Abstract
Chronic heart failure (CHF) represents one of the most severe and advanced stages of cardiovascular disease. Despite the critical importance of cardiac rehabilitation (CR) in CHF management, while studies have explored the effectiveness of various CR delivery modes and offered valuable context-specific insights, their relative efficacy remains inconsistent across different patient groups, healthcare environments, and intervention approaches. A clearer understanding requires comprehensive comparisons and in-depth analyses to address these variations. Systematic searches were conducted in databases including Pubmed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science, up to August 2024. Two researchers independently screened the literature according to strict inclusion criteria, extracted relevant data, and assessed the quality of included studies using Cochrane Collaboration tools and the Jadad scale. Subsequent pairwise and network meta-analyses were performed using statistical software, including Stata 17.0, to present the results graphically. The network meta-analysis included 9,552 articles, with 33 meeting the inclusion criteria and examining eleven different interventions. All interventions outperformed routine care. Combined CR with aerobic exercise and resistance training (HCR [AE + RE]) significantly improved Minnesota Living with Heart Failure Questionnaire (MLHFQ) scores and 6-min walk test (6MWT) performance, and reduced rehospitalization rates [SUCRA = 96%]. Center-based cardiac rehabilitation (CBCR) with high-intensity interval training (HIIT) was the most effective in enhancing left ventricular ejection fraction (LVEF), while CBCR(AE) demonstrated the greatest improvement in peak oxygen uptake (Peak VO2) [RR = 3.64, 95% CI: 1.66–7.95]. Our analysis identifies HCR (AE + RE) as the most effective intervention for improving quality of life (MLHFQ), exercise capacity (6MWT), and reducing hospital readmissions. CBCR (HIIT) was optimal for enhancing cardiac function through improved LVEF, while CBCR (AE) effectively boosted peak VO2.
PROSPERO: CRD42024517039, Review Completed not published.
Similar content being viewed by others
Introduction
Chronic heart failure (CHF), a complex clinical syndrome, arises from myriad etiologies that precipitate pathological alterations in cardiac structure and function. These alterations compromise ventricular systolic and/or diastolic function, culminating predominantly in symptoms such as dyspnea, fatigue, and fluid retention, manifesting as pulmonary and systemic circulatory congestion and peripheral edema1. CHF is recognized as the most severe and advanced manifestation of cardiovascular diseases2. It afflicts approximately 26 million adults worldwide, with the United States reporting about 5.7 million cases alone. Projections suggest a rise to over 8 million cases by 20303. Marked by elevated rates of hospitalization and mortality, diminished quality of life, and considerable economic impacts, CHF poses a significant public health challenge both in China and globally4.
While current pharmacological interventions form the foundation of CHF management—predominantly focusing on acute care and maintenance therapy during stable periods—these strategies often neglect aspects such as secondary prevention and long-term prognosis of CHF patients. A wealth of research underscores that CR, with its emphasis on structured exercise programs, can markedly bolster exercise tolerance, enhance the quality of life, alleviate depressive symptoms, and diminish the likelihood of rehospitalization, thus significantly ameliorating clinical outcomes5,6.
CR is not a singular intervention, but a comprehensive, tiered strategy for patients with cardiovascular diseases (CVD). It is a complex intervention that involves the collaborative efforts of multidisciplinary teams, addressing physiological, psychological, and social dimensions. The goal is to improve the overall quality of life for patients, particularly after the acute phases of their conditions. The essential elements of CR include comprehensive health assessments, tailored exercise regimens, pharmacological management, dietary counseling, psychological support, smoking cessation initiatives, management of concomitant risk factors, and educational activities to enhance patient adherence to therapeutic plans7. CR is a crucial component of non-pharmacological management for CHF, as recommended by both American and European guidelines for CHF patients8, and supported by the American College of Cardiology Foundation/American Heart Association Task Force and the European Society of Cardiology9. CHF patients should initiate CR in Phase I to optimize recovery and improve long-term outcomes.
CR programs are offered in various formats, including center-based, home-based, tele-based, and combined models, all proven effective in reducing cardiovascular mortality, morbidity, and hospitalization rates10,11,12. Nevertheless, previous meta-analyses have indicated that hospital-based CR programs generally exhibit lower effectiveness and adherence than home-based, tele-based, and combined models13,14,15. While some studies have evaluated the effectiveness of different CR delivery models and provided valuable insights in specific contexts, the relative efficacy of these models across various patient populations, healthcare settings, and intervention protocols remains heterogeneous. Therefore, further comprehensive comparisons and multidimensional analyses are crucial to clarify the relative effectiveness of these delivery methods. To address this gap, we conducted a network meta-analysis (NMA) aimed at comprehensively assessing and ranking the effectiveness of center-based, home-based, tele-based, and combined CR delivery models, with a focus on determining the most effective exercise modalities for patients with CHF.
Methods
This study rigorously adheres to the PRISMA-NMA guidelines for reporting NMA. The comprehensive study protocol, including detailed research strategies, has been registered on the PROSPERO platform (registration number CRD42024517039). The detailed PRISMA checklist is provided as Appendix 1.
Study types
The study encompasses randomized controlled trials (RCTs) on exercise-based CR that were published in English. These trials, irrespective of blinding or allocation concealment, included complete patient information.
Types of participants
The analysis included RCTs involving adults (≥ 18 years) with CHF, comparing different forms of exercise-based CR. Patients were categorized into three heart failure subtypes: reduced ejection fraction (HFrEF), preserved ejection fraction (HFpEF), and mid-range ejection fraction (HFmrEF). Consequently, the study population comprised adults diagnosed with one of these three subtypes of heart failure.
Types of interventions
In this NMA, various types of exercise-based CR are examined, either against each other or against standard care as part of the treatment. Exercise-based CR is categorized into center-based, home-based, tele-based, and combined interventions. Center-based CR(CBCR) is defined as CR conducted in a hospital or a similarly equipped facility. Home-based CR(HBCR) refers to interventions at the patient’s home or other non-hospital settings (e.g., community centers) using traditional follow-up methods such as telephone calls or regular visits. Tele-based CR(CTR) is performed at the patient’s home or outside hospital settings, monitored, and guided by health professionals using telemedicine technologies. Combined CR(HCR) refers to either (1) a combination of center and home-based CR or (2) center and tele-based CR. Patients receiving standard care are expected to maintain daily activities without CR interventions. The exercise interventions within center-based, home-based, tele-based, and combined CR included aerobic exercise (AE), high-intensity interval training (HIIT), and a combination of aerobic and resistance training (AE + RE), which were compared in this analysis.
Types of outcome
Primary outcomes include peak oxygen uptake(Peak O2), left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVEDD), and 6-min walk test (6MWT). Secondary outcomes include the Minnesota Living with Heart Failure quality of life(MLHFQ), and rehospitalization rates.
Exclusion criteria
Our study excludes non-RCTs such as conference abstracts, reviews (including systematic reviews), editorials, observational studies, animal studies, and pediatric trials. Studies deviating from the principles of randomization or lacking robust experimental design and those comparing the effectiveness of CR across different training periods are also excluded. Finally, studies with outcome measures inappropriate for this NMA are omitted.
Search strategy
We systematically searched the following databases: Pubmed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science. The search covered records from the inception of each database until August 2024. References of included studies were also retrospectively searched to supplement the search. The strategy adhered strictly to the PICOS principles, using keywords such as "heart failure," "cardiac failure," "heart decompensation," "cardiac rehabilitation," "exercises," "physical activity," "RCT," and "randomized controlled trial." These terms were combined using Boolean operators “and” and "or." The search strategy is outlined in Appendix 2.
Data extraction and screening
Duplicate studies were removed using EndNote, and two researchers initially screened potentially relevant citations based on titles and abstracts. Full texts were then evaluated based on inclusion and exclusion criteria. In cases of uncertainty, another researcher assessed the citations, and consensus determined inclusion. A detailed reading of the included studies facilitated data extraction into a predefined form, covering: (1) the first author’s name, publication year, methods, and country; (2) baseline characteristics of the patient groups (e.g., age); (3) mode of CR delivery; (4) sample size; (5) outcome measures. Data extraction was independently performed by authors who read each article in full.
Bias risk and quality assessment
We defined low overall risk of bias as low risk in the domains of randomization, blinding of outcome assessors, completeness of outcome data, and selective reporting without other domains at high risk. Unclear overall risk of bias is defined as having at least one unclear risk of bias in the domains of randomization, blinding, completeness of outcome data, or selective reporting, without high risk in any other domains. A high overall risk of bias is defined as having a high risk in at least one of these domains: randomization, blinding of outcome assessors, completeness of outcome data, or selective reporting. This stringent definition of bias risk was applied to ensure the credibility and trustworthiness of our research findings.
According to the Cochrane Handbook 5.1.016, the quality of our studies was assessed using the risk of bias tool for randomized controlled trials. Two researchers independently conducted the evaluations, with any disagreements resolved by a third reviewer. The evaluation covered key aspects such as the generation of random sequences, implementation of allocation concealment, blinding of participants, researchers, and outcome assessors, completeness of outcome data, selective reporting, and other biases. Each category was assessed as "low risk of bias," "unclear," or "high risk of bias." The assessment was graded on three levels: all entries assessed as "low risk of bias" (Grade A) indicated a lower likelihood of bias; any entries assessed as "high risk of bias" (Grade C) suggested a higher likelihood of bias; if some criteria were met, the risk of potential bias was considered moderate (Grade B).
Statistical analysis
Statistical analysis used StataSE 17.0 to perform network meta-analyses and generate network graphs, forest plots, funnel plots, league tables, and Surface Under the Cumulative Ranking Curve (SUCRA). The primary outcome measures, including peak oxygen uptake, ejection fraction, left ventricular end-diastolic diameter (LVEDD), 6-min walk distance, and Minnesota Living with Heart Failure quality of life, were analyzed as continuous variables, extracting mean differences and standard deviations post-intervention. The rehospitalization rates were analyzed as dichotomous variables.
In direct meta-analyses comparing pairs of interventions, heterogeneity was assessed using the I2 statistic. An I2 value ≤ 50% indicated no significant statistical heterogeneity, justifying a fixed-effect model. Conversely, an I2 value > 50% indicated significant heterogeneity, necessitating a random-effects model. For network meta-analysis, where each outcome measure forms a closed loop, it is imperative to perform a loop inconsistency test via node-splitting methods. This test compares direct and indirect estimates; a P-value < 0.05 suggests inconsistency between direct and indirect comparisons, warranting an inconsistency model, whereas a P-value > 0.05 supports using a consistency model.
The loop-specific approach evaluated inconsistency within loops, comparing direct and indirect estimates for each comparison. Node-splitting techniques were utilized to decompose the overall estimate at each node into its direct and indirect components. Sensitivity analyses were conducted for the primary and other available secondary outcomes: (1) including only studies with a low overall risk of bias; (2) excluding studies with a high overall risk of bias. Utilizing the network package, a network meta-analysis is conducted, producing SUCRA graphs to determine the relative efficacy of various types of exercise-based CR across different evaluation metrics. Subsequently, funnel plots are employed to detect publication bias within the incorporated literature and to perform bias analysis leveraging the obtained outcomes.
Results
Study selection
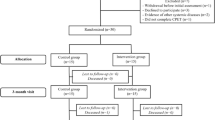
A total of 9,522 studies were identified and screened. After removing duplicates, 5,886 records were screened based on titles and abstracts, and 3,666 full-text articles were retained. A full-text review excluded 1,890 articles that did not meet the inclusion criteria, such as case reports, reviews, commentaries, animal studies, or poorly designed randomized controlled trials. An additional 328 studies were excluded for having non-conforming interventions, 468 for inadequate study design, 444 for lacking relevant outcomes, and 406 for not meeting intervention criteria. Ultimately, 33 studies were included. The detailed literature selection process is shown in Appendix 4 Fig. 4.1.
Study characteristics
Eight studies were conducted in the United States and five in China. Additional studies originated from Poland, Australia, Germany, the United Kingdom, Italy, Belgium, Canada, Switzerland, Finland, Greece, Athens, Turkey, Iran, Norway, and Japan. Of these, Two studies did not report the ages of participants. A total of 26 studies were single-center trials, and 7 were multicenter trials. The analysis included two studies on patients with HFmrEF, 29 studies on HFrEF, and two studies on HFpEF. The total sample size across all included studies was 5,900 participants, divided into 2,976 in the experimental groups and 2,924 in the control groups. All studies were dual-arm, with comparable baseline data of the patients. The basic characteristics of the included studies are presented in Appendix 3 Table 3.1.
Risk of bias of included studies
The duration of the individual CR programs varied: center-based programs averaged 3.375 months; home-based programs averaged 4.54 months; tele-based programs averaged 2.89 months; and combined programs averaged 5 months. The overall risk of bias for the included RCTs is detailed in Appendix 3 Table 3.2. A total of 33 RCTs were included, with 2 studies reporting random sequence generation using random number tables as their method17,18, and 28 studies reported using general randomization methods19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47, all considered at low risk of bias. One study was identified as using non-randomized group assignments26,30,32,42,45,46 and categorized as high risk due to the lack of randomization. Most interventions were non-blinded due to the practical requirements of exercise participation and the need to instruct patients, although a few studies implemented blinding26,30,32,38,44,46,47. (Appendix 4:Fig. 4.2 and 4.3). We analyzed heterogeneity at different intensities (high, medium, low) and frequencies (< 3 times per week, 3–5 times per week, > 5 times per week), which ranged from low to moderate. For details, please refer to Appendix 5 Table 5.1
Evaluation of consistency and heterogeneity
The I2 result did not identify any high heterogeneity in the network, and most comparisons were low or low-moderate levels of heterogeneity (Appendix 5 Table 5.2).
Synthesis of results
Network plot
This study included a total of 33 studies. Of these, 23 studies, all two-arm trials, reported 6MWT with two closed loops. Nine studies, all two-arm trials, reported LVEF and had one closed loop. Twenty studies, all two-arm trials, reported peak VO2 and had one closed loop. This study constructed an evidence network diagram encompassing all research indicators, as Appendix 4 Fig. 4.4 (A-F) detailed. In this network diagram, blue circles represent different interventions, with their size indicating the sample size included for each intervention. The lines connecting them represent studies that directly compared two interventions, and the thickness of these lines corresponds to the number of studies involving that comparison.
Loop inconsistency test
6MWT had two closed loops, LVEF and Peak VO2 each having one closed loop, and testing revealed that the p-values were all greater than 0.05, indicating good consistency within the closed loops. Thus, a consistency model was deemed appropriate for conducting network meta-analyses (Appendix 3:Table 3.3).
Network meta-analysis
MLHFQ
Among the 12 studies comparing five different interventions in the MLHFQ, all were dual-arm trials. The evidence network is shown in Appendix 4 Fig. 4.4 -A. Among all the interventions, HCR(AE + RE) demonstrated the most significant improvement in MLHFQ scores. The 95%CI did not include 1 in all cases, indicating statistically significant differences. The remaining pairwise comparisons had 95% CIs that included 1, suggesting no significant differences (see Appendix 4 Fig. 4.5-A). As the MLHFQ is a negative outcome measure, a lower SUCRA score indicates a more effective intervention. The ranking of interventions from most to least effective is as follows: HCR(AE + RE) (SUCRA = 100%) > HBCR(AE) (SUCRA = 71.4%) > CBCR(HIIT) (SUCRA = 70.9%) > CBCR(AE) (SUCRA = 47.1%) > CTR(AE) (SUCRA = 44%) > HBCR(AE + RE) (SUCRA = 37.6%) > CBCR(AE + RE) (SUCRA = 16.3%) > UC (SUCRA = 12.7%), as illustrated in Appendix 4 Fig. 4.6-A.
6MWT
A total of 23 studies compared eleven interventions in terms of differences in the 6MWT, all of which were dual-arm trials. The evidence network is illustrated in Appendix 4 Fig. 4.4 -B. Compared to usual care, HCR(AE + RE) [RR = 50.29, 95% CI (0.91, 100.09)] (SUCRA = 100%) was more effective in improving 6MWT performance, with all 95%CI excluding 1, indicating statistically significant differences. The remaining pairwise comparisons had 95% CIs that included 1, suggesting no significant differences (see Appendix 4 Fig. 4.5-B,4.6-B).
LVEF
Among the 26 studies, differences in LVEF were compared across five different intervention strategies, as shown in the evidence network diagram in Appendix 4 Fig. 4.4 -C, all of which were dual-arm studies. CBCR (HIIT) was the most effective intervention for improving LVEF among all treatments (SUCRA = 100%). None of the pairwise comparisons revealed statistically significant differences, as all 95%CIs included 1, as depicted in Appendix 4 Fig. 4.5-C,4.6-C.
LVEDD
Among the 3 studies, differences in LVEDD were compared across four different intervention strategies, as shown in the evidence network diagram in Appendix 4 Fig. 4.4 -D. All studies were dual-arm. For the pairwise comparisons, the 95% confidence intervals include 1, suggesting no statistically significant differences. These comparisons are depicted in Appendix 4 Fig. 4.5-D,4.6-D.
Peak VO2
Among the 20 studies, differences in Peak VO2 across five different intervention strategies were compared, as shown in the evidence network diagram in Appendix 4 Fig. 4.4 -E. All studies were dual-arm. CBCR(AE + RE) [RR = 2.46, 95% CI (1.03,5.89)] and HBCR(AE) [RR = 1.89, 95% CI (1.10,3.28)] both outperformed usual care, while CBCR(AE) demonstrated the best outcomes [RR = 3.64, 95% CI (1.66,7.95)]. The 95% confidence intervals do not include 1, indicating statistical significance. For the remaining pairwise comparisons, the 95% confidence intervals include 1, suggesting no statistically significant differences. These results are depicted in Appendix 4 Fig. 4.5-E. Peak VO2 is a positive outcome indicator, so a higher SUCRA score signifies better intervention efficacy. The ranking of interventions from most to least effective is as follows: CBCR(AE) (SUCRA = 80.6%) > CBCR(AE + RE) (SUCRA = 60.3%) > HBCR(AE) (SUCRA = 46.4%) > UC (SUCRA = 10.3%). This order is displayed in Appendix 4 Fig. 4.6-E.
Readmission rates
Among the 7 studies, differences in Readmission Rates across six different intervention strategies were compared, as shown in the evidence network diagram in Appendix 4 Fig. 4.4 -F. All studies were dual-arm. HCR (AE + RE) was the most effective intervention for reducing readmission rates among all treatments (SUCRA = 96%). The 95% confidence intervals do not include 1, indicating statistical significance. For the remaining pairwise comparisons, the 95% confidence intervals include 1, suggesting no statistically significant differences. These results are depicted in Appendix 4 Fig. 4.5-F,4.6-F.
Publication bias assessment
A funnel plot was generated using StataSE 17.0 software, with the horizontal axis representing the effect size of each study and the vertical axis representing the standard error. The funnel plot is a commonly used tool for detecting bias among studies included in a meta-analysis. Each point on the plot represents a pairwise comparison from one of the studies. Most studies display symmetrical distribution around the zero line, with a few scattered outside the funnel plot (except for Fig. 7-D,7-F), indicating the presence of some publication bias. Some scattered points lie close to the x-axis, suggesting a small sample size. Appendix 4 Fig. 4.7.
Meta-regressions
We also used meta-regression analysis to assess the potential impact of baseline factors on the primary outcomes. HFrEF, HFpEF, and HFmrEF were evaluated, with results showing no significant influence on the main outcomes (Appendix 6).
Discussion
Our study classifies exercise-based CR into different approaches based on location and exercise type. CR interventions are categorized as CBCR, HBCR, CTR, or HCR, and further divided by exercise modality: AE, AE + RE, and HIIT. This classification aims to determine the most effective treatment options for patients.
In this network meta-analysis, we evaluated 11 exercise modalities across 33 randomized controlled trials involving 5,900 participants with CHF. The primary outcomes assessed included MLHFQ, 6MWT, LVEF, LVEDD, Peak VO2, and readmission rates. The analysis showed that HCR(AE + RE) significantly improved MLHFQ scores, enhanced 6MWT performance, and reduced readmission rates, all supported by high-quality evidence. CBCR(HIIT) was the most effective in improving LVEF and overall cardiac function, while CBCR(AE) significantly enhanced Peak VO2. Although the efficacy of these CR delivery modes varied, all interventions demonstrated benefits, aligning with guidelines recommending exercise-based rehabilitation as a safe and effective treatment for heart failure, improving exercise capacity, cardiopulmonary function, and quality of life48.
HCR(AE + RE) emerged as a particularly effective intervention, improving MLHFQ scores, 6MWT, and reducing readmissions. Tailored combined CR programs offer a flexible and comprehensive approach to CHF care, overcoming limitations of hospital-based CR such as space constraints, travel difficulties, staff shortages, and high costs. Participants benefit from receiving professional guidance at the hospital while maintaining the flexibility to continue CR at home, increasing both participation and completion rates. Studies suggest that participants prefer this combined approach due to its adaptability49. Furthermore, combined CR is cost-effective, accounting for only 38% of the cost of hospital-based programs50. Previous studies have shown that combined CR significantly reduces risk factors, improves physical fitness, and facilitates a return to work, making it an ideal option for patients with preserved HFpEF or HFmrEF51,52,53.
Improving QoL is a key goal in CHF treatment, as it is a strong predictor of reduced hospitalization and mortality54. QoL reflects the broader impact of CHF on physical activity, psychological health, and social interactions. Using MLHFQ, our study found that combined CR significantly reduced MLHFQ scores and readmission rates, thereby improving QoL. HCR(AE + RE) further enhanced 6MWT performance, consistent with prior findings55.
CBCR (HIIT) was the most effective intervention for improving LVEF and cardiac function. High-intensity interval training enhances skeletal muscle mitochondrial content and metabolic function, boosts cardiac output, and strengthens heart function56. CBCR, particularly in hospital settings, maximizes improvements in LVEF, enhances exercise capacity, and alleviates heart failure symptoms. Its safety and reliable supervision make it an ideal choice for patients with HFrEF.
CBCR(AE) also showed significant improvements in Peak VO2. Aerobic exercise increases exercise tolerance and improves Peak VO2 in CHF patients, a finding confirmed by our network meta-analysis. The mechanism likely involves CR improving endothelial function and myocardial blood flow, increasing oxygen delivery to the heart, and enhancing exercise capacity in CHF patients57.
Strengths and limitations
This study is the first to use NMA to assess the impact of different exercise-based CR delivery methods—center-based, home-based, tele-based, combined CR, and standard care—on cardiac function, exercise capacity, and QoL in heart failure patients. We stratified the study population into three heart failure subtypes: HFrEF, HFpEF, and HFmrEF. Additionally, we compared various exercise modalities within each CR delivery method, including aerobic exercise (AE), high-intensity interval training (HIIT), and aerobic exercise combined with resistance training (AE + RE). Our analysis included studies from 16 countries, ensuring a wide geographic representation and consistency in baseline characteristics. The inclusion criteria focused on interventions strictly following established CR delivery models, excluding control groups with additional exercise interventions beyond standard care. Studies with incomplete outcome data were excluded to improve the reliability of our findings.
This NMA provides a comprehensive review of all RCTs related to exercise-based CR delivery methods. We applied rigorous quality assessments to ensure the strength of our conclusions. However, the study has certain limitations. Reporting on readmission rates was limited, with only six RCTs providing data on this outcome. This smaller sample size restricted our ability to fully evaluate the effects of different CR delivery methods on readmission rates.
Conclusion
Our NMA identified HCR(AE + RE) as the most effective intervention for improving MLHFQ scores, increasing 6MWT performance, and reducing readmission rates. CBCR(HIIT) proved most effective in enhancing LVEF and improving cardiac function in heart failure patients, while CBCR(AE) significantly increased Peak VO2. Despite these findings, data on readmission rates and adverse cardiovascular events are limited. Future research should prioritize large-scale, multicenter, high-quality randomized controlled trials to build a stronger evidence base for exercise-based CR delivery methods.
Data availability
Most of the data supporting the results of this study can be obtained by obtaining the full text from the search library, but some articles require permission from the original authors to obtain them, so the availability of these data is limited and therefore cannot be made public. If anyone wishes to obtain data on the submission system and paper from this study, please contact Professor Zhang .
References
Chinese Society of Cardiology, Chinese Medical Association, Chinese College of Cardiovascular Physician, Chinese Heart Failure Association of Chinese Medical Doctor Association, et al. Chinese guidelines for the diagnosis and treatment of heart failure 2024. Chin J Cardiol. 52(3), 235–275 (2024).
Butler, J. et al. Clinical course of patients with worsening heart failure with reduced ejection fraction. J. Am. Coll. Cardiol. 73(8), 935–944 (2019).
Savarese, G. & Lund, L. H. Global public health burden of heart failure. Card. Fail Rev. 3(1), 7–11. https://doi.org/10.15420/cfr.2016:25:2 (2017).
Savarese, G. et al. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc Res. 118(17), 3272–3287 (2023).
Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, Dalal H, Rees K, Singh SJ, Taylor RS. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. 1(1), CD003331 (2019).
Haykowsky, M. J. et al. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J. Am. Coll. Cardiol. 49(24), 2329–2336 (2007).
Committee of Cardiac Rehabilitation and Prevention of Chinese Association of Rehabilitation Medicine. Guidelines for cardiovascular rehabilitation and secondary prevention in China 2018 simplified edition. Chin. J. Intern. Med. 57(11), 802–810 (2018).
Papathanasiou, J. V. et al. Does group-based high-intensity aerobic interval training improve the inflammatory status in patients with chronic heart failure?. Eur. J. Phys. Rehabil. Med. 58(2), 242–250 (2022).
McMurray, J. J., Adamopoulos, S., Anker, S. D., Auricchio, A., Böhm, M., Dickstein, K., et al; ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 33(14), 1787–1847 (2012).
Xueping, Z., Peiying, Z., Bing, H., et al. Research progress on disease management in patients with chronic heart failure. Nurs. Res. China (2017).
Batalik, L. et al. Long-term exercise effects after cardiac telerehabilitation in patients with coronary artery disease: 1-year follow-up results of the randomized study. Eur. J. Phys. Rehabil. Med. 57(5), 807–814 (2021).
McDonagh, T. A., Metra, M., Adamo, M., Gardner, R. S., Baumbach, A. & Böhm, M., et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 24(1), 4–131 (2022).
Dalal, H. M., Zawada, A., Jolly, K., Moxham, T. & Taylor, R. S. Home based versus centre based cardiac rehabilitation: Cochrane systematic review and meta-analysis. BMJ. 19(340), b5631 (2010).
Rawstorn, J. C., Gant, N., Direito, A., Beckmann, C. & Maddison, R. Telehealth exercise-based cardiac rehabilitation: A systematic review and meta-analysis. Heart. 102(15), 1183–1192 (2016).
Bakhshayeh, S., Sarbaz, M., Kimiafar, K., Vakilian, F. & Eslami, S. Barriers to participation in center-based cardiac rehabilitation programs and patients’ attitude toward home-based cardiac rehabilitation programs. Physiother. Theory Pract. 37(1), 158–168 (2021).
Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J. & Welch, V. A. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane (2023).
Peng, X. et al. Home-based telehealth exercise training program in Chinese patients with heart failure: A randomized controlled trial. Medicine (Baltimore). 97(35), e12069 (2018).
Hambrecht, R. et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: A randomized trial. JAMA. 283(23), 3095–3101 (2000).
Corvera-Tindel, T., Doering, L. V., Woo, M. A., Khan, S. & Dracup, K. Effects of a home walking exercise program on functional status and symptoms in heart failure. Am. Heart J. 147(2), 339–346 (2004).
Chien, C. L., Lee, C. M., Wu, Y. W. & Wu, Y. T. Home-based exercise improves the quality of life and physical function but not the psychological status of people with chronic heart failure: a randomised trial. J. Physiother. 57(3), 157–163 (2011).
Oka, R. K. et al. Impact of a home-based walking and resistance training program on quality of life in patients with heart failure. Am. J. Cardiol. 85(3), 365–369 (2000).
Piotrowicz, E. et al. Effects of a 9-week hybrid comprehensive telerehabilitation program on long-term outcomes in patients with heart failure: The telerehabilitation in heart failure patients (TELEREH-HF) randomized clinical trial. JAMA Cardiol. 5(3), 300–308 (2020).
Dracup, K. et al. Effects of a home-based exercise program on clinical outcomes in heart failure. Am. Heart J. 154(5), 877–883 (2007).
Piotrowicz, E. et al. A new model of home-based telemonitored cardiac rehabilitation in patients with heart failure: effectiveness, quality of life, and adherence. Eur. J. Heart Fail. 12(2), 164–171 (2010).
O’Connor, C. M., Whellan, D. J., Lee, K. L., Keteyian, S. J., Cooper, L. S. & Ellis, S. J., et al.; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 301(14), 1439–50 (2009).
Hwang, R., Bruning, J., Morris, N. R., Mandrusiak, A. & Russell, T. Home-based telerehabilitation is not inferior to a centre-based program in patients with chronic heart failure: a randomised trial. J. Physiother. 63(2), 101–107 (2017).
Conraads, V. M. et al. Combined endurance/resistance training reduces NT-proBNP levels in patients with chronic heart failure. Eur. Heart J. 25(20), 1797–1805 (2004).
Belardinelli, R., Georgiou, D., Cianci, G. & Purcaro, A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: Effects on functional capacity, quality of life, and clinical outcome. Circulation. 99(9), 1173–1182 (1999).
Hambrecht, R. et al. Physical training in patients with stable chronic heart failure: Effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J. Am. Coll. Cardiol. 25(6), 1239–1249 (1995).
Du, H. et al. The Home-Heart-Walk study, a self-administered walk test on perceived physical functioning, and self-care behaviour in people with stable chronic heart failure: A randomized controlled trial. Eur. J. Cardiovasc. Nurs. 17(3), 235–245 (2018).
Chen, Y. W. et al. Home-based cardiac rehabilitation improves quality of life, aerobic capacity, and readmission rates in patients with chronic heart failure. Medicine (Baltimore). 97(4), e9629 (2018).
Dalal, H. M. et al. The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: The REACH-HF multicentre randomized controlled trial. Eur. J. Prev. Cardiol. 26(3), 262–272 (2019).
Piotrowicz, E. et al. Home-based telemonitored Nordic walking training is well accepted, safe, effective and has high adherence among heart failure patients, including those with cardiovascular implantable electronic devices: A randomised controlled study. Eur. J. Prev. Cardiol. 22(11), 1368–1377 (2015).
Austin, J., Williams, R., Ross, L., Moseley, L. & Hutchison, S. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. Eur. J. Heart Fail. 7(3), 411–417 (2005).
Willenheimer, R. et al. Effects on quality of life, symptoms and daily activity 6 months after termination of an exercise training programme in heart failure patients. Int. J. Cardiol. 77(1), 25–31 (2001).
Kiilavuori, K., Sovijärvi, A., Näveri, H., Ikonen, T. & Leinonen, H. Effect of physical training on exercise capacity and gas exchange in patients with chronic heart failure. Chest. 110(4), 985–991 (1996).
Koukouvou, G. et al. Quality of life, psychological and physiological changes following exercise training in patients with chronic heart failure. J. Rehabil. Med. 36(1), 36–41 (2004).
Giannuzzi, P., Temporelli, P. L., Corrà, U., Tavazzi, L.; ELVD-CHF Study Group. Antiremodeling effect of long-term exercise training in patients with stable chronic heart failure: Results of the Exercise in Left Ventricular Dysfunction and Chronic Heart Failure (ELVD-CHF) Trial. Circulation. 108(5), 554–9 (2003).
Xueyu, L., Hao, Y., Shunlin, X., Rongbin, L. & Yuan, G. Effects of low-intensity exercise in older adults with chronic heart failure during the transitional period from hospital to home in China: A randomized controlled trial. Res. Gerontol. Nurs. 10(3), 121–128 (2017).
Karapolat, H. et al. Comparison of hospital-based versus home-based exercise training in patients with heart failure: Effects on functional capacity, quality of life, psychological symptoms, and hemodynamic parameters. Clin. Res. Cardiol. 98(10), 635–642 (2009).
Chrysohoou, C., Angelis, A., Tsitsinakis, G., Spetsioti, S., Nasis, I. & Tsiachris, D., et al. Cardiovascular effects of high-intensity interval aerobic training combined with strength exercise in patients with chronic heart failure. A randomized phase III clinical trial. Int. J. Cardiol. 179, 269–74 (2015).
Brubaker, P. H., Moore, J. B., Stewart, K. P., Wesley, D. J. & Kitzman, D. W. Endurance exercise training in older patients with heart failure: Results from a randomized, controlled, single-blind trial. J. Am. Geriatr. Soc. 57(11), 1982–1989 (2009).
Nagatomi, Y. et al. Home-based cardiac rehabilitation using information and communication technology for heart failure patients with frailty. ESC Heart Fail. 9(4), 2407–2418. https://doi.org/10.1002/ehf2.13934 (2022).
Lundgren, K. M. et al. Feasibility of telerehabilitation for heart failure patients inaccessible for outpatient rehabilitation. ESC Heart Fail. 10(4), 2406–2417 (2023).
Kitzman, D. W. et al. Physical rehabilitation for older patients hospitalized for heart failure. N. Engl. J. Med. 385(3), 203–216. https://doi.org/10.1056/NEJMoa2026141 (2021).
Yeh, G. Y. et al. Tai chi exercise in patients with chronic heart failure: A randomized clinical trial. Arch. Intern. Med. 171(8), 750–757. https://doi.org/10.1001/archinternmed.2011.150 (2011).
Yeh, G. Y. et al. Effects of tai chi mind-body movement therapy on functional status and exercise capacity in patients with chronic heart failure: A randomized controlled trial. Am. J. Med. 117(8), 541–548. https://doi.org/10.1016/j.amjmed.2004.04.016 (2004).
McDonagh, T. A., Metra, M., Adamo, M., Gardner, R. S., Baumbach, A. & Böhm, M., et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 42(36), 3599–3726 (2021).
Grace, S. L., McDonald, J., Fishman, D. & Caruso, V. Patient preferences for home-based versus hospital-based cardiac rehabilitation. J. Cardiopulm. Rehabil. 25(1), 24–29 (2005).
Komasi, S. & Saeidi, M. Hybrid cardiac rehabilitation as an alternative to common hospital-based cardiac rehabilitation in Iran: An appropriate model for the Iranian health system limitations, culture, and patients. Res. Cardiovasc. Med. 6, e39367 (2017).
Korzeniowska-Kubacka, I., Dobraszkiewicz-Wasilewska, B., Bilińska, M., Rydzewska, E. & Piotrowicz, R. Two models of early cardiac rehabilitation in male patients after myocardial infarction with preserved left ventricular function: comparison of standard out-patient versus hybrid training programmes. Kardiol Pol. 69, 220–226 (2011).
Korzeniowska-Kubacka, I., Bilińska, M., Dobraszkiewicz-Wasilewska, B. & Piotrowicz, R. Hybrid model of cardiac rehabilitation in men and women after myocardial infarction. Cardiol. J. 22, 212–218 (2015).
Najafi, F. & Nalini, M. Hospital-based versus hybrid cardiac rehabilitation program in coronary bypass surgery patients in western Iran: Effects on exercise capacity, risk factors, psychological factors, and quality of life. J. Cardiopulm. Rehabil. Prev. 35, 29–36 (2015).
Burdese, E. et al. Usefulness of a telemedicine program in refractory older congestive heart failure patients. Diseases. 6(1), 10 (2018).
Heindl, B., Ramirez, L., Joseph, L., Clarkson, S., Thomas, R. & Bittner, V. Hybrid cardiac rehabilitation—The state of the science and the way forward. Prog. Cardiovasc. Dis. 70, 175–182 (2022).
MacInnis, M. J. & Gibala, M. J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 595(9), 2915–2930. https://doi.org/10.1113/JP273196 (2017).
Chen, Y. W., Wang, C. Y., Lai, Y. H., et al. Home-based cardiac rehabilitation improves quality of life, aerobic capacity, and readImission rates in patients with chronic heart failure. Medicine 97(4), e9629 (2018).
Author information
Authors and Affiliations
Contributions
HC and ZLM: conception and design. HC and HWR: acquisition and data.HC and CZ: analysis and interpretation of data. HC and CDM: drafting of manuscript. HC, JJ, CXM, and ZLM: critical manuscript revision for important intellectual content. HC, HWR, CZ and YJ: statistical analysis. ZLM: administrative, technical, or material support. HC and ZLM: supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hua, C., Huang, W., Chen, Z. et al. Effects of exercise based cardiac rehabilitation delivery modes on chronic heart failure: a systematic review and network meta-analysis. Sci Rep 14, 31246 (2024). https://doi.org/10.1038/s41598-024-82608-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82608-z
Keywords
This article is cited by
-
Serum CA125: a prognostic biomarker for mortality in chronic heart failure
BMC Cardiovascular Disorders (2025)
-
The impact of early rehabilitation program on exercise tolerance in post-myocardial infarction patients: a 5-week intervention study
BMC Cardiovascular Disorders (2025)