Abstract
Hemorrhagic shock is a significant cause of trauma-related mortality. Resuscitative endovascular balloon occlusion of the aorta (REBOA) is a less-invasive aortic occlusion maneuver for severe hemorrhagic shock but potentially inducing oxidative stress injuries. In an animal model, this study investigated hydrogen gas inhalation therapy’s potential to mitigate post-REBOA ischemia-reperfusion injuries (IRIs). Ten healthy female swine underwent REBOA placement after induced 40% hemorrhagic shock. They were observed during the IRI phase after a 60-minute Zone 1 occlusion for 180 minutes until euthanasia. 2% hydrogen gas inhalation was started simultaneously with REBOA inflation in the hydrogen group. We evaluated survival time, lactate, and 8-hydroxy-2’-deoxyguanosine (8-OHdG) biomarkers, gross findings, and pathological grades. One swine in the control group died at 90 min, and the remaining animals survived throughout the experiment. Survival analysis showed no significant differences between the two groups (control vs. hydrogen, 4/5 vs. 5/5; Log-rank, P = 0.317). Lactate levels during and after REBOA suggested a tendency towards lower levels in the hydrogen group (10.5 ± 4.2 vs. 7.6 ± 2.3 mmol/L, peak, T = 90). Serum 8-OHdG concentrations showed a lower trend in the hydrogen group (Range: 0.12–0.32 vs. 0.11–0.19 ng/mL). The villi of the ileum were destroyed during REBOA inflation and after reperfusion. Changes in the pathological grade of the ileum demonstrated no significant differences in both groups (2.8 ± 1.0 vs. 2.0 ± 1.0, proximal ileum, T = 240). Hydrogen gas inhalation therapy exhibited no significant difference compared to the control group in survival, lactate level, 8-OHdG, and intestinal mucosal injury following REBOA in a hemorrhagic shock model. Although it may slightly reduce mortality, biomarkers, and intestinal pathology, hydrogen gas inhalation therapy was not shown to have sufficient evidence to mitigate REBOA-IRI.
Similar content being viewed by others
Introduction
Traumatic deaths are an unexpected cause of death among young people, resulting in significant social losses. Among these, hemorrhagic shock is the most common preventable cause of trauma death1. Resuscitative endovascular balloon occlusion of the aorta (REBOA), in which a balloon catheter is inserted into the aorta to control bleeding and maintain central organ perfusion, has attracted attention as a resuscitation method for severe hemorrhagic shock2. REBOA can cause oxidative stress due to ischemia-reperfusion injury (IRI), leading to multiple organ failure and death3, so countermeasures are needed. To avoid trauma mortality, acute resuscitation, hemostatic treatment, and intensive care to reduce subsequent oxidative stress are necessary.
The intensity of aortic occlusion depends on the site of balloon occlusion (Zone 1 or Zone 3)2,4. Therefore, the organ or site that elicits an IRI in Zone 1 and Zone 3 is different. In Zone 3 REBOA, IRI occurs only in the lower extremities because abdominal organ blood flow is preserved. In Zone 1 REBOA, IRI also occurs in abdominal organs, particularly susceptible to the intestinal tract and liver5. It has been reported that partial REBOA reduces IRI in zone 1 REBOA6,7,8,9.
Abdominal organ damage due to REBOA-IRI can be fatal from circulatory failure with irreversible lactic acidosis. Therapeutic hypothermia for systemic IRI in post-cardiac arrest syndrome contributes to reducing oxidative stress. However, it is less feasible in patients with hemorrhagic shock who require REBOA because it induces hypothermia, one of the lethal triad of trauma which is comprised of the combination of hypothermia, acidosis, and coagulopathy. Novel therapies for oxidative stress in post-REBOA IRI are needed.
The efficacy and safety of hydrogen gas inhalation therapy have been reported as a treatment to reduce oxidative stress. Some animal studies have shown that hydrogen has anti-inflammatory effects and reduces the reactive oxygen species produced during ischemia-reperfusion injury. Hydrogen inhalation was associated with the reduction of necrotic tissues, post-cardiac arrest syndrome10,11, acute myocardial infarction12,13, contrast nephropathy14, hemorrhagic shock15, and cerebral infarction16. Recently, the efficacy and safety of hydrogen therapy in post-cardiac arrest syndrome (PCAS) in the clinical investigation were also reported17. The common point between PCAS and post-REBOA IRI is that extensive IRI occurred after the return of circulation. It is expected that hydrogen inhalation therapy could be applied to post-REBOA IRI.
This study aims to establish hydrogen gas inhalation therapy as a novel adjunct treatment for reducing oxidative stress caused by post-REBOA IRIs and to investigate whether the occlusion duration could be extended beyond the standard time (30–45 min). We evaluated survival time, biomarkers, gross and pathological findings of intestinal ischemia, and IRIs induced by REBOA. The efficacy and safety of hydrogen inhalation therapy in reducing intestinal ischemia was investigated.
Materials and methods
Overview
This study was conducted in an accredited animal research laboratory. Approval was obtained from the Animal Experiment Committee at the research institute before conducting the research. All methods were performed according to the relevant guidelines and regulations. Healthy domestic pigs (n = 10) were obtained from Sanesu Breeding Co., Ltd. (Chiba, Japan). We utilized the experimental model from our previous research that created hemorrhagic shock models to minimize technical error and the effects of subjective bias18. The animals were quarantined for a minimum of 7 days and fasted for 24 h with access to water before enrolment in the experimental protocol. At the time of experimentation, the animals were 3–4 months of age and weighed 30–40 kg.
Animal preparation
The swine were premedicated intramuscularly with 0.06 mg/kg medetomidine (Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan), 0.3 mg/kg midazolam (Astellas Pharma Inc., Tokyo, Japan), and 0.08 mg/kg atropine (Mitsubishi Tanabe Pharma Corporation, Osaka, Japan) in the animal cage in the early morning. After confirming sedation and endotracheal intubation in the animal operating room, maintenance anesthesia consisting of 1 to 3% sevoflurane was applied. The animals were mechanically ventilated with tidal volumes of 7–10 mL/kg and a respiratory rate of 10–15 breaths/min, sufficient to maintain the end-tidal CO2 at 40 ± 5 mmHg. FIO2 was titrated according to oxygenation during the experimental procedure. The oxygenation target was SpO2 at 95 to 99%. The swine were placed on a warming blanket set at 39 °C to maintain body temperature.
Surgical procedures and REBOA placement
After induction of general anesthesia, the right neck was exposed, and an arterial line was catheterized for proximal pressure monitoring and blood sampling into the right carotid artery. A central venous catheter was then inserted in the right jugular vein. A 10-Fr sheath was placed into the right femoral artery to insert a 7-Fr REBOA catheter (Rescue Balloon®; Tokai Medical Products, Aichi, Japan). The side arm of the 10-Fr sheath was used for distal pressure monitoring. The point at which the distal pulse pressure disappeared according to inflation was defined as the complete occlusion of the REBOA19. Acetated Ringer’s solution was infused, and a bolus injection was added when the blood pressure dropped. A REBOA catheter was placed in the thoracic aorta to maintain the balloon position in Zone 1. The REBOA catheter was fixed, and the balloon was gradually inflated with close distal pressure monitoring. We performed laparotomy to observe the gross findings of the intestine and prepared to obtain intestinal samples during the observation phase.
Induction of hemorrhagic shock
Using a 10-Fr arterial sheath in the right femoral artery, all animals were hemorrhaged a total of 30 mL/kg (approximately 40% blood loss) for 20 min. Based on the previous study, the first half of the volume was removed at 2.15 mL/kg/min for 7 min, and the remainder was removed at 1.15 mL/kg/min for 13 min20.
Hydrogen gas inhalation
A cylinder of mixed gas containing 4% hydrogen and 96% nitrogen was connected to the inspiratory side of anesthesia. Based on the previous reports, 1 to 2% of hydrogen gas was a safe and effective treatment range10,11,17, thus we employed 2% for the technical feasibility. After induction of general anesthesia, the hydrogen concentration was set to approximately 2% according to the tidal volume. In other words, the hydrogen/nitrogen gas mixture was adjusted as half of the tidal volume as volume control ventilation.
Time course of the experiment
Hemorrhagic shock was introduced 30 min before REBOA started (T = -30 min). Hydrogen gas inhalation was started at the same time as the onset of aortic occlusion in the hydrogen group, while conventional ventilation was continued in the control group. The aorta was fully occluded with REBOA from T = 0 to T = 60. The balloon was deflated at T = 60, and the animals were observed until euthanasia at T = 240. The animals were euthanized with the maximum dose of sevoflurane and intravenous injection of 1 mg/kg of potassium chloride. During the observation phase, the swine received a bolus infusion of fluids and noradrenaline with a maximum dose of 0.2 µg/kg/min to maintain hemodynamics (Fig. 1). The survival time was recorded if the experimental animal died of circulatory failure before sacrifice.
Time course of the experiment. Hemorrhagic shock was introduced 30 min before REBOA started (T = -30 min). Hydrogen gas inhalation was started at the same time as REBOA inflation (T = 0) in the Hydrogen group. The balloon was deflated at T = 60, and the animals were observed until T = 240. The blood gas analysis and intestinal tissue sampling were conducted every 60 min. Blood lactate was measured every 30 min.
Measurement and intestinal tissue sampling
Lactate measurements and gross observation of the intestine were recorded before phlebotomy (T=-30) and every 30 minutes from the start of phlebotomy (T = 0). Blood 8-hydroxy-2’-deoxyguanosine (8-OHdG), an oxidative stress marker, was measured at T = 240 or immediately before death (Japan Institute for the Control of Aging, Nikken SEIL Co). Intestinal tissue sampling was performed at T = 0, 60, 120, 180, and 240 min from three sites in the small intestine. Approximately 20-mm specimens were resected from the proximal ileum [200 cm to cecum], mid ileum [100 cm to cecum], and distal ileum [10 cm to ileum], and the adjacent oral intestinal portion was collected at every sampling time. The specimens’ mucosa were grossly observed, and pathological findings were evaluated after formalin fixation.
Grade of pathophysiological changes in the intestinal tissues
Based on previous reports, changes in intestinal histopathology were classified from grade 0 to grade 5 (Supplement Table 1)21. The length of villi extension was defined as the average of the lengths at three locations in the visual field, and the degree of destruction was calculated based on the length before the start of aortic occlusion (T = 0). Three researchers (YM, YI, and YH) selected a field of view in which the villi were captured on the long axis to measure their length and then determined the grade.
Statistical analysis
Biomarker and pathological grade and survival time were analyzed and plotted using GraphPad Prism 6.07 for Windows (GraphPad Software Inc., La Jolla, CA, USA). Survival time was analyzed using Kaplan Meier’s analysis.
Results
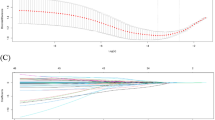
The experiment was conducted with five animals in each control and hydrogen group. The average body weight was 37.2 kg in the control group and 37.7 kg in the hydrogen group. Baseline blood gas analysis results were similar for both groups (Table 1). One swine in the control group died at 90 min, and the rest survived throughout the experiment. Kaplan-Meier survival analysis showed no significant difference in survival time (P = 0.317, Log-rank test) (Fig. 2). No adverse events related to the hydrogen gas inhalation were observed in the experimental period.
Before and after phlebotomy (T=-30 to T = 0), the mean (standard deviation) hemoglobin levels in the control and hydrogen groups decreased from 11.0 (1.5) to 8.7 (1.4) g/dL and from 12.0 (0.9) to 9.8 (1.0) g/dL, respectively (Supplement 1). The lactate levels increased from 1.2 (0.2) to 2.3 (1.2) mmol/L and from 1.1 (0.3) to 1.6 (0.6) mmol/L [T=-30 to T = 0], and then reached 8.4 (2.9) mmol/L and 6.3 (2.4) mmol/L at T = 60, respectively. Lactate peaked at 10.5 (4.2) mmol/L and 7.6 (2.3) mmol/L at T = 90 and then decreased gradually. Lactate levels tended to be lower in the hydrogen group at any point during occlusion and after deflation, although the changes over time were not statistically significant (Fig. 3). Potassium levels were 4.4 (0.3) mEq/L and 4.2 (0.1) mEq/L at T = 0, then increased in both groups to 6.2 (0.7) mEq/L and 5.9 (0.4) mEq/L [T = 60], 6.6 (1.9) mEq/L and 6.4 (1.3) mEq/L [T = 240] (Supplement 2). Calcium concentrations showed no marked changes in both groups (Supplement 3). Serum 8-OHdG concentrations ranged from 0.12 to 0.32 ng/mL in the control group and 0.11–0.19 ng/mL in the hydrogen group (Fig. 4).
The gross abdomen findings became pale blue to white during occlusion and recovered after deflation (Supplement 4). The villi of the ileum were destroyed during REBOA inflation and after reperfusion (Supplement 5). Changes in the pathological grade of the ileum tended to be lower in the hydrogen group from T = 120 to T = 240. This trend was evident in the proximal ileum but without significant differences (Fig. 5, Supplement Figs. 6, 7).
Discussion
An experimental animal model of complete aortic occlusion by REBOA for 60 min after the hemorrhagic shock was created and subsequent IRI was observed. Using this model, survival time, biomarkers such as lactate and 8-OHdG, and changes in the pathological grade of the ileum were compared in the two groups with and without hydrogen inhalation. Survival time and survival rate were better in the hydrogen group without significance (control vs. hydrogen, 4/5 vs. 5/5; Log-rank, P = 0.317). Although a slightly better trend was observed in blood lactate, 8-OHdG, and pathological changes, any parameters exhibited significant differences in both groups. There were no adverse events attributed to hydrogen inhalation.
It has been reported that subdiaphragmatic ischemia and IRI caused by REBOA induce systemic inflammation, organ damage, and lactic acidosis22,23,24. Adenosine, lidocaine, and magnesium (ALM) were reported as possible treatments to attenuate REBOA-IRI. ALM administration decreased plasma cytokine levels (IL-2, IL-4, and IL-10) and liver gene expression of IL1RN, MTOR, and LAMP3 but did not improve lactate level in a porcine IRI model induced after 45 min REBOA in the 20% hemorrhagic shock25. In a porcine model of cardiac arrest, a minor deterioration of lactate was observed in the hydrogen gas inhalation animals26. In this swine IRI model induced after 60-min aortic occlusion in 40% hemorrhagic shock, we observed a trend toward a less severe increase of serum lactate levels in the hydrogen group. Hydrogen gas inhalation may alleviate hyperlactatemia associated with extensive organ ischemia and IRI caused by Zone 1 REBOA.
Hydrogen gas inhalation therapy mitigated serum 8-OHdG concentration in post-cardiac arrest resuscitation patients27 and intestinal ischemia-reperfusion injury model in rats28. The hydrogen group observed a longer survival time in the rats’ post-cardiac arrest resuscitation model10. The present study observed a trend toward lower 8-OHdG concentrations and longer survival time in the hydrogen gas inhalation group without any significant differences. Unlike other oxidative stress pathologies, hydrogen gas inhalation therapy may not work sufficiently to alleviate systemic oxidative stress in REBOA-IRI.
Mild epithelial denudation was observed in the hydrogen group in a model of IRI of the rat intestinal membrane caused by SMA occlusion28. In the present study, pathological changes were slightly milder in the hydrogen group, especially in the proximal ileum. However, these changes did not demonstrate significant differences in any part. Thus, hydrogen gas inhalation therapy may not work to alleviate local tissue damage caused by REBOA-IRI. No rational explanation has been made as to why the effects of hydrogen inhalation and the degree of mucosal injury vary by site in the small intestine.
There are several limitations to this study. First, in previous studies of the REBOA experimental model in swine, there are reports of survival after 90 min of occlusion with resuscitation29. It is possible that swine are more tolerant to ischemia than humans and that the duration of occlusion and the intensity of the subsequent IRI differ from humans. Due to interspecies differences, a potential lack of correlation of results between human and swine models may exist. Second, the timing of hemorrhage and deployment of the REBOA may be shorter than actual trauma, physiologic insult may be more significant. Third, none of the changes in biomarkers, survival curves, and pathological changes were statistically significant. The trend may also be affected by individual differences and limited sample size. Hydrogen inhalation may not work efficiently to mitigate post-REBOA IRI. Despite these limitations, future research in hydrogen gas inhalation is warranted to accumulate scientific evidence in REBOA-IRI for safety and feasibility.
Conclusions
Hydrogen gas inhalation therapy exhibited no significant difference in survival, lactate level, 8-OHdG, and intestinal mucosal injury following REBOA in a hemorrhagic shock model. Although it may slightly reduce mortality, biomarkers, and intestinal pathology, hydrogen gas inhalation therapy may not work sufficiently to alleviate post-REBOA IRIs.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Tien, H. C., Spencer, F., Tremblay, L. N., Rizoli, S. B. & Brenneman, F. D. Preventable deaths from hemorrhage at a level I Canadian trauma center. J. Trauma 62, 142–146. https://doi.org/10.1097/01.ta.0000251558.38388.47 (2007).
Stannard, A., Eliason, J. L. & Rasmussen, T. E. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J. Trauma 71, 1869–1872. https://doi.org/10.1097/TA.0b013e31823fe90c (2011).
Davidson, A. J. et al. The pitfalls of resuscitative endovascular balloon occlusion of the aorta: Risk factors and mitigation strategies. J. Trauma Acute Care Surg. 84, 192–202. https://doi.org/10.1097/TA.0000000000001711 (2018).
Brenner, M. et al. Basic endovascular skills for trauma course: Bridging the gap between endovascular techniques and the acute care surgeon. J. Trauma Acute Care Surg. 77, 286–291. https://doi.org/10.1097/TA.0000000000000310 (2014).
Teeter, W. et al. Monitoring organs susceptible to Ischemia/Reperfusion Injury after prolonged resuscitative endovascular balloon occlusion of the Aorta in a hemorrhagic shock swine model. Panamerican J. Trauma Crit. Care Emerg. Surg. 9, 169–180 (2020).
Russo, R. M. et al. Extending the golden hour: Partial resuscitative endovascular balloon occlusion of the aorta in a highly lethal swine liver injury model. J. Trauma Acute Care Surg. 80, 372–378. https://doi.org/10.1097/TA.0000000000000940 (2016). discussion 378–380.
Russo, R. M. et al. Partial resuscitative endovascular balloon occlusion of the Aorta in Swine Model of hemorrhagic shock. J. Am. Coll. Surg. 223, 359–368. https://doi.org/10.1016/j.jamcollsurg.2016.04.037 (2016).
Williams, T. K. et al. Endovascular variable aortic control (EVAC) versus resuscitative endovascular balloon occlusion of the aorta (REBOA) in a swine model of hemorrhage and ischemia reperfusion injury. J. Trauma Acute Care Surg. 85, 519–526. https://doi.org/10.1097/TA.0000000000002008 (2018).
Forte, D. M. et al. Validation of a novel partial resuscitative endovascular balloon occlusion of the aorta device in a swine hemorrhagic shock model: fine tuning flow to optimize bleeding control and reperfusion injury. J. Trauma Acute Care Surg. 89, 58–67. https://doi.org/10.1097/TA.0000000000002718 (2020).
Hayashida, K. et al. Hydrogen inhalation during normoxic resuscitation improves neurological outcome in a rat model of cardiac arrest independently of targeted temperature management. Circulation 130, 2173–2180. https://doi.org/10.1161/CIRCULATIONAHA.114.011848 (2014).
Chen, G. et al. Hydrogen inhalation is Superior to mild hypothermia for improving neurological outcome and survival in a Cardiac arrest model of spontaneously hypertensive rat. Shock 50, 689–695. https://doi.org/10.1097/shk.0000000000001092 (2018).
Katsumata, Y. et al. The effects of Hydrogen Gas Inhalation on adverse left ventricular remodeling after percutaneous coronary intervention for ST-Elevated myocardial infarction- first pilot study in humans. Circ. J. 81, 940–947. https://doi.org/10.1253/circj.CJ-17-0105 (2017).
Nie, C. et al. Hydrogen gas inhalation ameliorates cardiac remodelling and fibrosis by regulating NLRP3 inflammasome in myocardial infarction rats. J. Cell. Mol. Med. 25, 8997–9010. https://doi.org/10.1111/jcmm.16863 (2021).
Homma, K. et al. Inhalation of Hydrogen Gas is Beneficial for preventing contrast-Induced Acute kidney Injury in rats. Nephron Exp. Nephrol. https://doi.org/10.1159/000369068 (2015).
Tamura, T. et al. Hydrogen Gas Inhalation attenuates endothelial glycocalyx damage and stabilizes Hemodynamics in a rat hemorrhagic shock model. Shock 54, 377–385. https://doi.org/10.1097/SHK.0000000000001459 (2020).
Ono, H. et al. Hydrogen gas inhalation treatment in acute cerebral infarction: a randomized controlled clinical study on safety and neuroprotection. J. Stroke Cerebrovasc. Dis. 26, 2587–2594. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.06.012 (2017).
Tamura, T., Suzuki, M., Homma, K. & Sano, M. Efficacy of inhaled hydrogen on neurological outcome following brain ischaemia during post-cardiac arrest care (HYBRID II): a multi-centre, randomised, double-blind, placebo-controlled trial. EClinicalMedicine 58, 101907. https://doi.org/10.1016/j.eclinm.2023.101907 (2023).
Matsumura, Y., Higashi, A., Izawa, Y. & Hishikawa, S. Organ perfusion during partial REBOA in haemorrhagic shock: dynamic 4D-CT analyses in swine. Sci. Rep. 12, 18745. https://doi.org/10.1038/s41598-022-23524-y (2022).
Matsumura, Y. et al. Distal pressure monitoring and titration with percent balloon volume: feasible management of partial resuscitative endovascular balloon occlusion of the aorta (P-REBOA). Eur. J. Trauma Emerg. Surgery: Off. Publ. Eur. Trauma Soc. 47, 1023–1029. https://doi.org/10.1007/s00068-019-01257-4 (2021).
Frankel, D. A. et al. Physiologic response to hemorrhagic shock depends on rate and means of hemorrhage. J. Surg. Res. 143, 276–280. https://doi.org/10.1016/j.jss.2007.01.031 (2007).
Gomes, O. M. et al. Ischemia-reperfusion histopathology alterations of the rabbit intestinal wall with and without ischemic preconditioning. Acta Cir. Bras. 26, 285–288. https://doi.org/10.1590/s0102-86502011000400007 (2011).
Kuckelman, J. et al. Efficacy of intermittent versus standard resuscitative endovascular balloon occlusion of the aorta in a lethal solid organ injury model. J. Trauma Acute Care Surg. 87, 9–17. https://doi.org/10.1097/TA.0000000000002307 (2019).
Kauvar, D. S. et al. Effect of partial and complete aortic balloon occlusion on survival and shock in a swine model of uncontrolled splenic hemorrhage with delayed resuscitation. J. Trauma Acute Care Surg. 87, 1026–1034. https://doi.org/10.1097/TA.0000000000002439 (2019).
Sadeghi, M. et al. Total resuscitative endovascular balloon occlusion of the aorta causes inflammatory activation and organ damage within 30 minutes of occlusion in normovolemic pigs. BMC Surg. 20 https://doi.org/10.1186/s12893-020-00700-3 (2020).
Franko, J. J. et al. Adenosine, lidocaine, and magnesium for attenuating ischemia reperfusion injury from resuscitative endovascular balloon occlusion of the aorta in a porcine model. J. Trauma Acute Care Surg. 92, 631–639. https://doi.org/10.1097/TA.0000000000003482 (2022).
Astapenko, D. et al. Protection of the endothelium and endothelial glycocalyx by hydrogen against ischaemia-reperfusion injury in a porcine model of cardiac arrest. Clin. Hemorheol Microcirc. https://doi.org/10.3233/ch-231768 (2023).
Tamura, T. et al. Hydrogen gas inhalation alleviates oxidative stress in patients with post-cardiac arrest syndrome. J. Clin. Biochem. Nutr. Advpub. https://doi.org/10.3164/jcbn.19-101 (2020).
Yamamoto, R. et al. Hydrogen gas and preservation of intestinal stem cells in mesenteric ischemia and reperfusion. World J. Gastrointest. Surg. 14, 1329–1339. https://doi.org/10.4240/wjgs.v14.i12.1329 (2022).
Morrison, J. J. et al. The inflammatory sequelae of aortic balloon occlusion in hemorrhagic shock. J. Surg. Res. https://doi.org/10.1016/j.jss.2014.04.012 (2014).
Acknowledgements
We thank all Center for Development of Advanced Medical Technology members, Jichi Medical University.
Author information
Authors and Affiliations
Contributions
YM, the corresponding author, was responsible for drafting, editing, and submitting the manuscript. YM, YH, MA, and YI conducted the animal experiments and collected data. YI critically appraised this manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Grant-in-Aid for Scientific Research (B), Japan Society for the Promotion of Science (JSPS), Grant Number 21H03029.
Ethical approval
This study was conducted in an accredited animal research laboratory (Center for Development of Advanced Medical Technology, Jichi Medical University, Tochigi, Japan). Approval was obtained before conducting the research from the Animal Experiment Committee of the Center for Experimental Medicine, Jichi Medical University (authorization no. 17045-05; October 29, 2020). This study is reported following ARRIVE guidelines (https://arriveguidelines.org).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Matsumura, Y., Hayashi, Y., Aoki, M. et al. Hydrogen gas inhalation therapy may not work sufficiently to mitigate oxidative stress induced with REBOA. Sci Rep 14, 32128 (2024). https://doi.org/10.1038/s41598-024-83934-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83934-y