscale 2D crystalline grains are surrounded by an amorphous 3D structure with short range order. The final material can have many applications including as a green cement since it does not produce carbon dioxide during its transformation, as is the case of traditional Portland cement.
Materials and methods
Materials
The precursor and reagent used for the synthesis of AAM are a commercially available metakaolin under the brand name Metamax®. (ii) The liquid alkali silicate is a mixture of Na and K silicates, M = 1.5 and water content of 56 wt%. Na and K silicates were supplied by ISPL (www.ispl.com.sg, brand ISPL 51) and Noble Alchem (http://noblealchem.com, brand PSLP K13), respectively. (iii) The silica fume was supplied by Elkem (www.elkem.com, brand 920D). (iv) The Slag (GGBFS) was supplied by Engro (www.engro-global.com, brand P8000). (v) NaOH and KOH were supplied by Sigma Aldrich.
Methods
Formulation and processing of the reagent
The reagent used for binder synthesis is a mixture of Na and K liquid silicates (composition described in the materials section), the modulus M of both being ~ 1.5. The original M of the as-delivered Na and K silicates have been altered to M = 1.5 by adding NaOH and KOH, respectively. After correction and mixing, the resulting alkali solution was left to rest for at least 24 h prior use.
Formulation and processing of AAM (binder)
The AAM used as a binder comprises metakaolin, composite liquid alkali silicate (described above), and silica fume and GGBFS as minor components. Materials in the mix design were proportioned to result in a theoretical composition in which (molar ratios) SiO2/Al2O3 = 4, Al2O3/(Na,K)2O = 1, CaO/SiO2 = 0.05, and H2O/(Na,K) = 11. More specifically for the composition characterized in this study, the mass fractions used were as follows: Metakaolin: 33.5 wt%, liquid alkali silicates: 52 wt% (M = 1.5), silica fume: 7 wt%, GGBFS: 7.5 wt%. After mixing the solids and the liquid silicate using an IKA EUROSTAR 40 mechanical stirrer at 600 rpm, the stirring was kept long enough to remove all visible agglomerates.
Formulation and processing of the mortar
The mortar, which is a combination of a cementitious agent and aggregates, was prepared mixing the binder above described and river sand (quartz, d90% = 2 mm, d50% = 0.5 mm and d10% = 0.04 mm) in the proportion of 1L of binder to 3L of aggregates, using a Matest E092N lab mortar mixer (Matest, Italy). After mixing, the mortar was cast in prismatic molds (15 × 15x30mm) and left to cure at room temperature for 28 days before use. All characterization of the powder AAM were performed using this mortar after finely grinded.
Liquid biphasic exfoliation (LBFE)
MK and AAM were exfoliated using an adaptation of a liquid biphasic exfoliation system 4,54, containing water and dichloromethane (DCM) as the exfoliation media. Briefly, 1 g of MK or finely grinded mortar containing AAM were added to a 250 mL round bottom flask containing 90 mL of water and 10 mL of DCM. The round bottom flask with the mixture was then sonicated, using a 32 kHz/500W bath ultrasound (Bandelin) for 4 h, with a constant temperature of 10 °C. After exfoliation, the dispersion was left to rest overnight, for the large particulates to settle, forming a phase separation at the bottom. Then, 100μL of the water-phase (top) supernatant was collected close from the solvent interphase and dripped on SiO2 coated Si substrates, which were left to dry at 45 °C for ~ 6 h. These substrates were used for AFM and SEM characterizations.
Characterization methods
XRD: X-ray powder diffraction data were collected in a standard laboratory diffractometer Rigaku-Dairix with CuKα radiation and Bragg–Brentano geometry. The diffractometer was set in step-scan mode with steps of 0.01° and with a counting time of 5 s. The powder samples were prepared by back-mounting to avoid preferential orientation. Solid samples were cut into slices with the proper dimensions to collect diffraction data in the same conditions. The QPA and crystal structure refinement were performed by the Rietveld method using the General Structure Analysis System-II (GSAS – II). The software uses an axial-divergence-broadened pseudo-Voigt as the peak profile function and allows refinement of the parameters of Gaussian and Lorentzian widths for each individually fitted peak. The instrumental parameters for profile refinement were initially determined using the diffraction data of a silicon standard sample. This allows the refinement of physically meaningful parameters for the analyzed samples56, producing reliable quantitative measurements without the use of internal standards57.
SAXS/WAXS: Powder samples were analyzed for Wide and Small-angle X-ray scattering (WAXS and SAXS) using a standard laboratory small- and wide-angle X-ray scattering equipment Xenocs Xeuss 2.0 SAXS/WAXS with wavelength λ = 1.5418 Å. The samples were disposed of in stainless steel washers of 1 cm diameter and covered with a Kapton tape, which is commonly used for its high transparency to X-rays in the SAXS region. The analysis’s q-range extends from 0·05 Å−1 to 0.9 Å−1.
SEM-FESEM: The characterization by field emission scanning electron microscopy (FESEM) with energy dispersive x-ray (EDX) was performed at an equipment FEI Verios 460 L. Samples of the precursor MK and AAM were coated with a gold layer of approximately 5 nm thickness and analyzed using Carl Zeiss AG—SUPRA 40 equipment.
Elemental analysis to quantify carbon, nitrogen, hydrogen, sulphur, and oxygen (CNHS-O) was performed using an organic elemental analyzer Vario El cube (Elementar – Germany).
AFM: Drop-casted dispersions on SiO2/Si substrate were imaged using a Bruker Dimension FastScan® Atomic Force Microscope (AFM) in a tapping mode with a silicon tip on silicon nitride cantilever (T: 0.6 um, L: 27 µm, W: 32 µm, f0: 1400 kHz, k: 18 N/m). The images were obtained with a pixel resolution of 512 sample/line. The image processing and height profiles were performed using NanoScope Analysis software.
X-ray photoelectron spectroscopy (XPS): The powder samples were analyzed by XPS on a Thermo Escalab 250Xi using AlKα radiation. Survey spectra were collected with a pass energy of 150 eV and a step size of 1 eV. High-resolution spectra were collected with a pass energy of 30 eV and a step size of 0.05 eV. During each scan, an ion gun was utilized to neutralize the charging effects.
Chemical elemental analysis (CNHS-O and ICP-OES): Quantification of carbon, nitrogen, hydrogen, sulphur, and oxygen (CNHS-O) was performed in an organic elemental analyzer, Vario El cube -Elementar. For analysis by Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES), the powder samples were digested with HNO3/HCl (3:1) in a microwave oven at 240 °C for 15 min and topped up to 14 mL with H2O. A Perkin Elmer Avio 500 equipment was used for the analysis of the metal species. In total, 65 atomic species were analyzed, while 28 were detectable and the rest were below the detection limits of the method.
Computational methods
Density functional theory calculations
We have modelled kaolinite and MK using density functional theory calculations based on the models proposed in Ref.34. The calculations were carried out using the SIESTA package58. The core electrons were modelled using pseudopotentials of the Troullier-Martins type59. The bases set for the Kohn–Sham states are linear combinations of numerical atomic orbitals (a single zeta polarized basis for hydrogen and double zeta polarized basis for all other species). The charge density was projected on a real-space grid with an equivalent cut-off energy of 250 Ry to calculate the exchange–correlation and Hartree potentials. Structural relaxations were performed using conjugate gradient optimization.
The lattice parameters of kaolinite, obtained with the PBE functional60, are more accurate than those obtained with the LMKLL functional, which is more commonly used for van der Waals bonded systems61 (see Table 1). Both functionals predicted accurately the inter-layer distance. We therefore will use the PBE functional for the calculations unless otherwise stated.
Classical molecular dynamics simulations
Classical molecular dynamics simulations were performed using the LAMMPS code62, using the REAX potential developed by50. The amorphous structure of NaAlSi2O6 was prepared by starting from a 3 × 3x3 supercell of analcime with 6 × 32 formula units, which was first expanded to double its equilibrium size for an initial amorphization step, followed by annealing at 900 K for 8 ns at the fixed volume corresponding to the low temperature lattice constant, following by a 20 ps quench to 300 K. The amorphous structure of NaAlSi3O8 was prepared by starting from a 3 × 2x3 supercell of albite. It was initially amorphized by a short annealing with all the lattice parameters expanded by double. Then, it was annealed at 900 K at the experimental volume, with orthogonal lattice parameters 24.4, 26.0 and 19.2 Å.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Courland, R. Concrete Planet: The Strange and Fascinating Story of the World’s Most Common Man-Made Material. (Prometheus, 2022).
Kriven, W. M. et al. Why geopolymers and alkali‐activated materials are key components of a sustainable world: A perspective contribution. Journal of the American Ceramic Society n/a (2024).
IEA. Technology Roadmap - Low-Carbon Transition in the Cement Industry. (2018).
Beneš, H. et al. Direct delamination of graphite ore into defect-free graphene using a biphasic solvent system under pressurized ultrasound. RSC Adv. 6, 6008–6015 (2016).
Liu, T. et al. Preparation of organic-free two-dimensional kaolinite nanosheets by in situ interlayer fenton reaction. ChemistrySelect 4, 11604–11608 (2019).
Donato, K. Z. et al. Graphene oxide classification and standardization. Sci Rep 13, 6064 (2023).
Zhu, Y., Ji, H., Cheng, H.-M. & Ruoff, R. S. Mass production and industrial applications of graphene materials. Natl Sci Rev 5, 90–101 (2018).
Owuor, P. S. et al. Roadblocks faced by graphene in replacing graphite in large-scale applications. Oxford Open Materials Science 1, itab004 (2020).
Björk, J., Zhou, J., Persson, P. O. Å. & Rosen, J. Two-dimensional materials by large-scale computations and chemical exfoliation of layered solids. Science 1979(383), 1210–1215 (2024).
Islam, M. A. et al. Exfoliation mechanisms of 2D materials and their applications. Appl Phys Rev 9, 41301 (2022).
Kaur, H. & Coleman, J. N. Liquid-phase exfoliation of nonlayered non-Van-Der-Waals crystals into nanoplatelets. Advanced Materials 34, 2202164 (2022).
Puthirath Balan, A. et al. Exfoliation of a non-van der Waals material from iron ore hematite. Nat Nanotechnol 13, 602–609 (2018).
Seymour, L. M. et al. Hot mixing: Mechanistic insights into the durability of ancient Roman concrete. Sci Adv 9, eadd1602 (2023).
Luukkonen, T. Alkali-activated materials in environmental technology: introduction. in Alkali-Activated Materials in Environmental Technology Applications 1–12 (Elsevier, 2022).
Handbook of Clay Minerals. (CALLISTO REFERENCE, 2015).
Bergaya, F. & Lagaly, G. General Introduction. in Developments in Clay Science vol. 5 1–19 (Elsevier, 2013).
Brigatti, M. F., Galán, E. & Theng, B. K. G. Structure and Mineralogy of Clay Minerals. in Developments in Clay Science vol. 5 21–81 (Elsevier, 2013).
Leonelli, C. & Palomo, A. Chemistry and materials science of alkali-activated materials. in Alkali-Activated Materials in Environmental Technology Applications 13–40 (Elsevier, 2022).
Liu, T., Zhou, H., Graham, N., Yu, W. & Sun, K. 2D kaolin ultrafiltration membrane with ultrahigh flux for water purification. Water Res 156, 425–433 (2019).
Zhang, X. et al. Improved sodium adsorption by modified kaolinite at high temperature using intercalation-exfoliation method. Fuel 191, 198–203 (2017).
Zhang, Q. et al. Chemically modified kaolinite nanolayers for the removal of organic pollutants. Appl Clay Sci 157, 283–290 (2018).
Zhang, Y. et al. Intercalated 2D nanoclay for emerging drug delivery in cancer therapy. Nano Res 10, 2633–2643 (2017).
Kauling, A. P. et al. The worldwide graphene flake production. Advanced Materials 30, 1803784 (2018).
Paton, K. R. et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat Mater 13, 624–630 (2014).
Nizri, G., Makarsky, A., Magdassi, S. & Talmon, Y. Nanostructures formed by self-assembly of negatively charged polymer and cationic surfactants. Langmuir 25, 1980–1985 (2009).
Detellier, C. & Letaief, S. Kaolinite–Polymer Nanocomposites. in Developments in Clay Science vol. 5 707–719 (Elsevier, 2013).
van Deventer, J. S. J., Provis, J. L., Duxson, P. & Lukey, G. C. Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. J Hazard Mater 139, 506–513 (2007).
Duxson, P., Mallicoat, S. W., Lukey, G. C., Kriven, W. M. & van Deventer, J. S. J. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf A Physicochem Eng Asp 292, 8–20 (2007).
Ma, G., Bai, C., Wang, M. & He, P. Effects of Si/Al Ratios on the Bulk-Type Zeolite Formation Using Synthetic Metakaolin-Based Geopolymer with Designated Composition. Crystals (Basel) 11, 1310 (2021).
Wan, Q. et al. Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem Concr Compos 79, 45–52 (2017).
Bish, D. L. Rietveld refinement of non-hydrogen atomic positions in kaolinite. Clays Clay Miner 37, 289–296 (1989).
Sperinck, S., Raiteri, P., Marks, N. & Wright, K. Dehydroxylation of kaolinite to metakaolin—A molecular dynamics study. J. Mater. Chem. 21, 2118–2125 (2011).
Wang, W. et al. Measurement of the cleavage energy of graphite. Nat Commun 6, 7853 (2015).
Meral, C., Benmore, C. J. & Monteiro, P. J. M. The study of disorder and nanocrystallinity in C-S–H, supplementary cementitious materials and geopolymers using pair distribution function analysis. Cem Concr Res 41, 696–710 (2011).
Cutini, M., Maschio, L. & Ugliengo, P. Exfoliation energy of layered materials by DFT-D: Beware of dispersion!. J Chem Theory Comput 16, 5244–5252 (2020).
Bish, D. L. & Johnston, C. T. Rietveld refinement and fourier-transform infrared spectroscopic study of the dickite structure at low temperature. Clays Clay Miner 41, 297–304 (1993).
Lee, S., Kim, Y. J. & Moon, H.-S. Phase transformation sequence from kaolinite to mullite investigated by an energy-filtering transmission electron microscope. Journal of the American Ceramic Society 82, 2841–2848 (1999).
Chakraborty, A. K. Phase Transformation of Kaolinite Clay. (Springer India, New Delhi, 2014).
Kovářík, T. et al. Structural and physical changes of re-calcined metakaolin regarding its reactivity. Constr Build Mater 80, 98–104 (2015).
Moya, J. S., Cabal, B., Lopez-Esteban, S., Bartolomé, J. F. & Sanz, J. Significance of the formation of pentahedral aluminum in the reactivity of calcined kaolin/metakaolin and its applications. Ceram Int 50, 1329–1340 (2024).
Davidovits, J. Geopolymers Based on Natural and Synthetic Metakaolin a Critical Review. in Proceedings of the 41st International Conference on Advanced Ceramics and Composites 201–214 (John Wiley & Sons, Ltd, 2018). https://doi.org/10.1002/9781119474746.ch19.
N’Guessan, N. E. et al. Role of cations on the dissolution mechanism of kaolinite in high alkaline media. Appl Clay Sci 205, 106037 (2021).
Caballero, L. R., Paiva, M. D. D. M., Fairbairn, E. D. M. R. & Toledo Filho, R. D. Thermal, mechanical and microstructural analysis of metakaolin based geopolymers. Materials Research 22, e20180716 (2019).
Scarlett, N. V. Y. & Madsen, I. C. Quantification of phases with partial or no known crystal structures. Powder Diffr 21, 278–284 (2006).
Hong, F. et al. Study on the mechanical properties, gelling products and alkalization process of alkali-activated metakaolin: From experiment to molecular dynamics simulation. Journal of Building Engineering 79, 107705 (2023).
Hou, D. et al. Molecular insights into the reaction process of alkali-activated metakaolin by sodium hydroxide. Langmuir 38, 11337–11345 (2022).
Garg, N. & Skibsted, J. Dissolution kinetics of calcined kaolinite and montmorillonite in alkaline conditions: Evidence for reactive Al(V) sites. Journal of the American Ceramic Society 102, 7720–7734 (2019).
Scherb, S., Köberl, M., Beuntner, N., Thienel, K.-C. & Neubauer, J. Reactivity of metakaolin in alkaline environment: correlation of results from dissolution experiments with XRD quantifications. Materials 13, 2214 (2020).
Sarnthein, J., Pasquarello, A. & Car, R. Structural and electronic properties of liquid and amorphous SiO2: An ab initio molecular dynamics study. Phys Rev Lett 74, 4682–4685 (1995).
Psofogiannakis, G. M., McCleerey, J. F., Jaramillo, E. & van Duin, A. C. T. ReaxFF reactive molecular dynamics simulation of the hydration of Cu-SSZ-13 Zeolite and the formation of Cu dimers. The Journal of Physical Chemistry C 119, 6678–6686 (2015).
High-resolution neutron powder diffraction. Line, C. M. B., Dove, M. T., Knight, K. S. & Winkler, B. The low-temperature behaviour of analcime. 1. Mineral Mag 60, 499–507 (1996).
Davidovits, J. GEOPOLYMERS: Man-made rock geosynthesis and the resulting development of very early high strength cement. Journal of Materials Education 16, 91–139 (1994).
Zhao, H., Yang, Z. & Guo, L. Nacre-inspired composites with different macroscopic dimensions: strategies for improved mechanical performance and applications. NPG Asia Mater 10, 1–22 (2018).
Godoy, A. P. et al. Ultrasound exfoliation of graphite in biphasic liquid systems containing ionic liquids: A study on the conditions for obtaining large few-layers graphene. Ultrason Sonochem 55, 279–288 (2019).
Chen, S., Wu, C. & Yan, D. Binder-scale creep behavior of metakaolin-based geopolymer. Cem Concr Res 124, 105810 (2019).
Toby, B. H. & Von Dreele, R. B. GSAS-II : The genesis of a modern open-source all purpose crystallography software package. J Appl Crystallogr 46, 544–549 (2013).
Madsen, I. C., Scarlett, N. V. Y., Cranswick, L. M. D. & Lwin, T. Outcomes of the international union of crystallography commission on powder diffraction round robin on quantitative phase analysis: Samples 1 a to 1 h. J Appl Crystallogr 34, 409–426 (2001).
Soler, J. M. et al. The SIESTA method for ab initio order- N materials simulation. Journal of Physics: Condensed Matter 14, 2745–2779 (2002).
Troullier, N. & Martins, J. L. Efficient pseudopotentials for plane-wave calculations. Phys Rev B 43, 1993–2006 (1991).
Perdew, J. P. et al. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46, 6671–6687 (1992).
Lee, K., Murray, É. D., Kong, L., Lundqvist, B. I. & Langreth, D. C. Higher-accuracy van der Waals density functional. Phys Rev B 82, 081101 (2010).
Thompson, A. P. et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput Phys Commun 271, 108171 (2022).
Acknowledgements
This research, including the computational calculations, was carried out at the Centre for Advanced 2D Materials (CA2DM), funded by the National Research Foundation, Prime Minister’s Office, Singapore, under its Medium-Sized Centre Programme, and by the Singapore Ministry of Education under its Research Centre of Excellence award to the Institute for Functional Intelligent Materials, National University of Singapore (I-FIM, project No. EDUNC-33-18-279-V12). The National Supercomputing Centre, Singapore (NSCC) is acknowledged for providing computational resources.
Author information
Authors and Affiliations
Contributions
J.V.E., J.E.L. and A.H.C.N. conceptualized the project. D.K., V.R., J.J., K.G., J.V.E., and R.K.D. performed the synthesis. R.K.D. and K.Z.D. performed the exfoliation protocol. J.A.G.C., G.K.W.K., K.Z.D., X.H.Y., D.K., K.L., V.R., J.J., K.G., J.V.E., and R.K.D. performed the characterization. A.C. performed the simulations. J.A.G.C., A.C., R.K.D., and A.H.C.N. led the manuscript writing with the assistance of all co-authors. A.H.C.N. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carrio, J.A.G., Donato, R.K., Carvalho, A. et al. From 2D kaolinite to 3D amorphous cement. Sci Rep 15, 1669 (2025). https://doi.org/10.1038/s41598-024-81882-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-81882-1