Abstract
Vaginal reconstruction is necessary for various congenital and acquired conditions, including vaginal aplasia, trauma, tumors, and gender incongruency. Current surgical and non-surgical treatments often result in significant complications. Decellularized vaginal matrices (DVMs) from human tissue offer a promising alternative, but require effective sterilization to ensure safety and functionality. This study aimed to develop a sterilization method for decellularized human vaginal wall scaffolds. Based on our previously implemented decellularization technique with minor modifications, we designed and examined three sterilization methods consisting of (i) chemical decellularization, (ii) decellularization with additional peracetic acid/hydrogen peroxide (PAA/H2O2); (iii) decellularization with antibiotic and antimycotic (AAE) based treatment. Sterilization efficacy was evaluated through controlled contamination with common vaginal microbes and sterility testing subsequent to each sterilization method. The extracellular matrix (ECM) structure was assessed via histological staining. Decellularization alone reduced some added bacterial contaminants but did not achieve complete sterilization. PAA/H2O2-sterilization resulted in severe ECM damage, rendering it unsuitable. The AAE-treatment demonstrated effective sterilization without compromising the ECM structure. Combined decellularization and AAE-based treatment forms a viable sterilization method for human vaginal wall tissue, maintaining ECM integrity and achieving effective micro-organism elimination. This method holds potential for clinical application in vaginal transplantation.
Similar content being viewed by others
Introduction
The vagina wall is a fibromuscular tube, that plays an essential role in sexual intercourse, discharge of menstrual blood and in childbirth1. Vagina reconstruction is needed for many congenital or acquired diseases, including vaginal aplasia, trauma, tumor growth (with radiation damage) and feminine gender incongruency2. Several surgical and nonsurgical treatments are available, but these are all associated with serious drawbacks3,4,5. Long-term nonsurgical dilation might not be optional and causes pain, mental and emotional distress and high risk of stenosis3,4,6,7, whilst surgical treatments carry risk of stenosis, necrosis, vaginal prolapse, and donor site morbidity5,8,9,10,11,12. Furthermore, surgery with use of a non-vaginal graft results in complications and complaints related to dyspareunia13,14,15, insensibility16,17, and excessive or lack of vaginal discharge18,19. Therefore, alternative treatment options based on transplantation or tissue engineering techniques are explored.
Approximately 90,000 whole organ transplantations are performed worldwide each year20. Amongst transplantation, a 1% occurrence of transmitted infections is reported, but these are generally only recognized after clusters of infections are observed in recipients with a common donor. Furthermore, in case of transmitted infections the risks are severe and include sepsis, graft rejection, and postoperative morbidity and mortality20. Thus, success of transplantation relies on effective sterilization. In answer to a shortage of organ donors, recent developments in the field of tissue engineering and regenerative medicine have resulted in promising new biomaterials for tissue replacement of various organs21, including the vagina. Specifically for reconstruction of the vagina, decellularized matrices from skin22,23,24, small intestinal submucosa25,26,27,28,29,30 (SIS) and urinary bladder matrix31,32 (UBM) have been applied. However, xenogeneic-derived decellularized matrix may cause severe immunologic reactions33 and autologously-derived materials may cause donor site morbidity34. Alternatively, decellularized vaginal matrix (DVM) could be used for vagina reconstruction1,35,36,37, which is an extracellular matrix (ECM)-derived bioscaffold. In a previously conducted study, we have developed a decellularized matrix from healthy human vagina donor tissue conform established criteria for acellular biomaterials2. Compared with previously mentioned biomaterials, DVM can promote formation of neo-vagina rapidly1.
Our method and several other decellularization procedures result in acellular vaginal wall tissue with retained biomechanical integrity, important ECM proteins, and tissue aspects essential for cell adhesion, survival and proliferation1,35,36,37. Although current immunosuppression therapy has improved the chance of graft survival by preventing tissue rejection, clinical application of tissue-engineered transplants in immunocompromised hosts is challenging due to a higher susceptibility to infections. Therefore, a terminal sterilization method prior to implantation is required38. The vaginal microbiota contains vaginal Lactobacillus species (Lactobacillus crispatus, Lactobacillus iners, Lactobacillus gasseri or Lactobacillus jensenii)39,40,41,42,43. In the human vaginal microbiota typically also a predominance of Actinobacteria, Prevotella, Veillonellaceae, Streptococcus, Probacteria, Bacteroides, Bifidobacteriaceae, and Burkholderiales is observed42. However, many aspects can temporally change the vaginal microbiota, causing differences among individuals due to variation in sexual activity, douching, chronic stress, race, medication, diet, smoking or pregnancy40,44. Also an abnormal vaginal microflora may occur due to the presence of for example (sexually transmitted) infections i.e., Streptococcus pneumoniae, Haemophilus influenzae, Listeria monocytogenes, or due to an overgrowth of naturally occurring species such as Escherichia coli or Staphylococcus aureus42,43. Among non-bacterial species, Candida albicans is a well-known fungus to cause vaginal infections44. To minimize the risk of disease transmission to clinically acceptable levels, sterilization of biomaterials is required45. This extends their potential use beyond the sole treatment of life-threatening conditions, to patients who could benefit from their use for reconstruction of other organs45, such as vagina or skin. Sterilization of vaginal matrix would thus minimize risks and increase the clinical acceptability of human donor vagina.
A sterilization method should be validated according to a predefined applicable standard for that specific medical device or biomaterial. To this aim, an ideal sterilization method should be able to eliminate all bioburden present in a biomaterial and prevent rigorous changes to its biological and mechanical properties that could compromise the integrity and function of an implant46.
A multitude of validated processing methods have been published to render a biomaterial sterile. Gamma irradiation is known to cause major structural damage47,48. Ultraviolet (UV) irradiation has a weak penetration capacity and is ideal for surface disinfection, but unsuitable for use on liquid medicines and for internal disinfection of solids46. Hydrogen peroxide (H2O2) is a disinfectant that can achieve a sterilization effect under certain conditions49, and sterilization by hydrogen peroxide low-temperature plasma (HPLP) is a common method in hospitals46. However, H2O2 generates many active oxygen and high-energy free radicals, that cause cytomembrane disruption and denaturation of proteins and nucleic acids50. The strong oxidation may further affect the physical and chemical properties of biomaterials51. Ethylene oxide (ETO) is an effective sterilization agent, but requires the use of an ETO device and further produces toxic reaction products, such as ethylene chlorohydrin52,53. Both ETO and these reaction products can cause severe pathological changes in implants45. ETO has been classified as mutagen and carcinogen, which has caused strict regulations around its use for sterilization45. Sporadically, supercritical carbon dioxide (ScCO2) is used to disinfect or sterilize biomaterials. However, its exact effect on killing pathogens is unknown and ScCO2 may have effect on the physical and chemical properties of a biomaterial54,55, and could further cause partial dissolution of proteins by acidification46.
For these reasons we looked at an alternative sterilization procedure for human DVM. Peracetic acid (PAA) is a common disinfectant, but can also act as sterilizing agent with a demonstrated effect against bacteria, viruses, and spores56,57,58. Use of PAA has been approved by the Food and Drug Administration (FDA)59, and has been applied at concentrations of 0.05–0.1% for sterilization of a variety of organs (i.e., cardiac and arterial valves60, lung61, liver62,63, bladder64,65,66, spleen67,68, kidney69,70, small intestine71,72,73, tendon74,75 and nerve76) and biomaterial tissues (i.e., bone, heart valves, pericardium, liver, small intestine, lung, kidney, spleen, bladder, tendon and porcine vagina)35,46. PAA-sterilization does carry concerns about residual reactions after biomaterial implantation, and its potential hazard to PAA handling staff and the environment need to be considered45,46. Furthermore, the oxidation and acidity of PAA can potentially affect the physical and chemical properties of a biomaterial64,73.
Alcohol is a disinfectant known to cause denaturation of proteins77. However, short soaking (5 min to 4 h) of biomaterials in alcohol combined with other disinfectant and sterilization agents have previously resulted in successful sterilization of biomaterial scaffolds for reconstruction (i.e., pericardium78, heart valve79, blood vessel80, liver81,82, pancreas83, trachea84, bladder83, kidney69, bone85, tendon86,87 and nerve88). Long exposure to alcohol at high concentrations is further known to potentially denature proteins89. Antibiotics are not typically used for disinfection or sterilization, but can kill micro-organisms through various mechanisms90. The most common antibiotics applied for cell cultures, tissue engineering and regenerative medicine are penicillin and streptomycin. Penicillin inhibits synthesis of bacterial cell walls, whereas streptomycin prevents protein translation of bacteria through its binding to a ribosome subunit46. Amphotericin-B is an antimycotic agent often added to cell cultures (in combination with antibiotics), and inhibits the growth of fungi and some protozoa by affecting the permeability of cell membranes46. Antibiotics and antimycotics do not affect tissue properties, but streptomycin is one of the antibiotics that could cause toxic reactions in the human body at high residual concentrations46.
The aim of this study was to overcome limitations associated with previous sterilization methods, by developing a sterilization method for human DVM. Three methods were designed and tested: (i) sterilization by chemical decellularization2, (ii) sterilization by chemical decellularization and additional 0.01%, 0.05% or 0.1% PAA/H2O2 treatment and (iii) decellularization and additional mixed antibiotic, antimycotic and alcohol treatment. A 0.01–0.1% PAA range was examined to minimize structural damage. The developed protocols were histologically assessed in terms of structural extracellular matrix preservation. Furthermore, controlled microbial contamination of vaginal wall tissue was performed prior to decellularization combined with sterilization, and the induced level of sterility was evaluated.
Materials and methods
Surgical procedure
Vaginal tissue was retrieved from six healthy transmasculine donors during colpectomy at Amsterdam UMC (the Netherlands) between December 2023 and June 20242. No tissue was procured from prisoners. As previously described, robotic-assisted laparoscopic colpectomy (daVinci XI system, Intuitive) was performed as part of the donor’s medical transition according to an established method91. Vaginal epithelium was dissected with monopolar scissors, and bleeding was minimized with fenestrated bipolar forceps92. Tissue from vaginal epithelium, lamina propria and smooth muscle was removed. Vaginal adventitia was preserved to prevent fistula to bladder, urethra or rectum, bleeding from perivaginal plexus and nerve injury to adjacent structures92. Per donor, three vaginal wall rings were obtained. After surgical retrieval, the vaginal tissue was directly stored in phosphate buffered saline (PBS—Fresenius Kabi), pH 7.4, on ice for a maximum of 4 h until decellularization. Written informed consent for experimental use of resected vaginal tissue was obtained from all donors. Tissue collection and experimental usage was approved by the institutional Medical Ethical Examination Committee of Amsterdam UMC location VU Medical Center (Amsterdam; IRB approval by METc VUmc registration number 2018/3190, October 2018) and performed in accordance with relevant guidelines and regulations.
Experimental design
Decellularized vaginal scaffolds were prepared from retrieved human vaginal wall tissue utilizing a previously established protocol2. In order to validate sterilization efficacy, an extensive assessment protocol was established (Fig. 1). It includes (i) identification of naturally present micro-organisms and residual bioburden, (ii) controlled contamination of vaginal tissue blocks with confirmation test, (iii) histological assessment of ECM architectural damage; (iv) quantitative efficacy validation of sterilization.
Flowchart assessment of sterilization efficacy. Naturally occurring micro-organisms in donated vaginal wall tissue were identified in negative controls. After controlled contamination with L. crispatus, S. aureus, E. coli and C. albicans strains at known concentrations, contamination was confirmed. The efficacy of decellularization with and without sterilization on these contaminants was assessed through blood agar plating of tissue stamps and tissue homogenate.
This experimental design ensures initial investigation of negative controls of donated vaginal wall tissue for naturally occurring micro-organisms and potential bacterial and fungal infection by blood agar stamps, and by plating of homogenized vaginal tissue on blood agar. Next, a controlled contamination was performed with micro-organisms commonly found in the human vagina microbiota. Controlled contamination was performed with one micro-organism per sample and confirmed by plating of the last wash medium on blood agar, and plating of homogenized tissue on blood agar. Tissue samples were then exposed to our sterilization methods. As extensive ECM damage can be inflicted during sterilization, the structural architecture of samples was assessed by Haematoxylin and Eosin (H&E) staining. Finally, sterilized test samples were stamped on blood agar and homogenized tissue was plated on blood agar to test. Residual micro-organisms from controlled contamination were quantified (number of colony-forming units per material) and identified by mass spectroscopy. All experiments were conducted thrice.
Sterilization
Three sterilization methods were developed. These are based on (i) our original decellularization protocol2, (ii) our decellularization protocol combined with PAA/H2O2 treatment and (iii) our decellularization protocol with addition of a 1% and 10% mixture containing penicillin, streptomycin and amphotericin-B, and a final washing step with 70% ethanol.
Controlled contamination
A controlled contamination of the human vaginal wall tissue was performed. The selected bacterial (aerobic or anaerobic, gram+ or gram−) and fungal strains are commonly found in the healthy and diseased vaginal microbiota. Full thickness tissue blocks of 5 by 5 mm2 were inoculated prior to sterilization and decellularization by submersion in 1 mL solution of approximately 108–109 CFU. This was performed to achieve a minimum attachment of L. crispatus (anaerobic Gram+), S. aureus (aerobic Gram+, ATCC 49230), E. coli (aerobic Gram−, ATCC 25922) or C. albicans at 106 CFU per strain, to allow assessment of the sterilization efficiency based on the 6 log-reduction criterium (see “Sterilization by antibiotics and antimycotics treatment” section) and to test worst-case contamination scenarios. The differences in controlled contaminant concentrations are due to limitations on the quantity of cultured CFUs that could be acquired at experiment initiation, and differences in optical density and the surface attachment ability. The added contaminant was allowed to attach to human vaginal wall blocks for 3 h at 37 °C in Dulbecco’s Modified Eagle Medium (DMEM—Gibco, ThermoFisher Scientific) with constant agitation at 120 rotations per minute (RPM). Three washes of 5 min at 37 °C and 120 RPM were performed in sterile PBS to remove non-adhered, added contaminants. Control groups without controlled contamination were included to assess resident microbiota of the samples and to serve as a reference to assess the efficacy of the respective sterilization method to eliminate the microbiota. All individual samples of all groups were homogenized in 500 µL of 0.5% Tween 80 (Sigma-Aldrich) in PBS using 2 mm zirconia beads (Biospecs) for 2 times 1 min at 7.000 RPM in a MagNA Lyser (Roche). Dilutions of the homogenate were cultured on blood agar (Colombia agar containing 5% sheep blood, ThermoFisher Scientific) and, depending on the microbial species, incubated for 1–7 days at 37 °C under appropriate aerobic or anaerobic conditions. All blood agar plates were quantitatively assessed by visual examination for the number of colonies that were formed. Controlled contamination was performed with tissue from five donors and measured in triplicates (total n = 15 samples per sterilization method).
Sterilization by decellularization [DC]
After controlled contamination, decellularization was initiated by 24 h incubation at 37 °C with constant agitation (100 rotations/min) in 0.18% w/w Triton x-100 (Sigma-Aldrich) and 0.015% w/w sodium-deoxycholate (Sigma-Aldrich) in PBS93. Tissue was washed twice for 20 min in PBS, followed by a 72 h wash at 4 °C with constant agitation in PBS with 1% PS (100 U/mL penicillin and 100 µg/mL streptomycin). Enzymatic digestion involved 24 h incubation at 37 °C with DNase I (150 IU/mL; Sigma-Aldrich) and 50 mmol MgCl2 (Sigma-Aldrich) in PBS with constant agitation (100 rotations/min). Tissue was washed twice for 20 min in PBS. A 24 h incubation at 4 °C was performed with constant agitation in PBS with 1% PS, followed by twice 20 min in PBS at room temperature with constant agitation (100 rotations/min). The duration of the total protocol was 6 days. All solutions were sterilized before use with a 0.22 μm sterile membrane filter (Merck Millipore). A non-sterilized control group was included.
Sterilization by peracetic acid/hydrogen peroxide treatment [PAA/H2O2-sterilization]
Decellularization was performed as described above in “Sterilization by decellularization [DC]” section. After enzymatic digestion, tissue was washed twice in PBS for 20 min each. Tissue was treated with PAA, acetic acid (AcA) and H2O2 in a 1:1:1 ratio. Several sterilization conditions were tested, which included concentrations of 0.01, 0.05 or 0.1% PAA/H2O2, sterilization durations of 2–3 h, and constant agitation at either 125 or 250 rotations/min. These solutions were prepared by diluting a PAA-mixture (Neodisher endo SEPT PAC, Dr. Weigert) 25 times in 100% ethanol, with further dilution in PBS to respectively 0.96%, 4.8% or 9.6% ethanol. A PAA/H2O2 concentration range was tested to minimize structural damage with sufficient sterilization action, and a control group with no PAA/H2O2 sterilization was included. Tissue was washed twice for 20 min in PBS. A 24 h incubation at 4 °C was performed with constant agitation in PBS with 1% PS, followed by twice 20 min in PBS at room temperature with constant agitation (100 rotations/min). The duration of the total protocol was 6 days. All solutions were sterilized before use with a 0.22 μm sterile membrane filter (Merck Millipore).
Sterilization by antibiotics and antimycotics treatment [AAE-based]
After controlled contamination, a 1 h wash was performed on a rollerbench at room temperature in PBS containing high concentrations of antimicrobials, i.e., 10% PS (1000 U/mL penicillin and 1,000 µg/mL streptomycin; Life Technologies Europe BV) and 10% amphotericin-B (25 µg/mL, Bio-Reagent, Sigma-Aldrich). Next, decellularization was performed in accordance to “Sterilization by decellularization” Sect94. However, for this protocol 1% PS washing solutions were supplemented with 1% amphotericin-B. After AA incubation, tissue was washed twice for 20 min in PBS, followed by 5 min in 70% ethanol and again twice in PBS for 20 min at room temperature with constant agitation (100 rotations/min). During experiments, DVM was soaked in 70% ethanol for 5 min to ensure adequate effect. Lastly, a 24 h incubation at 4 °C was performed with constant agitation in PBS with 1% PS and 1% amphotericin-B, followed by twice 20 min in PBS at room temperature with constant agitation (100 rotations/min). A control groups without decellularization was included. The duration of the total protocol was 7 days. All solutions were sterilized before use with a 0.22 μm sterile membrane filter (Merck Millipore).
Embedding by semi-automated tissue processing
Tissues were dehydrated and embedded in paraffin using the Epredia™ Excelsior™ AS Tissue Processor (Thermo Fisher Scientific). This sequence involved 1 h in 70% ethanol, 1 h in 90% ethanol, 1 h in 96% ethanol and thrice for 1 h in 100% ethanol at RT. Three sequential xylene steps at 37 °C, 40 °C and 45 °C were performed, followed by sequential paraffin baths 1, 2 and 3 in three steps of each 1 h and 20 min at 62 °C. Next, tissue was manually embedded in liquid paraffin of 55 °C by 1 h solidification at RT on a cooled plate of − 5 °C. After overnight 4 °C paraffin wax hardening, samples are ready for microtome sectioning.
Haematoxylin with eosin staining
Samples were fixed overnight at RT with constant agitation (on a roller) in 4% w/v formaldehyde (ROTI® Histofix 4%; Carl Roth). Tissue was washed 30 min in PBS, followed by three cycles of 30 min in 70% ethanol (Sigma-Aldrich) at RT. Tissue was embedded according to “Embedding by semi-automated tissue processing” (above). Samples were sliced to 5 μm-thick sections using a microtome with a 6° knife angle. Sections were mounted on microscope slides in a water bath at 37.2 °C. The slide-sections were deparaffinized twice for 5 min in xylene, followed by hydration for 2 min in 100% ethanol (twice), 96% ethanol and 70% ethanol. Slides were H&E stained at RT in 1 mg/mL of Haematoxylin (Sigma-Aldrich) in 70% ethanol and 5 mg/mL of Eosin (Sigma-Aldrich) in 70% ethanol. Slides were embedded with 1x Entellan (Sigma-Aldrich) and dried overnight before inspection with the Leica DM5000B (Leica Biosystems) fluorescence microscope. H&E staining was performed on triplicates from three donors (n = 9 samples per sterilization method).
Assessment of sterilization efficacy
Sterility was evaluated conform the bioburden 6-log reduction criteria and in accordance with the ISO sterility guidelines, WHO-guidelines, guidelines of the European Pharmacopoeia 2.6.1 and the international code of practice95,96. In line with these guidelines, sterilization efficacy was quantified by plating of tissue homogenates on blood agar plates. After controlled contamination and sterilization, test samples were stamped on blood agar and then homogenized in 500 µL of PBS containing 0.5% Tween 80 (Sigma-Aldrich) using 2 mm zirconia beads (Biospecs) twice for 1 min at 7.000 RPM in a MagNA Lyser (Roche). The homogenates, and 10-fold serial dilutions thereof in PBS, were cultured on blood agar and incubated at 37 °C under aerobic and anaerobic conditions. Incubation was performed for 7 days for controls without controlled contamination, for 24 h after controlled contamination with S. aureus and E. coli, and for 48 h after controlled contamination with L. crispatus and C. albicans. The tissue residue after homogenization was placed in liquid TSB medium, and tubes were incubated at 37 °C shaking at 120 RPM for identical periods as the corresponding blood plates. All blood agar plates were quantitatively assessed by visual examination for the number of colonies that were formed. For assessment of the efficacy, successful sterilization was defined as complete extermination of all controlled contaminants with at least a 6-log reduction. TSB cultures were visually inspected and an aliquot of 10 µl was plated on blood agar plates in case of visible growth. Assessment of sterilization efficacy was performed on triplicates from three donors (n = 9 samples per sterilization method).
Identification of endogenous micro-organisms in tissue samples
Identification of endogenous micro-organisms in unprocessed vaginal wall tissue and in vagina wall tissue after controlled contamination and sterilization was carried out by plating of the last washing solutions on blood agar using a streak plate procedure and incubation for 24–48 h at 37 °C. After incubation, micro-organism identification was performed by mass spectrometry by a Bruker Biotyper MALDI-TOF MS system (Bruker Daltonics). Identification of endogenous micro-organisms was conducted on triplicates from three donors (n = 9 samples per sterilization method).
Statistical analysis
The GraphPad Prism 10.2.3 (GraphPad Software) and Microsoft Office Excel 2019 (Microsoft Corporation) software for Windows were used for statistical analysis of the results, to identify any significant differences between inoculated controls and test specimens after sterilization. Homogeneity of variances was analyzed using the Barlett’s test. Sterilization results were then analyzed using the two-sample t-test with pooled variance, or by the Welch’s t-test in case of unequal variances. Data was plotted as mean ± standard deviation (SD). An obtained statistically significance was defined in terms of the following P-value: *for P ≤ 0.05, **for P ≤ 0.01 and ***for P ≤ 0.001.
Results
Controlled contamination and naturally occurring micro-organisms
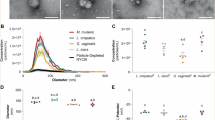
Quantitative assessment of applied contaminants (Fig. 2) confirmed a mean adhesion of 1.4 × 106 CFU/material, 2.6 × 107 CFU/material, 1.8 × 106 CFU/material and 2.9 × 106 CFU/material of added contaminants per human vaginal wall tissue sample for either L. crispatus, S. aureus, E. coli or C. albicans, respectively. A negative control without controlled-contamination demonstrated the presence of 4 × 101 CFU naturally occurring micro-organisms per human vaginal wall tissue sample.
By quantification, controlled contamination with L. crispatus, S. aureus, E. coli and C. albicans strains was confirmed. After 3 h of incubation with inoculum, the number of colony-forming unit (CFU) per material was assessed. Depicted mean values (n = 15, triplicates from 5 donors) are 5.3 × 101, 1.4 × 106, 2.6 × 107, 1.8 × 106 and 2.9 × 106 CFU/material, respectively.
Conditions of the AAE-sterilization protocol were pre-determined by controlled contamination experiments. For 1 h incubation with PS and amphotericin-B (Supplement 1), effective elimination of controlled bacterial contamination and largely effective elimination of added fungal contaminants was only seen for 10% AA concentrations. C. albicans was reduced from 1 × 107 CFU/material to 17 CFU/material and from 1 × 108 CFU/material to 40 CFU/material, thereby demonstrating a 6 log-reduction. Therefore, an AA concentration of 10% was considered sufficient. Concentrations of the digestion medium were tested for 24 h incubation (Supplement 2), and effective treatment was found at 100% of the DC concentrations. Furthermore, for 24 h incubation with PS and amphotericin-B (Supplement 3), effective removal of controlled bacterial and fungal contamination was seen at concentrations of 1%. Lastly, effective removal of controlled bacterial and fungal contaminants with 70% ethanol (Supplement 4), was seen within 2 min of incubation.
Micro-organism identification in native and processed vaginal tissue
In donated tissue from healthy masculine transgender individuals, anaerobic gram+ pathogens and micro-organisms were most common. A high variability was observed (Table 1; Supplement 5), but most micro-organisms found in native controls are part of the normal, healthy microbiota of the human vagina. These were Gardnerella vaginalis, Prevotella timonensis, Finegoldia magna, Winkia neuii, Peptoniphilus lacydonensis, Peptoniphilus lacrimalis, Streptococcus agalactiae and Staphylococcus lugdunensis39,40,42,44,97. Furthermore, the rare species Facklamia hominis was identified, with the vagina (reported as female genitourinary tract) suggested as its natural habitat98. Anaerococcus vaginalis and Corynebacterium tuberculostericum were further found and are generally less prominent in the vaginal microbiota, but have been reported as part of the vaginal microbiota in masculine transgender individuals due to long-term androgen exposure99. Pathogens detected in native controls were Cutibacterium acnes, Cutibacterium avidum, Propionimicrobium lymphophilum, Actinomyces timonensis and Bacillus cereus / thuringiensis. Upon decellularization, only controlled contaminants were observed but no native microbes. Upon AAE-based sterilization, controlled contaminants as well as native bacteria (both pathogens and commensals) were eliminated.
Assessment ECM structure of sterilized DVM
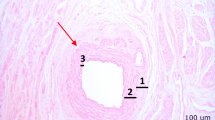
The ECM architecture was investigated by histological assessment after sterilization (Fig. 3). After decellularization, the ECM architecture was well preserved. The ECM architecture was further assessed for potential damaging effects by PAA/H2O2-sterilization for various concentrations of 0.01%, 0.05% and 0.1%. Figure 3 demonstrates that the ECM structure of decellularized vaginal wall tissue is diminished for sterilization with all of these PAA/H2O2 concentrations. Furthermore, with respect to PAA/H2O2-sterilization of various durations and agitation frequencies, the ECM architectural damage is visible after both 2 h and 3 h of sterilization at either 125 or 250 rot/min, as clear holes or completely dissolved tissue. Damage is more prominent for increased PAA/H2O2 concentrations and longer sterilization times. Histological assessment further demonstrated a maintained ECM architecture after AAE-based treatment.
Structural analysis of decellularized vaginal wall matrix after chemical decellularization (DC) only, sterilization with 0.1%, 0.05% or 0.01% PAA/H2O2 treatment and AAE-based sterilization. Histological staining with Haematoxylin and Eosin (H&E) demonstrates damage to the ECM structure (red arrowheads) for sterilization with 0.1%, 0.05% or 0.01% PAA/H2O2 for 2 h sterilization at 125 rotations/min or 250 rotations/min constant agitation and for 3 h sterilization at 125 rotations/min, compared to a decellularized vaginal wall control sample without exposure to a sterilization procedure. Scalebars are 100–200 μm in length. Depicted histological images are a representation of triplicates from 3 donors (n = 9 per test condition). Scalebars are 100 μm in size.
Assessment sterility treatment by decellularization
The efficiency of our sterilization methods was quantified after decellularization (Fig. 4) and for the AAE-based treatment (Fig. 5). Efficiency of PAA/H2O2-sterilization was not assessed because the major damage of the ECM structure would not allow translation to clinical settings. Chemical decellularization demonstrated no significant reduction of L. crispatus, S. aureus, E. coli or C. albicans. Although the quantitative reduction of L. crispatus and S. aureus did comply with the log-6 reduction criterium, this was insufficient for complete sterilization.
Controlled contamination with L. crispatus, S. aureus, E. coli and C. albicans strains was performed and the number of colony-forming unit (CFU) per material was assessed for untreated specimen and after chemical decellularization (n = 3, triplicate from 1 donor). No significant elimination of contaminants was observed.
Controlled contamination with L. crispatus, S. aureus, E. coli and C. albicans strains was performed and the number of colony-forming unit (CFU) per material was assessed for untreated specimen and after chemical decellularization with complementary AAE-based sterilization treatment (n = 9, triplicates from 3 donors). A significant elimination of contaminants was observed for species of L. crispatus (P < 0.01), S. aureus (P < 0.001), E. coli (P < 0.01) and C. albicans (P < 0.05).
The sterilization effect of decellularization in combination with AAE-based treatment demonstrated complete eradication as judged from lack of growth on both the agar plates and the liquid TSB cultures of L. crispatus (P < 0.01), S. aureus (P < 0.001), E. coli (P < 0.01) and C. albicans (P < 0.05), which is in compliance with the log-6 reduction criterium.
Discussion
Sterilization of vagina wall tissue and the need for efficacy validation
Approximately 90,000 transplantations are performed worldwide each year, yet a donor organ shortage remains20. Recently, tissue engineering and regenerative medicine have resulted in promising new decellularized scaffolds for tissue replacement of various organs, including the vagina. Moreover, the chance of graft survival has improved by preventing tissue rejection through immunosuppression therapy. However, clinical application of tissue engineered transplants in immunocompromised hosts is challenging, as their diminished inflammatory response makes them more susceptible to infections. Therefore, risk of transplant transmitted diseases is of considerable concern. Guidelines and methods for microbiological screening of transplants vary across countries and even national regions100. Despite the official registration of transplant-transmitted infections, only a 1% occurrence is reported as this is generally only recognized after clusters of infections are observed in recipients with a common donor. However, in case of transmitted infections, the risks are severe and include sepsis, graft rejection, and postoperative morbidity and mortality20. Therefore, success of vagina transplantation relies on the formulation and utilization of an effective sterilization procedure prior to implantation.
To reliably confirm efficient sterilization, validation is required by controlled contamination at high bioburden concentrations to simulate worse-case scenarios. High bioburdens on the scaffold can either originate from high endogenous microbe concentrations in the donor tissue, or from later contamination by micro-organisms that are resistant to specific disinfection or sterilization approaches. Quality control for sterility validation should include both identification (qualification) and quantification of potential contaminants, to allow risk assessment and any further optimization to guarantee successful sterilization before implantation.
In general, testing of mass-produced biomaterials for sterility validation is performed through large quantity, random sampling with complete sample loss. Sterility assessment of large-size biomaterials typically involves the constant removal, and consequent loss, of a biomaterial section from one sample. For biomaterials that are manufactured at small quantities and volumes, like our matrix, both approaches would not be feasible. Under these conditions, the European Pharmacopeia guidelines allow sterility testing on the final washing or storage solution. In this study, this last form of sterility testing was conducted.
Aim and study design in accordance to official guidelines
This study aimed to compare three of our tailored sterilization methods for safe implantation of decellularized human vagina wall tissue in future clinical applications. Sterilization efficacy was evaluated in accordance to internationally established guidelines95,96, the bioburden reduction criteria (6-log numbers of CFU reduction and ISO 14973) for residual microbes and contaminants at low tissue quantity and volume, and preservation of the ECM structure. Although over the years, various decellularization techniques for vaginal tissue have been designed, tissue disinfection is often not considered, is outside the scope of the particular study, or lacks validation. Standard chemical and physical sterilization approaches consist of antibiotics, antimycotics, PAA, ethanol, freeze-drying and/or gamma-irradiation29,35,36,37. For vagina reconstruction from dermis, polyglycolic acid-poly(lactic-co-glycolic acid), poly(L-lactide-co-caprolactone) and amniotic membrane, this has involved freeze-drying, gamma-irradiation and EO gas101,102,103,104. However, most studies did not assess sterilization efficacy nor the induced structural damage. Therefore, we aimed to perform a well standardized and complete study to develop a safe and reliable method to sterilize human decellularized vaginal scaffolds for vaginal reconstruction, and thereby bridging the gap towards their clinical application.
Decellularization carries insufficient disinfection potential
We studied the sterilization effect of decellularization, which previously demonstrated to form complete acellular tissue with preservation of ECM architecture, suitable biomechanical tissue properties, and retained ECM proteins and vascular architecture2. Upon controlled contamination, DC did not affect fungal contamination and was incapable of complete bacterial elimination. All pathogenic and non-pathogenic micro-organisms of the native microbiota were present at low CFU quantities and eliminated by DC, but no endogenous fungi were present to demonstrate the anti-fungal action of DC. Of the identified endogenous micro-organism, the F. hominis is rare, but considered a non-pathogen for the female genitourinary tract98. Both A. vaginalis and C. tuberculostericum are less prominent in the vagina, but are increased in the vaginal microbiota of masculine transgender individuals due to long-term androgen exposure. Furthermore, some bacterial infections were identified (P. lymphophilum, C. acnes, C. avidum, (A) timonensis and (B) cereus/thuringiensis). As DC demonstrated incapable of complete microbial contaminant removal and thereby does not comply with sterilization criteria, infections would be of concern especially for clinical application of this method, considering the associated risk for transplant transmitted diseases. Although other studies on tissue-engineered constructs have applied similar decellularization protocols for porcine aorta93, human muscle105, and rat vagina29, validation of any potential sterilization effects by a similar chemical DC protocol has to our knowledge not been previously reported. Regarding this limited data availability in the literature and prospective application of decellularized scaffolds, the insufficient sterilization effect of decellularization is a noteworthy finding.
Additional PAA/H2O2-sterilization destructive to ECM structure
The second sterilization method consisted of the decellularization protocol a PAA/H2O2-sterilization. PAA is a well-known and broadly applied decontaminant, but has serious health and environmental risks. Among others, PAA contact is irreversible destructive to skin106 and eyes, its inhalation irritates nose107, throat and lungs, and animal studies further indicate neurotoxicity, reproductive toxicity and carcinogenic action108. Therefore, our medical center applies strict regulations regarding the use of PAA for experimental work. Consequently, we had to replace pure PAA by an endoscope disinfectant solution that consists of a mixture of PAA, acetic acid and H2O2 diluted 25 times at a 1:1:1 ratio in ethanol. PAA/H2O2-sterilization demonstrated considerable damage, manifested as clear holes or completely dissolved tissue in the histological sections, that increased with PAA/H2O2-concentration and incubation time. Tissue samples were thereby softened and became difficult to process. These findings are contrasting previous reports regarding 2 h PAA-treatment of acellular vaginal matrix with additional freeze-drying and gamma-irradiation35, and regarding non-destructive sterilization by 0.1% PAA of decellularized cardiac and arterial valves60, lung61, liver62,63, bladder64,65,66, spleen67,68, kidney69,70, small intestine71,72,73, tendon74,75 and nerve76. Furthermore, the absence of damage after similar decellularization and 0.1% PAA sterilization of porcine vagina37 and heart109, suggests that our observed ECM damage can be best explained by the combined exposure to PAA, H2O2, acetic acid and ethanol. Strong oxidation of PAA and the synergistic effect with acetic acid and hydrogen peroxide both destroy the cell membranes, as well as matrix proteins and enzymes46. Ethanol causes protein denaturation, destruction of enzymes and reduction of collagen content, and H2O2 oxidation affects the physical and chemical biomaterial properties46. We considered the observed ECM damage after our PAA/H2O2-sterilization unacceptable for future clinical applications even for short durations of 2 h and at low concentrations of 0.01%. Further biochemical quantification of ECM components (i.e., collagen, elastin, laminin, fibronectin, or glycosaminoglycans) could be used to determine the extent of damage and to validate our qualitative findings.
Additional AAE-based treatment for effective sterilization
As third sterilization approach, the decellularization protocol was complemented with antibiotics, antimycotics and ethanol. This AAE-based sterilization combines action against aerobic and anaerobic species of Gram+ and Gram− bacteria and fungi, and can further act against protozoa38. This last effect was not investigated in this study. Our AAE-based protocol demonstrated efficient sterilization of controlled-contaminated human vagina wall tissue without any visual destructive effects on the ECM structure. Similar decellularization and sterilization of human vaginal tissue94 without tissue damage has previously been reported, but without further validation of sterilization efficiency. Regarding our findings, this treatment carries potential for translation towards tissue banks and clinical settings, and for usage of our sterile DVM in future clinical applications.
Limitations
Whether our AAE-based sterilization treatment impacts the biomechanical properties of vagina wall tissue or its ECM constituent proteins, has been outside the scope of this study but are fundamental for in vivo tissue regeneration and durability of the implant. Modifications to morphology and ECM components cause alterations in cell adhesion and cell-biomaterial interactions, and we have previously demonstrated that these ECM proteins are crucial to maintain elasticity. Therefore, the impact of AAE-based treatment and the action of residual sterilization agents on cell adhesion, cytotoxicity, preservation of ECM constituent proteins and tissue biomechanical properties will be addressed in an upcoming study.
In this study, controlled contamination was performed with excessive amounts (108–109 CFU/ml) of either L. crispatus, S. aureus, E. coli or C. albicans to test worse-case scenarios. These micro-organisms are representatives of common Gram+ and Gram− bacteria, anaerobic bacteria and a fungal species of the microbiota of the human vagina. The significance of micro-organism elimination by AAE-based treatment was tested and confirmed by a two-sample t-test with pooled variance, or by the Welch’s t-test for unequal variance. Alternatively, the Wilcoxon rank sum w test can be performed as nonparametric variant to decrease sensitivity to big deviations from normality distribution and exclude the impact of heterogeneity of variances. To this end, we performed a second statistical analysis with the Wilcoxon rank sum w test, that confirmed significant elimination of L. crispatus, S. aureus, E. coli and C. albicans by the AAE-based treatment.
Although all controlled-contaminants and naturally occurring micro-organisms were successfully eliminated by our AAE-based treatment, this might not be the case for all bacteria, fungi and especially protozoa forming the vaginal microbiota. Despite the broad disinfection and sterilization range of ethanol, penicillin, streptomycin, amphotericin-B and our decellularization agents combined, we do advise validation testing to ensure elimination of, for example, infrequent human vagina commensals or pathogens, protozoa or combinations of multiple species at extremely high concentrations. Although ethanol is reported capable of inactivating enveloped viruses that includes influenza, Ebola and coronaviruses110, it is less or not effective against other viruses such as human papillomavirus (HPV) or acquired immunodeficiency virus (AIDS)110,111. There are currently no medical or natural treatments to cure HPV or AIDS112,113, therefore extensive testing prior to transplantation of human donor vagina wall tissue is required. Next, although elimination of L. crispatus, S. aureus, E. coli and C. albicans was achieved for endogenous micro-organisms combined with one controlled-contaminant, the AAE-based treatment might be less efficient for contaminations that occur in unison as is the case in the authentic vaginal microbiota. However, in light of the full removal of all endogenous micro-organisms combined with a worse-case controlled-contamination, we believe that AAE-based treatment will in most cases be sufficient for sterilization of donated vagina wall tissue.
We further want to emphasize that AAE-based treatment was validated for vagina wall tissue blocks of 5 by 5 mm2. We previously validated our decellularization protocol to be effective for tissue blocks, rings, and full organs. Together with the validation guideline principles, successful translation of our AAE-method to sterilization of complete organs is expected. Lastly, we want to caution for the misuse and overuse of antibiotics-based sterilization, as this can result in drug-resistant pathogens. Globally, antimicrobial resistant bacteria cause millions of deaths114. Furthermore, these resistant pathogens harm modern medicine by making infections harder to treat and thereby increasing the risks of medical procedures and treatments. In terms of economic costs, the yearly losses due to antimicrobial resistance is estimated at trillions of US dollars114. In our protocol, addition of ethanol will help prevent resistance development.
Future perspective
Future investigations on human decellularized vagina wall need to be performed to unravel whether cell attachment or proliferation is impaired by the AAE-treatment and induces changes to the surface topography. Furthermore, changes in biomechanical properties due to modification of surface chemistry and texture or biomaterial integrity of sterilized decellularized vagina wall should be examined. These studies will provide insight into possible side effects caused by the AAE-based sterilization protocol. Moreover, prior to clinical application, a comprehensive evaluation of the ECM integrity is advised, including biochemical quantification of ECM components (such as elastin, collagen, fibronectin, laminin and glycosaminoglycans). Additionally, immunofluorescent imaging may be included to facilitate the evaluation of ECM component organization and preservation.
Conclusion
This research demonstrated that treatment of human vaginal wall tissue by chemical decellularization has disinfection potential with preserved ECM structure, but with insufficient sterilization efficacy. Additional sterilization with 0.01%, 0.05% and 0.1% PAA/H2O2 resulted in major ECM damage, that is considered unacceptable for clinical settings. Our decellularization protocol combined with AAE-based treatment demonstrated effective in producing sterile tissue with histological preservation of the ECM structure. This method is suitable for translation into clinical settings, and could be used for sterilization of other biomaterials such as other acellular biological scaffolds, or even biomaterials of polymeric or metallic origin. Furthermore, the sterile decellularized vaginal scaffold developed here, carries potential for first transplantation of human vagina after future optimization of biochemical and biomechanical characteristics.
Data availability
Data is available upon request from the corresponding author.
References
Tian, Y. et al. A stereological study of 3D printed tissues engineered from rat vaginas. Ann. Transl. Med. 8, 1490–1490 (2020).
Sueters, J. et al. Creation of a decellularized vaginal matrix from healthy human vaginal tissue for potential vagina reconstruction: experimental studies. Int. J. Surg. 109, 3905–3918 (2023).
Liao, L. M., Doyle, J., Crouch, N. S. & Creighton, S. M. Dilation as treatment for vaginal agenesis and hypoplasia: A pilot exploration of benefits and barriers as perceived by patients. J. Obstet. Gynaecol. (Lahore) 26, 144–148 (2006).
Callens, N. et al. Vaginal dilation treatment in women with vaginal hypoplasia: a prospective one-year follow-up study. Am. J. Obstet. Gynecol. 211, 228–229 (2014).
Sueters, J. et al. Vaginoplasty for gender dysphoria and Mayer-Rokitansky-Kuster-Hauser syndrome: A systematic review. Fertil. Steril. Rev. 4, 219–236 (2023).
Bombard, I. I., Mousa, S. A. & D. S. & Mayer–Rokitansky–Kuster–Hauser syndrome: complications, diagnosis and possible treatment options: a review. Gynecol. Endocrinol. 30, 618–623 (2016).
Callens, N. et al. An update on surgical and non-surgical treatments for vaginal hypoplasia. Hum. Reprod. Update 20, 775–801 (2014).
Gigante Gomes, T., Agostinho, M., Cardoso, M. C., Nunes da Costa, J. & Matias, J. Xcm biologic tissue matrix xenograft and autologous micromucosa graft for vaginal reconstruction in Mayer–Rokitansky–Küster–Hauser syndrome. Arch. Plast. Surg. 48, 185–188 (2021).
Manrique, O. J. et al. Complications and patient-reported outcomes in male-to-female vaginoplasty-where we are today: A systematic review and meta-analysis. Ann. Plast. Surg. 80, 684–691 (2018).
Ferrando, C. A. Vaginoplasty complications. Clin. Plast. Surg. 45, 361–368 (2018).
Ferrando, C. A. Adverse events associated with gender affirming vaginoplasty surgery. Am. J. Obstet. Gynecol. 223, e1–e6 (2020).
Bustos, S. S. et al. Complications and patient-reported outcomes in transfemale vaginoplasty: An updated systematic review and meta-analysis. Plast. Reconstr. Surg. Glob Open 9, E3510 (2021).
Blanchard, R., Legault, S. & Lindsay, W. R. N. Vaginoplasty outcome in male-to-female transsexuals. J. Sex. Marital Ther. 13, 265–275 (1987).
di Summa, P. G. et al. Colic-based transplant in sexual reassignment surgery: Functional outcomes and complications in 43 consecutive patients. J. Sex. Med. 16, 2030–2037 (2019).
Gentile, G. et al. Penile-scrotal flap vaginoplasty versus inverted penile skin flap expanded with spatulated urethra: A multidisciplinary single-centre analysis. Arch. Ital. Urol. Androl. 92, 186–191 (2020).
Brotto, L. A. et al. Psychophysiological and subjective sexual arousal to visual sexual stimuli in new women. J. Psychosom. Obstet. Gynecol. 26, 237–244 (2005).
Lawrence, A. A. Patient-reported complications and functional outcomes of male-to-female sex reassignment surgery. Arch. Sex. Behav. 35, 717–727 (2006).
Hayashida, S. A. et al. The clinical, structural, and biological features of neovaginas: A comparison of the Frank and the McIndoe techniques. Eur. J. Obstet. Gynecol. Reprod. Biol. 186, 12–16 (2015).
Zhong, C. X., Wu, J. X., Liang, J. X. & Wu, Q. H. Laparoscopic and gasless laparoscopic sigmoid colon vaginoplasty in women with vaginal agenesis. Chin. Med. J. (Engl.) 125, 203–208 (2012).
Timsit, J. F. et al. Diagnostic and therapeutic approach to infectious diseases in solid organ transplant recipients. Intens. Care Med. 45, 573–591 (2019).
Cao, D. & Ding, J. Recent advances in regenerative biomaterials. Regen. Biomater. 9, 1–37 (2022).
Zhu, L., Zhou, H., Sun, Z., Lou, W. & Lang, J. Anatomic and sexual outcomes after vaginoplasty using tissue-engineered biomaterial graft in patients with Mayer–Rokitansky–Küster–Hauser syndrome: A new minimally invasive and effective surgery. J. Sex. Med. 10, 1652–1658 (2013).
Stany, M. P., Sunde, J., Bidus, M. A., Rose, G. S. & Elkas, J. C. The use of acellular dermal allograft for vulvovaginal reconstruction. Int. J. Gynecol. Cancer 20, 1079–1081 (2010).
Wang, Z., Huang, J., Zeng, A., Wu, M. & Wang, X. Vaginoplasty with acellular dermal matrix after radical resection for carcinoma of the uterine cervix. J. Investig. Surg. 32, 180–185 (2019).
Ding, J. X., Zhang, X. Y., Chen, L. M. & Hua, K. Q. Vaginoplasty using acellular porcine small intestinal submucosa graft in two patients with Meyer-Von-Rokitansky-Küster-Hauser syndrome: A prospective new technique for vaginal reconstruction. Gynecol. Obstet. Investig. 75, 93–96 (2013).
Ding, J. X., Chen, L. M., Zhang, X. Y., Zhang, Y. & Hua, K. Q. Sexual and functional outcomes of vaginoplasty using acellular porcine small intestinal submucosa graft or laparoscopic peritoneal vaginoplasty: A comparative study. Hum. Reprod. 30, 581–589 (2015).
Raya-Rivera, A. M. et al. Tissue-engineered autologous vaginal organs in patients: A pilot cohort study. Lancet 384, 329–336 (2014).
de Moreira Lemos, N. L. Small intestinal submucosa patch for extensive vaginal endometriosis resection. J. Minim. Invas. Gynecol. 16, 765–767 (2009).
Wefer, J. et al. Homologous acellular matrix graft for vaginal repair in rats: A pilot study for a new reconstructive approach. World J. Urol. 20, 260–263 (2002).
Zhang, X., Qiu, J., Ding, J. & Hua, K. Comparison of neovaginoplasty using acellular porcine small intestinal submucosa graft or Interceed in patients with Mayer–Rokitansky–Küster–Hauser syndrome. Arch. Gynecol. Obstet. 300, 1633–1636 (2019).
Liu, L. et al. Porcine urinary bladder matrix-polypropylene mesh: A novel scaffold material reduces immunorejection in rat pelvic surgery. Int. Urogynecol. J. 23, 1271–1278 (2012).
Gupta, A., Francis, S., Stewart, R., Hobson, D. & Meriwether, K. V. Repair of colonic neovaginal stenosis using a biological graft in a male-to-female transgender patient. Int. Urogynecol. J. 30, 661–663 (2019).
Fu, R. H. et al. Decellularization and recellularization technologies in tissue engineering. Cell. Transplant. 23, 621–630 (2014).
Atala, A. Tissue engineering of reproductive tissues and organs. Fertil. Steril. 98, 21–29 (2012).
Zhang, J. K. et al. A new material for tissue engineered vagina reconstruction: Acellular porcine vagina matrix. J. Biomed. Mater. Res. A 105, 1949–1959 (2017).
Hou, C. et al. Printing 3D vagina tissue analogues with vagina decellularized extracellular matrix bioink. Int. J. Biol. Macromol. 180, 177–186 (2021).
Greco, K. V., Jones, L. G., Obiri-Yeboa, I. & Ansari, T. Creation of an acellular vaginal matrix for potential vaginal augmentation and cloacal repair. J. Pediatr. Adolesc. Gynecol. 31, 473–479 (2018).
Fidalgo, C. et al. A sterilization method for decellularized xenogeneic cardiovascular scaffolds. Acta Biomater. 67, 282–294 (2018).
Lamont, R. et al. The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG Int. J. Obstet. Gynaecol. 118, 533–549 (2011).
Chen, X., Lu, Y., Chen, T. & Li, R. The female vaginal microbiome in health and bacterial vaginosis. Front. Cell Infect. Microbiol. 11, 1–15 (2021).
Serrano, M. G. et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med. 25, 1001–1011 (2019).
Aagaard, K. et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS ONE 7, e36466 (2012).
Wrønding, T. et al. Antibiotic-free vaginal microbiota transplant with donor engraftment, dysbiosis resolution and live birth after recurrent pregnancy loss: a proof of concept case study. eClinicalMedicine 61, 102070 (2023).
Sadeghpour Heravi, F. Host-vaginal microbiota interaction: shaping the vaginal microenvironment and bacterial vaginosis. Curr. Clin. Microbiol. Rep. 1, 1–15. https://doi.org/10.1007/s40588-024-00227-8 (2024).
Huang, Q., Dawson, R. A., Pegg, D. E., Kearney, J. N. & Macneil, S. Use of peracetic acid to sterilize human donor skin for production of acellular dermal matrices for clinical use. Wound Repair. Regen. 12, 276–287 (2004).
Tao, M. et al. Sterilization and disinfection methods for decellularized matrix materials: Review, consideration and proposal. Bioact. Mater. 6, 2927–2945 (2021).
Ghosh, M. M. et al. A comparison of methodologies for the preparation of human epidermal-dermal composites. Ann. Plast. Surg. 39, 390–404 (1997).
Harrell, C. R., Djonov, V., Fellabaum, C. & Volarevic, V. Risks of using sterilization by gamma radiation: The other side of the coin. Int. J. Med. Sci. 15, 274–279 (2018).
Hayrapetyan, H., Nederhoff, L., Vollebregt, M. & Mastwijk, H. Nierop Groot, M. Inactivation kinetics of Geobacillus stearothermophilus spores by a peracetic acid or hydrogen peroxide fog in comparison to the liquid form. Int. J. Food Microbiol. 316, 108418 (2020).
Linley, E., Denyer, S. P., McDonnell, G., Simons, C. & Maillard, J. Y. Use of hydrogen peroxide as a biocide: new consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 67, 1589–1596 (2012).
Shah, D. D. et al. Effect of peroxide- versus alkoxyl-induced chemical oxidation on the structure, stability, aggregation, and function of a therapeutic monoclonal antibody. J. Pharm. Sci. 107, 2789–2803 (2018).
Mendes, G. C. C., Brandão, T. R. S. & Silva, C. L. M. Ethylene oxide sterilization of medical devices: A review. Am. J. Infect. Control 35, 574–581 (2007).
Kearney, J. N., Bojar, R. & Holland, K. T. Ethylene oxide sterilisation of allogenic bone implants. Clin. Mater. 12, 129–135 (1993).
Baldini, T., Caperton, K., Hawkins, M. & McCarty, E. Effect of a novel sterilization method on biomechanical properties of soft tissue allografts. Knee Surg. Sport Traumatol. Arthrosc. 24, 3971–3975 (2016).
Choe, J. A. et al. Biomaterial characterization of off-the-shelf decellularized porcine pericardial tissue for use in prosthetic valvular applications. J. Tissue Eng. Regen. Med. 12, 1608–1620 (2018).
Wutzler, P. & Sauerbrei, A. Virucidal efficacy of a combination of 0.2% peracetic acid and 80% (v/v) ethanol (PAA-ethanol) as a potential hand disinfectant. J. Hosp. Infect. 46, 304–308 (2000).
Kline, L. B. & Hull, R. N. The virucidal properties of peracetic acid. Am. J. Clin. Pathol. 33, 30–33 (1960).
Baldry, M. G. C. The bactericidal, fungicidal and sporicidal properties of hydrogen peroxide and peracetic acid. J. Appl. Bacteriol. 54, 417–423 (1983).
Rutala, W. A., Gergen, M. F. & Weber, D. J. Comparative evaluation of the sporicidal activity of new low-temperature sterilization technologies: Ethylene oxide, 2 plasma sterilization systems, and liquid peracetic acid. Am. J. Infect. Control 26, 393–398 (1998).
Luo, J. et al. Development and characterization of acellular porcine pulmonary valve scaffolds for tissue engineering. Tissue Eng. A 20, 2963–2974 (2014).
Zvarova, B. et al. Residual detergent detection method for nondestructive cytocompatibility evaluation of decellularized whole lung scaffolds. Tissue Eng. C Methods 22, 418–428 (2016).
Sun, D. et al. Novel decellularized liver matrix-alginate hybrid gel beads for the 3D culture of hepatocellular carcinoma cells. Int. J. Biol. Macromol. 109, 1154–1163 (2018).
Agarwal, T., Narayan, R., Maji, S., Ghosh, S. K. & Maiti, T. K. Decellularized caprine liver extracellular matrix as a 2D substrate coating and 3D hydrogel platform for vascularized liver tissue engineering. J. Tissue Eng. Regen. Med. 12, e1678–e1690 (2018).
Rosario, D. J. et al. Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract. Regen. Med. 3, 145–156 (2008).
O’Neill, J. D., Freytes, D. O., Anandappa, A. J., Oliver, J. A. & Vunjak-Novakovic, G. V. The regulation of growth and metabolism of kidney stem cells with regional specificity using extracellular matrix derived from kidney. Biomaterials 34, 9830–9841 (2013).
Bolland, F. et al. Development and characterisation of a full-thickness acellular porcine bladder matrix for tissue engineering. Biomaterials 28, 1061–1070 (2007).
Gao, R. et al. Hepatocyte culture in autologous decellularized spleen matrix. Organogenesis 11, 16–29 (2015).
Liu, P. et al. Hemocompatibility improvement of decellularized spleen matrix for constructing transplantable bioartificial liver. Biomed. Mater. 14, 025003 (2019).
Poornejad, N. et al. Comparison of four decontamination treatments on porcine renal decellularized extracellular matrix structure, composition, and support of renal tubular epithelium cells. J. Biomater. Appl. 30, 1154–1167 (2016).
Moradi, L. et al. Evaluation of different sterilization methods for decellularized kidney tissue. Tissue Cell 66, 101396 (2020).
Dew, L., English, W. R., Chong, C. K. & MacNeil, S. Investigating neovascularization in rat decellularized intestine: An in vitro platform for studying angiogenesis. Tissue Eng. A 22, 1317–1326 (2016).
Nowocin, A. K., Southgate, A., Gabe, S. M. & Ansari, T. Biocompatibility and potential of decellularized porcine small intestine to support cellular attachment and growth. J. Tissue Eng. Regen. Med. 10, E23–E33 (2016).
Gosztyla, C. et al. A comparison of sterilization techniques for production of decellularized intestine in mice. Tissue Eng. C Methods 26, 67–79 (2020).
Jones, G. et al. Decellularization and characterization of porcine superflexor tendon: A potential anterior cruciate ligament replacement. Tissue Eng. A 23, 124–134 (2017).
Whitaker, S., Edwards, J. H., Guy, S., Ingham, E. & Herbert, A. Stratifying the mechanical performance of a decellularized xenogeneic tendon graft for anterior cruciate ligament reconstruction as a function of graft diameter. Bone Jt. Res. 8, 518–525 (2019).
Sridharan, R., Reilly, R. B. & Buckley, C. T. Decellularized grafts with axially aligned channels for peripheral nerve regeneration. J. Mech. Behav. Biomed. Mater. 41, 124–135 (2015).
Thomas, P. Long-term survival of bacillus spores in alcohol and identification of 90% ethanol as relatively more spori/bactericidal. Curr. Microbiol. 64, 130–139 (2012).
Wei, H. J. et al. Construction of varying porous structures in acellular bovine pericardia as a tissue-engineering extracellular matrix. Biomaterials 26, 1905–1913 (2005).
Dijkman, P. E., Driessen-Mol, A., Frese, L., Hoerstrup, S. P. & Baaijens, F. P. T. Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials 33, 4545–4554 (2012).
Jiang, B. et al. Vascular scaffolds with enhanced antioxidant activity inhibit graft calcification. Biomaterials 144, 166–175 (2017).
Kim, M. K. et al. Decellularized extracellular matrix-based bio-ink with enhanced 3D printability and mechanical properties. Biofabrication 12, 025003 (2020).
Lewis, P. L. et al. Complex bile duct network formation within liver decellularized extracellular matrix hydrogels. Sci. Rep. 8, 12220 (2018).
Jiang, K. et al. 3-D physiomimetic extracellular matrix hydrogels provide a supportive microenvironment for rodent and human islet culture. Biomaterials 198, 37–48 (2019).
Singh, A. et al. Tissue-engineered neo-urinary conduit from decellularized trachea. Tissue Eng. A 24, 1456–1467 (2018).
Nie, Z., Wang, X., Ren, L. & Kang, Y. Development of a decellularized porcine bone matrix for potential applications in bone tissue regeneration. Regen. Med. 15, 1519–1534 (2020).
Aeberhard, P. et al. Efficient decellularization of equine tendon with preserved biomechanical properties and cytocompatibility for human tendon surgery indications. Artif. Organs 44, E161–E171 (2020).
Roth, S. P. et al. Transforming growth factor beta 3-loaded decellularized equine tendon matrix for orthopedic tissue engineering. Int. J. Mol. Sci. 20, 5474 (2019).
Zhu, M. et al. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. 10, 4620 (2019).
Hussein, K. H. et al. Sterilization using electrolyzed water highly retains the biological properties in tissue-engineered porcine liver scaffold. Int. J. Artif. Organs 36, 781–792 (2013).
Calvo, J. & Martínez-Martínez, L. Mecanismos de acción de los antimicrobianos. Enferm Infect. Microbiol. Clin. 27, 44–52 (2009).
Nikkels, C. et al. Robotic-assisted laparoscopic colpectomy combined with a hysterectomy and bilateral salpingo-oophorectomy versus a vaginal colpectomy in trans masculine individuals. Int. J. Transgend. Health 1, 1–10 (2023).
Groenman, F., Nikkels, C., Huirne, J., van Trotsenburg, M. & Trum, H. Robot-assisted laparoscopic colpectomy in female-to-male transgender patients; technique and outcomes of a prospective cohort study. Surg. Endosc. 31, 3363–3369 (2017).
Fitzpatrick, J. C., Clark, P. M. & Capaldi, F. M. Effect of decellularization protocol on the mechanical behavior of porcine descending aorta. Int. J. Biomater. 2010, 1–11 (2010).
Ruiz-Zapata, A. M. et al. Extracellular matrix stiffness and composition regulate the myofibroblast differentiation of vaginal fibroblasts. Int. J. Mol. Sci. 21, 1–15 (2020).
Cohen, H. et al. WHO Expert Committee on Biological Standardization (1973).
Hendry, J. Radiation Sterilization of Tissue Allografts: Requirements for Validation and Routine Control—A Code of Practice (2007).
Amabebe, E. & Anumba, D. O. C. The vaginal microenvironment: The physiologic role of Lactobacilli. Front. Med. 5, 1–11 (2018).
LaClaire, L. & Facklam, R. Antimicrobial susceptibilities and clinical sources of facklamia species. Antimicrob. Agents Chemother. 44, 2130–2132 (2000).
Winston McPherson, G. et al. The vaginal microbiome of transgender men. Clin. Chem. 65, 199–207 (2019).
Fishman, J. A. & Grossi, P. A. Donor-derived infection—the challenge for transplant safety. Nat. Rev. Nephrol. 10, 663–672 (2014).
De Filippo, R. E., Bishop, C. E., Filho, L. F., Yoo, J. J. & Atala, A. Tissue engineering a complete vaginal replacement from a small biopsy of autologous tissue. Transplantation 86, 208–214 (2008).
Dorin, R. P., Atala, A. & Defilippo, R. E. Bioengineering a vaginal replacement using a small biopsy of autologous tissue. Semin. Reprod. Med. 29, 38–44 (2011).
Sartoneva, R. et al. Porous poly-L-lactide-co-1-caprolactone scaffold: A novel biomaterial for vaginal tissue engineering. R. Soc. Open Sci. 5, 180811 (2018).
Brindeau, A. Creation d’un vagin artificiel a l’aide des membranes ovulaires d’un oeuf a terme. Gynecol. Observ. 29, 385 (1934).
Porzionato, A. et al. Decellularized human skeletal muscle as biologic scaffold for reconstructive surgery. Int. J. Mol. Sci. 16, 14808–14831 (2015).
French, M. S. Solvay Pharmaceutical Internal Memo—lrritancy Testing of Peracetic Acid to Skin (1993).
Fraser, J. A. L. & Thorbison, A. Fogging Trials with Tenneco Organics at Collards Farm (Solvay InterTox, 1986).
ECETOC. Peracetic Acid and Its Equilibrium Solutions (2001).
Methe, K. et al. An alternative approach to decellularize whole porcine heart. Biores. Open Access 3, 327–338 (2014).
Watts, S., Ramstedt, M. & Salentinig, S. Ethanol inactivation of enveloped viruses: structural and surface chemistry insights into Phi6. J. Phys. Chem. Lett. 12, 9557–9563 (2021).
Kampf, G. Efficacy of ethanol against viruses in hand disinfection. J. Hosp. Infect. 98, 331–338 (2018).
Hathaway, J. K. HPV. Clin. Obstet. Gynecol. 55, 671–680 (2012).
Bailon, L., Mothe, B., Berman, L. & Brander, C. Novel approaches towards a functional cure of HIV/AIDS. Drugs 80, 859–868 (2020).
Murray, C. J. L. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655 (2022).
Author information
Authors and Affiliations
Contributions
J.S.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, visualization, writing—original draft. L.d.B.: conceptualization, data curation, investigation, methodology, project administration, visualization. F.G.: writing—review & editing. T.S.: conceptualization, supervision, writing—review & editing. J.H.: funding acquisition, supervision, writing—review & editing. S.Z.: conceptualization, investigation, methodology, resources, supervision, validation, writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sueters, J., de Boer, L., Groenman, F. et al. A sterilization method for human decellularized vaginal matrices. Sci Rep 14, 31728 (2024). https://doi.org/10.1038/s41598-024-82409-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82409-4