Abstract
Hydroxyapatite (HA) is an engineered biomaterial that closely resembles the hard tissue composition of humans. Biological HA is commonly non-stoichiometric and features lower crystallinity and higher solubility than stoichiometric HA. The chemical compositions of these biomaterials include calcium (Ca), phosphorus (P), and trace amounts of various ions such as magnesium (Mg2+), zinc (Zn2+), and strontium (Sr2+). Significantly, these ions are essential for the metabolic processes of hard tissues. This study involved the application of Co2+-doped HA coatings at different concentrations (5%, 10%, and 20% by weight) onto Ti-6Al-4V, utilizing the spin-coating method. The FTIR, XRD, FESEM, EDS, and AFM techniques were utilized to analyze the coated substrates. Tetraethyl orthosilicate (TEOS (T)) was employed as a binding agent to enhance adhesion and reduce surface cracks in the coating. The adhesion strength of coatings applied to Ti-6Al-4V was assessed for use in biomedical applications. Polarization and electrochemical impedance spectroscopy (EIS) studies in a simulated body fluid (SBF) solution were conducted to evaluate the corrosion behavior of the coatings. The corrosion behavior of the coated samples increased significantly compared to the substrate. The 10Co/HA/T coating demonstrated the highest charge transfer resistance (Rct) value of 13.40 MΩ × cm2, whereas the uncoated substrate exhibited the lowest Rct of 0.14 MΩ × cm2. A cell viability assay was conducted utilizing MG-63 cells for the Ti-6Al-4V and coatings, which prepared coatings demonstrated outstanding biocompatibility. Based on this study, the 10Co/HA/T coating was identified as the most promising sample. These findings suggest that surface modification of Ti-6Al-4V through Co2+-doped HA coatings offers a viable strategy for enhancing its performance in biomedical applications.
Similar content being viewed by others
Introduction
Annually, millions of patients worldwide undergo hospitalization for bone grafting procedures. Bone grafting involves a surgical process where damaged bone is substituted with synthetic or biological grafts1. Therefore, significant efforts have been made to synthesize novel biocompatible materials with improved biological properties for bone grafting2. Titanium and its alloys have long been extensively utilized in the fields of medicine for implant purposes3. These metallic biomaterials exhibit excellent biocompatibility and possess strong mechanical characteristics, such as fatigue strength, tensile strength, and hardness, which establish them as the favored option for implants4,5. Nevertheless, titanium (Ti) alloys frequently exhibit infection in the implant region or poor bone formation abilities following direct implantation into the human body6. To mitigate the risk of corrosion products released from Ti alloys into neighboring tissues, a protective biocompatible coating can be applied7,8. Calcium phosphate (CaP) coatings can enhance the biological characteristics of Ti implants through several techniques, including vapor deposition, sol–gel methods, sputtering, and electrophoretic deposition9,10,11,12. The Sol–Gel coatings are widely chosen for the surface improvement of various metal substrates because of their low annealing temperatures and economic advantages13,14,15. The key factors contributing to the widespread application of the sol–gel method are its relative simplicity compared to other coating processes and its ability to significantly enhance the coating layers of biomaterials on substrates16,17,18. Overall, the sol–gel method’s versatility and ecological advantages make it a highly promising substitute for conventional coating techniques.
Bioceramics are ceramic materials that are biocompatible and utilized for the replacement or repair of damaged tissues19. They possess unique characteristics of being both bioactive and biodegradable, which are deliberately engineered to elicit cellular reactions19. The bioactivity of ceramic materials is a crucial factor that facilitates a bond between the material and living tissues, thereby preventing fibrous encapsulation20. Various materials, such as CaP, hydroxyapatite (HA), and bioglass (BG), have demonstrated bioactive properties21,22,23. The CaP family is the most common type of bioceramic and is highly regarded for its excellent biocompatibility properties, making it a preferred choice in biomedical applications24. When biocompatible materials enter the human body, they should not harm the adjacent tissues25. Hydroxyapatite (HA: Ca10(PO4)6 (OH)2) is one of the extensively studied CaP bioceramics for biomedical applications26,27,28. HA is a vital bone mineral known for its remarkable biocompatibility, ability to enhance bone-to-implant bonding, osteoconductive properties, and excellent stability in the human body29,30,31,32. Furthermore, HA exhibits comparable biological, structural, and chemical, characteristics to human bone12. Characterization of the hydroxyapatite, including dissolution rate, solubility, and crystallization, can be modified through the substitution of cationic and anionic elements33,34. These modifications enhance its bonding capabilities with bone, impart antibacterial properties, and promote osteointegration33.
Cobalt (Co) is commonly found in nature in both organic and inorganic forms and acts as an essential element for the correct functioning inside the human body35. The incorporation of cobalt ions into various ceramic materials has a wide range of applications across multiple fields, including biomedicine36, catalytic properties37, optical and magnetic materials38, environmental applications39, and supercapacitors28. Oxide films doped with cobalt on titanium surfaces not only enhanced corrosion resistance but also improved biocompatibility, thereby facilitating cell proliferation40. Kermani et al.41 have shown that cobalt plays a significant role in promoting angiogenesis and is a valuable element for bone tissue regeneration. Currently, several investigations have been reported regarding the integration of cobalt as a biological enhancer in bioactive glasses. The majority of these investigations have focused on the regeneration of bone tissue42,43. Krame et al. successfully synthesized Co2+-doped HA through a straightforward ion exchange44. Numerous investigations have shown that the release of cobalt stimulates the growth of new blood vessels (angiogenesis) and enhances the process of bone regeneration, both in laboratory settings (in vitro) and within living organisms (in vivo)45,46.
In this experimental study, we have produced coatings on Ti-6Al-4V by different concentrations of cobalt in the HA structure. The aim is to improve the biological properties of implants for biomedical applications. Co2+-doped HA coatings were fabricated on Ti-6Al-4V through spin-coating method at 2500 rpm to analyze the surface modification. The corrosion behavior and biocompatibility of samples were investigated along with the evaluation of their microstructures. This research uniquely explores cobalt-doped hydroxyapatite coatings on Ti-6Al-4V, a widely used titanium alloy in biomedical implants. While cobalt is recognized for enhancing angiogenesis and bone tissue regeneration, its application in HA coatings for biomedical use is not well studied. This study systematically assesses the impact of various cobalt concentrations on coating properties, including corrosion resistance, biocompatibility, adhesion strength, and surface morphology. It identifies the 10Co/HA/T coating as the most promising regarding charge transfer resistance, biocompatibility, and overall performance.
Experimental procedure
Chemicals and reagents
All materials were purchased from Merck: Cobalt (II) chloride hexahydrate (CoCl₂0.6 H₂O, 99%), di-phosphorus pentoxide (P2O5, 98%), calcium nitrate tetrahydrate (Ca(NO3)2.4H2O, 99%), tetraethyl orthosilicate (TEOS, 99%), hydrofluoric acid (HF, 48%), acetone (CH₃COCH₃, 99%), ethanol (C₂H₅OH, 99.9%), and sodium hydroxide (NaOH, 97%). SBF47 was used as an electrolyte for electrochemical tests. This study used analytical-grade solvents and chemicals in their original form, without additional purification.
Synthesis of pure HA, HA/T and Co/HA/T
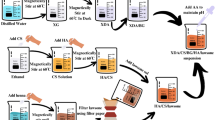
The synthesis of pure HA, HA/T, and Co/HA/T nanoparticles was conducted through Sol–Gel technique, based on our previous research but with minor modifications27,28. Viscous sols were prepared for pure HA, HA/T, and Co/HA/T with cobalt concentrations of 5%, 10%, and 20% by weight for coating production. Specifically, to synthesize 5Co/HA/T, a solution comprising 0.479 M Ca(NO3)2.4H2O and 0.025 M CoCl₂0.6 H₂O was stirred in ethanol for 2 h. Simultaneously, a separate solution containing 0.3 M P2O5 was stirred in ethanol for the same duration. The synthesized nanoparticles’ molar ratios of (Ca + Co)/P are set to 1.67. Following this, the P2O5 solution was carefully added dropwise into the calcium-cobalt solution and stirred vigorously for 2 h. Then, after adding a small quantity of tetraethyl orthosilicate (T) as a binder, the resulting solution was agitated for an additional 2 h. The solution underwent reflux for 18 h at a temperature of 80°C following a 24-h aging period at room temperature. The resulting solution was then kept to an 80°C water bath to produce a viscous sol for coatings. The synthesis process for pure HA, HA/T, 10Co/HA/T, and 20Co/HA/T was identical, as amounts of precursors summarized in Table 1. Figure 1 illustrates the schematic representation of the preparation process for Co2+-doped HA/T nanoparticles.
Substrates preparation
The chemical combination of Ti-6Al-4V is presented in Table 2. The Ti-6Al-4V sheets were cut into specimens measuring 10 mm × 10 mm × 3 mm and subsequently underwent polishing with sandpaper varying in grit sizes from 300 to 2500. Following this, the metal sheets underwent a cleaning process involving rinsing in deionized water and ultrasonic treatment in acetone for 15 min. The surface functionalization of substrate was enhanced by utilizing an acid etching technique to eliminate oxide layers and impurities from the substrate surface. The procedure involved an alkali treatment using 8M NaOH at 60°C for 1 h, followed by a 20-min treatment with 2M HF at room temperature. After carefully rinsing every substrate with ethanol and double-distilled water, each substrate was dried in an oven.
Spin coating on Ti-6Al-4V
The spin-coating technique is superior for producing coatings, as it enables the creation of homogeneous and uniform thin layers while allowing for precise control over their thickness. In contrast to plasma, dip-coating, and spraying techniques, spin-coating employs centripetal force to evenly distribute sols on the surface of the substrate, achieving layer thickness from a few nanometers to several microns with high reproducibility and uniformity48. This approach allows for the modification of parameters such as fluid viscosity and spin speed, directly affecting the characteristics of the final layer49. The prepared sols were applied onto the substrate utilizing a spin coating device at room temperature. The Ti-6Al-4V was coated with a thin film of sol through a centrifugal force at a speed of 2500 rpm for 20 s. Subsequently, the coatings were sintered at 450°C for 3 h. The heat treatment administered to the coated substrates plays a significant influence on the qualities of the coatings. The adhesion between the Ti-6Al-4V and coating is enhanced when the hydroxyapatite coatings are sintered within temperatures range of 375°C to 500°C50,51. The main benefit of the sol–gel technique lies in its ability to achieve low sintering temperatures, which allows the coating to crystallize at reduced temperatures while preventing the formation of surface cracks. Figure 2 illustrates the sequential stages in the spin-coating procedure. Each step is clearly outlined to provide a visual representation of the process.
Physical characterization
The produced samples were examined using Fourier transform infrared (FTIR; Bruker TENSOR 27, Germany). The X-ray diffraction (XRD; Siemens, D500-Germany) analysis with Cu-Kα radiation of 1.5406Å, was used to determine the phase purity, and crystallinity. The following is the Debye–Scherrer equation52 that can be used to calculate the average crystallite size of the synthesized samples:
where θ is the Bragg angle in degrees, β is their entire width at half maximum in radians, and λ is the X-ray wavelength. Additionally, the unit cell volume (V) and hexagonal lattice parameters of the produced HA were computed using the following formulas32:
where a and c are the lattice constants and h, k, and l are the Miller indexes.
The surface morphology and shape of the nanoparticles were evaluated with field emission scanning electron microscopy (FESEM; Can Scan-Tescan MIRA3 FEG, check), cross-section images were utilized to estimate the thickness of coatings, and elemental analysis was examined using EDS. The surface roughness of Ti-6Al-4V, both before and after etching, as well as all coatings, was analyzed using atomic force microscopy (AFM) with the Nanosurf Mobile S system, employing a scan size of 5 µm × 5 µm for all samples. The adhesion test with a universal testing machine using a pull-out method was conducted on the coatings. The tool includes two metal jigs that hold one side of the Ti-6Al-4V substrates in place, while the other side on the coating surface is attached with the 3M Scotch-Weld epoxy adhesive. The specimens were cured at a temperature of 180 °C for 2 h before the pull-out test was conducted at a crosshead speed of 5 mm/min. To minimize errors, an average of three measurements was recorded.
In vitro electrochemical studies
The electrochemical data was obtained using the Autolab PGSTAT30 Potentiostat–Galvanostat at the open circuit potential (OCP). This device allows for data collection and subsequent data mathematical analysis. The experiments were carried out in a standard three-electrode cell with an SBF as the electrolyte at room temperature, following a 5-h immersion period. The working, counter, and reference electrodes included coated samples, platinum wire, and Ag/AgCl (3 M KCl), respectively. The coatings were examined using the potentiodynamic polarization technique with a scan rate of 1 mV·s-1. Furthermore, electrochemical impedance tests were conducted at OCP, spanning a frequency range from 100 kHz to 10 mHz with an amplitude of ± 5 mV. The resulting impedance data were then fitted and analyzed using ZView® (II) software.
Biocompatibility evaluation
The proliferation of MG-63 cells (Pasteur Institute, NCBI code: C555) was assessed using the MTT assay, following ISO 10,993–5 standards, over 24, 48, and 72 h. The MG-63 cells were cultured in a humidified environment with 5% CO2 at a temperature of 37 °C. In this study, the cells were seeded at a density of 1 × 104 cells into every well. After 24, 48, and 72 h, the culture medium was removed, and 10% MTT was added to each well. The cells were then incubated at 37 °C for 4 h. By absorbing MTT, living cells convert it into insoluble formazan crystals. 50 µl of DMSO were added to dissolve the formazan crystals. All experiments were conducted three times for each sample. Cell viability was determined using the following equations53:
The mean OD of sample refers to its measured optical density, while the mean OD of control indicates the optical density of the control group (usually untreated cells). Toxicity% represents the percentage of non-viable cells determined by the MTT assay, and viability% indicates the percentage of viable cells. These equations calculate the cell viability percentage using the mean optical density (OD) of the sample and control in the MTT assay.
Results and discussions
FT-IR spectra
FTIR analysis was conducted on the HA, HA/T, and Co/HA/T powder samples with varying concentrations of cobalt. Specifically, the powder samples of the viscous sol used for the coatings were prepared at the same temperature. The FTIR peaks depicted in Fig. 3 show that the prepared samples have very similar peaks. The vibration modes of tetrahedral phosphate were related to the peaks that appeared at 471, 559, 603, 1054, and 1127 cm−154,55.The weak band at 471 cm−1 was ascribed to the bending mode (ν2) of PO43−, whereas sharp bands at 559 cm−1 and 603 cm−1 can be attributed to the bending asymmetric vibration mode (ν4) of the phosphate group56. The strong peak at 1054 cm−1 was attributed to the asymmetric stretching vibration modes (ν3) of PO43− groups57. Additionally, peaks at 1634 and 3200–3700 cm−1 signify the presence of water molecules58,59. A weak band at 1453 cm−1 denotes O–H bending vibrations in HA, while the weak peak at 816 cm-1 suggests the presence of the Si–O–Si bond60. Based on the FTIR analysis, the synthesized samples exhibit excellent purity and quality.
X-ray diffraction patterns
The XRD patterns of the synthesized powder samples (HA, HA/T, 5Co/HA/T, 10Co/HA/T, and 20Co/HA/T) are illustrated in Fig. 4. The powder samples for the XRD analysis were prepared from the viscous sol used for the coatings at the same temperature. The XRD experiments were done to evaluate purity and crystal structures. The XRD patterns of the HA/T and Co2+-doped HA/T samples depicted in Fig. 4b–e exhibit comparable diffraction patterns to that of pure HA shown in Fig. 4a. The main characteristic peaks associated with the hexagonal structure of HA diffraction planes were identified in each of the five samples at 2θ angles of 23.05°, 25.89°, 28.34°, 31.92°, 32.53°, 33.05°, 39.68°, 42.25°, 44.76°, 46.62°, 49.51°, and 53.18°. These results align with the research conducted by Prakash et al.52,61. Each peak observed in the HA, HA/T and Co/HA/T samples closely matched the standard International Center for Diffraction Data (ICDD) file number 09-043227. Incorporating cobalt ions in the HA structure resulted in a reduction in the intensity of the peaks and their broadening in the XRD patterns. This indicates structural strain likely due to the difference in ionic radii between calcium ions (1 Å) and cobalt ions (0.65 Å)62. Table 3 presents the unit cell volume, lattice constants, and average crystallite size of each sample. According to Table 3, the obtained data for the samples are in very high agreement with the standard hydroxyapatite revealing the high purity of the prepared samples. All mentioned parameters for HA/T and Co/HA/T samples were decreased compared to pure HA sample. The Debye–Scherrer equation was used to calculate the average crystallite size of the samples. The particle size of the samples decreases from 17.68 nm to 12.23 nm. These results are attributed to the ionic radii mismatch between calcium and cobalt ions. The smaller ionic radius of cobalt compared to calcium may have contributed to the reduced nanoparticle size in Co/HA/T relative to pure HA. The obtained results are consistent with previous reports44,62.
Assessment of microstructure
FESEM images are a highly effective method for investigating the morphology of coatings, including shape, particle size, and size distribution of nanoparticles on their surface. Figure 5 shows the morphology of the coatings before and after electrochemical measurements, while Fig. 6 presents the particle size distribution of pure HA, HA/T, and Co/HA/T coatings with different cobalt concentrations from ion exchange. Figure 6a–d shows that the average size of nanoparticles decreased from 41.55 nm for pure HA to 27.40 nm for 10Co/HA/T. Also, the FESEM images illustrate the synthesized nanoparticles with a spherical shape and a uniform size distribution. In Fig. 5a1, the surface morphology of pure HA exhibits a rough surface with varying pore sizes and cracks, while the HA/T coating in Fig. 5b1, the pore sizes disappeared after adding TEOS and displays uniform surface with cracks. The surface morphology of Co/HA/T coatings with varying cobalt concentrations is depicted in Fig. 5c1–e1. As illustrated in Fig. 5b1–e1, the TEOS-based coatings exhibit uniform surfaces without holes in comparison to the pure HA coating, thus the utilization of TEOS has improved the quality of the coating surface. This integration of TEOS facilitates the even distribution of nanoparticles on the Ti-6Al-4V surface, leading to strong adhesion between the Ti-6Al-4V and coating. Interestingly, FESEM images reveal small aggregates and uneven surfaces in the 5% cobalt coating (Fig. 5c1), whereas the 10% and 20% cobalt coatings exhibit smooth surfaces without pores (Fig. 5d1 and e1). The particle size distribution was determined by measuring 50 particles from the FESEM images with a log–normal function28 (Fig. 6a–e):
where the dispersion is denoted by σ and the median diameter by D0. The standard deviation \({\sigma }_{D}=\langle D\rangle .{[\text{exp}\left({\sigma }^{2}\right)-1]}^{1/2}\) and mean diameter \(\langle D\rangle ={D}_{0}.\text{exp}({\sigma }^{2}/2)\) were computed using the fit parameters σ and D0, which are shown in Table 3. According to the data displayed in Table 3, the ⟨D⟩SEM values obtained are larger than ⟨D⟩XRD values. This discrepancy can be attributed to the possible aggregation of several crystallites within a single grain. The particle size distribution of the 10% cobalt sample has shown a significant improvement in uniformity of particle size compared to all other coatings that were produced (Fig. 6d). FESEM investigations confirm that the 10Co/HA/T coating features a homogeneous surface without pores, outperforming the other coatings. Additionally, EDS analysis of samples (Fig. 7) confirmed the existence of elements oxygen (O), cobalt (Co), phosphorus (P), calcium (Ca), silicon (Si), titanium (Ti), vanadium (V), and aluminum (Al) in the coatings. The thickness of coatings was determined from the cross-sectional images obtained through FESEM, as illustrated in Fig. 8A. The optimal coating thickness was approximately 642 nm for the 10Co/HA/T sample. According to the FESEM cross-sectional images and corresponding data, the thickness of the coatings for pure HA, HA/T, and Co/HA/T coatings (with different cobalt concentrations) remained relatively close but still exhibited some variation (Fig. 8A). Cobalt ions (Co2⁺) have a smaller ionic radius (0.65 Å) than calcium ions (1 Å). Their incorporation into the HA structure affects lattice parameters and crystal growth. This substitution can lead to a denser structure, which slightly impacts the thickness of the coating. The thickness of the coating has significant influences in promoting bone tissue growth at implant-bone interfaces26,63. Also, coating thickness greatly impacts electrochemical behavior such as corrosion resistance. Thicker organic coatings improve corrosion resistance but can crack under deposition tensions, allowing electrolyte infiltration and undermining the coating’s protective qualities64. Figure 8B demonstrates the elemental mapping of the optimal coating. This analysis aimed to confirm the presence of Co, Ca, P, Si, and O elements in the samples. The elemental distribution was qualitatively investigated, revealing that these elements are completely spread across the coating surface.
Surface roughness
The utilization of AFM analysis proves is an effective method for assessing coatings in biomedical applications. AFM offers detailed information into the morphology, topography, and surface roughness of coatings, playing a critical role in assessing biocompatibility and the potential for bacterial adhesion12,65. Figure 9 displays AFM images of the surface of Ti-6Al-4V, both before and after etching, as well as all coatings. Table 4 presents a summary of the measurements related to the surface roughness of samples. These measurements include root mean square roughness (Rq), average roughness (Ra), and total roughness (Rt) surface of substrate and coatings, all providing valuable insights into the surface characteristics of the coatings. The average roughness of the titanium substrate before/after etching was obtained as 9.05 and 21.50 nm, respectively, and this increase in roughness can improve the adhesion of the coating to the substrate. Table 4 shows that the Ra of the 10Co/HA/T coating is 5.62 nm, considerably lower than that of the pure HA coating, which measures 30.96 nm. This reduction can be attributed to the decrease in nanoparticle size, pores, and aggregates for this sample. The examination of the results shown in Table 4 confirms that the inclusion of cobalt ions into the HA structure leads to a reduction in the surface roughness. Surface modifications hold promise in enhancing the performance of titanium implants by promoting osteoblast cell proliferation, safeguarding against chemical corrosion from bodily fluids, facilitating bone growth, and reducing bacterial adhesion26,65. Studies show that smoother surfaces demonstrate enhanced resistance to corrosion. Valadez-Martínez et al. showed that reducing the surface roughness increases the corrosion resistance of coatings66.
Adhesion strength
The adhesion strength was evaluated through a pull-out test, under the ASTM D4541 standard. This method provides reliable and useful results for comparing different coatings. The adhesion strength of different hydroxyapatite coatings, including Pure HA, HA/T, and different concentrations of cobalt-doped (5%, 10%, and 20Co/HA/T), was assessed both before and following a 10-day immersion in SBF (Fig. 10). The results of the adhesion strength tests for the coatings are compiled in Table 4. The coating with the highest adhesion strength was 10Co/HA/T, whereas the pure HA demonstrated the lowest adhesion strength before immersion. The findings demonstrate the impact of the presence of cobalt ions and TEOS within HA coatings. The increase in adhesion strength of coatings up to 43.2 MPa occurs due to the formation of a chemical bond between the substrate and the coating. The reduction of adhesion strength for pure HA to 19.2 MPa can attributed to non-uniform coating surfaces and the presence of surface holes, as confirmed by FESEM images. The strength of the chemical bond is a consequence of the native aggregation of O2 on the surface of Ti-6Al-4V. Exposure to air leads to the formation of bondings Ti–OH by hydroxide ions and water on the Ti-6Al-4V surface26. After immersion in SBF, all coatings exhibited a reduction in adhesion strength. Particularly, pure HA showed a marked reduction to 10.1 ± 1.23 MPa, indicating a significant reduction of adhesion after exposure to SBF. The 10-day immersion in SBF negatively impacted the adhesion strength of all coatings, emphasizing the necessity of assessing coating performance under conditions that simulate the physiological environment. The different concentrations of cobalt-doped, particularly 10Co/HA/T, showed better retention of adhesion strength after immersion than other coatings, indicating that cobalt doping may improve the coating’s stability in biological fluids. Research on cobalt-modified HA shows that the mechanisms of cobalt immobilization are crucial for material dispersion and stability, both of which are essential for preserving the integrity of coatings under physiological conditions67. The incorporation of cobalt ions into hydroxyapatite coatings has been associated with enhanced stability and improved mechanical properties in biological conditions, as cobalt ions contribute to the strengthening of ionic interactions in the coating matrix67. Therefore, it can be predictable that the adhesion strength of the Co/HA/T coatings on the Ti-6Al-4V is superior to pure HA, and the 10% cobalt coating was identified as the optimal sample for adhesion strength.
In vitro corrosion tests
Tafel analysis
The use of metal alloys as implants in the human body may lead to their corrosion. Thus, harmful and undesirable elements from implants may be released into the surrounding tissues, which can have negative effects. To assess this behavior, corrosion tests were conducted on pure HA, HA/T, and Co/HA/T coatings in a corrosive environment. The coatings underwent Tafel polarization analysis by immersion in the SBF solution for five hours. Figure 11A presents the results, including the corrosion current density (icorr) and corrosion potential (Ecorr), with their values detailed in Table 5. The plots depicted in Fig. 11B demonstrate the variations in polarization resistance (Rp) and corrosion current density obtained from Tafel analyses. Variations in polarization resistance and corrosion current density were observed in the coatings. These data indicate that Co/HA/T coatings with varying cobalt concentrations have high polarization resistance. Notably, the polarization resistance significantly increased from 0.421 MΩ × cm2 for pure HA to 14.706 MΩ × cm2 after integrating cobalt ions into the HA structure. Figure 11A shows that increasing the cobalt amount from 5 to 10% decreases the current density, attributed to the formation of a more uniform coating. Thus, the integration of cobalt ions into the HA structure improves the passive layer coating. However, when the cobalt amount is increased to 20%, the uniformity of the surface decreases, and that leads to an increase in Icorr again. The reduction in the Icorr of the coatings suggests an improvement in their corrosion protection in the SBF68,69. The optimal coating, containing 10% cobalt (10Co/HA/T), shows a crack-free surface, so the aggressive solution cannot easily penetrate the substrate. The corrosion protection efficiency of the coatings was evaluated using Eq. (7)70:
η% represents the percentage of corrosion protection efficiency, where Rc is the polarization resistance of the coated sample and Rb is that of the bare (uncoated) Ti-6Al-4V substrate. This equation evaluates how effective the coating is at improving the polarization resistance compared to the uncoated sample. Tafel polarization was conducted within the potential range of − 0.25V to + 0.25V vs. OCP at a scan rate of 0.001 V/s. The icorr was determined using the Butler-Volmer equation, and the Stern-Geary equation was used to obtain the Rp as outlined in Eq. (8)71.
Rp is the polarization resistance, βa is the anodic Tafel slope (indicating the rate of the anodic reaction), βc is the cathodic Tafel slope (indicating the rate of the cathodic reaction), Icorr is the corrosion current density, and 2.303 is a constant derived from the logarithmic conversion between the Tafel slopes and current. This equation calculates polarization resistance using the Tafel slopes and corrosion current density. Based on Table 5, the 10Co/HA/T coating displayed superior performance in terms of protective efficiency (98.51%) compared to the other coatings.
EIS studies
EIS studies serve as an important method for assessing the resistance of coatings in corrosion solutions and have numerous applications in the biomedical field. The EIS experiments were conducted on all samples after a 5-h immersion period, as well as on the optimal coating with varying immersion times in SBF solution, illustrated in Fig. 12a,b. The EIS data was analyzed using equivalent circuits, as depicted in Fig. 12c,d. Also, Fig. 13 schematically illustrates the corrosion process of the coatings in the electrolyte solution (SBF) on the Ti-6Al-4V. A larger capacitive loop diameter observed in the Nyquist plots signifies an enhancement in the corrosion resistance of the coatings72,73,74. The Nyquist plots in Fig. 12a showed a notable increase in diameter when Co2+ ions were present, in comparison to pure HA. This improvement in corrosion behaviour is attributed to the integration cobalt ions into the hydroxyapatite structure, which functions as a protective barrier against corrosion. The charge transfer resistance (Rct) is a key parameter to evaluate the corrosion properties of coatings70,75. Figure 12a demonstrated that the 10Co/HA/T sample had a higher Rct value of 13.40 MΩ × cm2, in contrast to the bare substrate, which exhibited the Rct value of 0.14 MΩ × cm2. The rise of Rct can be attributed to the formation of a safeguarding passive layer on samples. Additionally, the microstructure of coatings, including holes, cracks, non-uniformity, and coating thickness impacts their corrosion behaviour in EIS studies. Figure 5a2–e2 presents FESEM images of the coatings’ surfaces after electrochemical measurements. The pure HA coating exhibited a nonuniform morphology with microcracks and holes (Fig. 5a2), while the surfaces of the HA/T and Co/HA/T coatings at varying cobalt concentrations (Fig. 5b2–e2) were relatively uniform and free of holes. Moreover, as noted earlier, coating thickness significantly affects electrochemical properties like corrosion resistance. Pure HA is the thickest among the prepared coatings (Fig. 8A); however, its large holes and uneven surfaces result in low corrosion resistance. The absence of holes and defects in the HA/T and Co/HA/T coatings after electrochemical tests indicates high corrosion resistance and effective prevention of electrolyte solution (SBF) penetration into the coating. Notably, the 10% cobalt coating (Fig. 5d2) displayed a smoother, more uniform, and flawless surface compared to the other coatings. Thus, the FESEM results confirm the findings from the electrochemical measurements. FESEM images can help in evaluating the uniformity and defect density of coatings, factors that significantly affect their corrosion performance. For example, coatings characterized by rough textures and elevated defect densities tend to demonstrate diminished corrosion behaviour76. Furthermore, the structural integrity of these coatings is associated with their ability to resist permeation by corrosive substances, where alterations in the composition of the coating can result in decreased permeability and enhanced resistance77. The 10Co/HA/T coating demonstrates high resistance to corrosion due to its increased capacitance loop diameter and uniform surface, validating its exceptional passivation capabilities.
The electrochemical impedance data, obtained from analyzing the experimental results using two distinct equivalent circuits, are outlined in Tables 6 and 8, which align with prior literature reports26,78. The equivalent circuit model utilized for the substrate differs from the coatings (Fig. 12c-d). Within this model, Rs symbolizes the electrolyte resistance, Rct represents the charge transfer resistance, and Rcoat corresponds to the resistance of the coatings. The capacitance of the coatings is associated with the first constant phase element (CPEcoat), whereas the second time constant signifies the electrical double-layer capacitance (CPEdl). Also, Y0 and n are used to represent CPE. Table 7 presents a comparison of the results related to Rct, Icorr, coating technique, and nanoparticle size from this study with those documented in the existing literature. The outcomes suggest that the results of this study are better than those of earlier studies.
In addition, the evaluation of the corrosion behaviour of the 10Co/HA/T coating, identified as the most optimal specimen was performed over different immersion durations, as illustrated in Fig. 12b. Initially, on the first day, the Rct of the 10Co/HA/T coating was measured at 13.40 MΩ × cm2. However, extending the immersion time resulted in a reduction in the corrosion behaviour provided by the optimal coating. The reduction in Rct over the duration of immersion may be ascribed to various factors, including electrochemical reactions and the penetration of aggressive ions into the samples, as shown in Table 8. After 40 days, the Rct value of 10% cobalt coating decreased to 0.75 MΩ × cm2, although its corrosion behaviour exceeded that of the bare substrate and pure HA. Coatings perform optimally after immersion in an electrolyte solution, forming a protective layer that enhances the corrosion resistance. However, prolonged immersion leads to degradation, reducing the coatings’ resistance. Extended immersion deteriorates the protective layer, raising permeability and vulnerability to corrosion79. The presence of cobalt ions at the ideal concentration improves the corrosion behaviour of the coatings by minimizing the formation of cracks and ensuring surface uniformity. Hence, the EIS investigations validate that the 10Co/HA/T coating demonstrated exceptional resistance against corrosion in comparison to other samples.
Cell viability
The results of cell proliferation, as determined by MTT assay analyses conducted at 24, 48, and 72 h on Ti-6Al-4V, pure HA, HA/T, and different concentrations of cobalt-doped (5%, 10%, and 20Co/HA/T), are illustrated in Fig. 14. The coatings’ cell viability was determined using Eqs.1 and 2, and the obtained data are summarized in Table 9. The results of the MTT assay demonstrated that pure HA, HA/T, and Co/HA/T coatings exhibited no toxicity. The cellular activity of the HA coatings, particularly 10Co/HA/T coating, showed an enhancement in biocompatibility over time compared to the other samples. The cell viability percentages for Ti-6Al-4V were 67.21 ± 2.48% at 24 h, 71.07 ± 2.51% at 48 h, and 74.30 ± 1.86% at 72 h. MG-63 cells show very good cell activity on different hydroxyapatite coatings except for 20Co/HA/T coating. The cell viability of the 20% cobalt coating showed a smaller increase during the long-term test (Fig. 14). Among all samples, the 10Co/HA/T coating obtained the highest cell density, which cell viability percentages of 88.47 ± 2.42% at 24 h, 98.79 ± 2.03% at 48 h, and 101.27 ± 1.91% at 72 h. The increased proliferation of MG-63 cells on the 10Co/HA/T coating can be due to the optimal cobalt concentration and its incorporation into the calcium site of the HA structure, as well as the presence of TEOS. Generally, the HA coatings, including pure HA, HA/T, and different concentrations of cobalt (5%, 10%, and 20Co/HA/T), demonstrated comparable MG-63 cell viability to Ti-6Al-4V, indicating the higher bioactive properties of HA coatings. This bioactivity enhances the stimulation of bone cells by facilitating the proliferation of osteoblasts. Coatings based on silicon in hydroxyapatite markedly improve the proliferation of osteoblasts, positioning them as a promising option for bone tissue engineering80. The findings indicate that Co/HA samples demonstrate outstanding biocompatibility, significantly enhancing the proliferation of bone marrow stromal cells in comparison to pure hydroxyapatite81. This research confirms the excellent cell proliferation of 10Co/HA/T coating as an optimal sample for bioengineering applications.
Conclusion
In summary, we utilized the spin coating technique to produce hydroxyapatite coatings on Ti-6Al-4V. A comprehensive range of analytical methods was employed to gather detailed information on the coatings, including assessments of sample purity, structural investigations, morphological examinations, and corrosion performance evaluations. The FTIR analysis confirmed that the synthesized samples exhibited exceptional purity and quality. X-ray diffraction patterns of the coatings showed peaks similar to pure HA, suggesting the absence of additional silicon and cobalt phases. FESEM images showcased that coatings using TEOS as a binder had uniform surfaces and lacked any holes. The analysis conducted by EDS confirmed the existence of silicon, phosphorus, calcium, cobalt, and oxygen elements in the samples. The optimal sample, the 10Co/HA/T coating, displayed a thickness of 642 nm. The AFM results indicated a reduction in coating surface roughness from 30.96 nm to 5.62 nm. The adhesion strength of various hydroxyapatite coatings was evaluated both before and after a 10-day immersion in SBF to assess their long-term performance. The 10Co/HA/T coating exhibited the highest adhesive strength among the samples at 43.2 ± 0.93 MPa. Electrochemical tests showed that incorporating Co2+ ions into the HA structure significantly improved the corrosion resistance of the coatings in SBF. In vitro biological evaluation demonstrated that the prepared coatings had appropriate cell viability compared to the bare substrate. The Co/HAP/T coatings demonstrate significant advantages in biocompatibility and corrosion resistance, particularly the 10Co/HA/T, which exhibited enhanced cell viability and adhesion strength compared to pure HA coating after immersion in SBF. However, limitations include a notable reduction in adhesion strength post-immersion, with pure HA showing a decrease to 10.1 ± 1.23 MPa, indicating potential challenges in long-term stability and performance in physiological environments. This research offers precious insights into the characterizing and optimizing Co/HA/T coatings, emphasizing their potential biomedical applications.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Shakeel, A. & Corridon, P. R. Mitigating challenges and expanding the future of vascular tissue engineering—Are we there yet?. Front. Physiol. 13, 1079421 (2023).
Oryan, A., Alidadi, S., Moshiri, A. & Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 9, 1–27 (2014).
Liu, X., Chen, S., Tsoi, J. K. H. & Matinlinna, J. P. Binary titanium alloys as dental implant materials—A review. Regen. Biomater. 4, 315–323 (2017).
He, D. et al. Effect of hydrothermal treatment temperature on the hydroxyapatite coatings deposited by electrochemical method. Surf. Coatings Technol. 406, 126656 (2021).
Sykaras, N., Iacopino, A. M., Marker, V. A., Triplett, R. G. & Woody, R. D. Implant materials, designs, and surface topographies: their effect on osseointegration. A literature review. Int. J. Oral Maxillofac. Implants 15, (2000).
Nicholson, J. W. Titanium alloys for dental implants: A review. Prosthesis 2, 11 (2020).
Koju, N., Sikder, P., Ren, Y., Zhou, H. & Bhaduri, S. B. Biomimetic coating technology for orthopedic implants. Curr. Opin. Chem. Eng. 15, 49–55 (2017).
Arifin, A., Sulong, A. B., Muhamad, N., Syarif, J. & Ramli, M. I. Material processing of hydroxyapatite and titanium alloy (HA/Ti) composite as implant materials using powder metallurgy: A review. Mater. Des. 55, 165–175 (2014).
Eliaz, N. & Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials (Basel) 10, 334 (2017).
Priyadarshini, B., Rama, M., Chetan & Vijayalakshmi, U. Bioactive coating as a surface modification technique for biocompatible metallic implants: A review. J. Asian Ceram. Soc. 7, 397–406 (2019).
Cha, S. & Park, Y. S. Plasma in dentistry. Clin. Plasma Med. 2, 4–10 (2014).
Singh, S., Singh, G. & Bala, N. Characterization, electrochemical behavior and in vitro hemocompatibility of hydroxyapatite-bioglass-iron oxide-chitosan composite coating by electrophoretic deposition. Surf. Coatings Technol. 405, 126564 (2021).
Yang, J., Sultana, R., Hu, X. & Ichim, P. Novel layered hydroxyapatite/tri-calcium phosphate-zirconia scaffold composite with high bending strength for load-bearing bone implant application. Int. J. Appl. Ceram. Technol. 11, 22–30 (2014).
Anjaneyulu, U., Pattanayak, D. K. & Vijayalakshmi, U. The facile and phase pure evaluations of nano hydroxyaptite powder by sol-gel method. Int. J. ChemTech Res. 7, 1516–1520 (2014).
Owens, G. J. et al. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 77, 1–79 (2016).
Dehghanghadikolaei, A., Ansary, J. & Ghoreishi, R. Sol-gel process applications: A mini-review. Proc. Nat. Res. Soc. 2, 2008–2029 (2018).
Dikici, B., Niinomi, M., Topuz, M., Koc, S. G. & Nakai, M. Synthesis of biphasic calcium phosphate (BCP) coatings on β-type titanium alloys reinforced with rutile-TiO 2 compounds: Adhesion resistance and in-vitro corrosion. J. Sol-Gel Sci. Technol. 87, 713–724 (2018).
Dikici, B. et al. Synthesis and characterization of hydroxyapatite/TiO 2 coatings on the β-type titanium alloys with different sintering parameters using sol-gel method. Prot. Met. Phys. Chem. Surfaces 54, 457–462 (2018).
Jodati, H., Yılmaz, B. & Evis, Z. A review of bioceramic porous scaffolds for hard tissue applications: Effects of structural features. Ceram. Int. 46, 15725–15739 (2020).
Singh, S., Singh, G. & Bala, N. Synthesis and characterization of iron oxide-hydroxyapatite-chitosan composite coating and its biological assessment for biomedical applications. Prog. Org. Coatings 150, 106011 (2021).
Singh, S., Singh, G., Bala, N. & Aggarwal, K. Characterization and preparation of Fe3O4 nanoparticles loaded bioglass-chitosan nanocomposite coating on Mg alloy and in vitro bioactivity assessment. Int. J. Biol. Macromol. 151, 519–528 (2020).
Farrokhi-Rad, M., Shahrabi, T., Mahmoodi, S. & Khanmohammadi, S. Electrophoretic deposition of hydroxyapatite-chitosan-CNTs nanocomposite coatings. Ceram. Int. 43, 4663–4669 (2017).
Topuz, M. Investigation of halloysite nanotube effect in poly–(lactic acid)/hydroxyapatite coatings on Ti–6Al–4V biomedical alloy. J. Polym. Environ. 31, 4112–4126 (2023).
Yilmaz, B., Alshemary, A. Z. & Evis, Z. Co-doped hydroxyapatites as potential materials for biomedical applications. Microchem. J. 144, 443–453 (2019).
Topuz, M., Yigit, O., Kaseem, M. & Dikici, B. Synthesis of implantable ceramic coatings and their properties. Adv. Ceram. Coatings Biomed. Appl. https://doi.org/10.1016/B978-0-323-99626-6.00008-1 (2023).
Priyadarshini, B. & Vijayalakshmi, U. In Vitro bioactivity, biocompatibility and corrosion resistance of multi-ionic (Ce/Si) co-doped hydroxyapatite porous coating on Ti-6Al-4 V for bone regeneration applications. Mater. Sci. Eng. C 119, 111620 (2021).
Gezaz, M. S., Aref, S. M. & Khatamian, M. Investigation of structural properties of hydroxyapatite/zinc oxide nanocomposites; an alternative candidate for replacement in recovery of bones in load-tolerating areas. Mater. Chem. Phys. 226, 169–176 (2019).
Safari-Gezaz, M., Mirzaei-Saatlo, M., Asghari, E. & Parhizkar, M. The incorporation of cobalt ions into hydroxyapatite nanostructure for a novel range of electrochemical energy storage applications. J. Phys. Chem. Solids 112118 (2024).
Zhang, B. G. X., Myers, D. E., Wallace, G. G., Brandt, M. & Choong, P. F. M. Bioactive coatings for orthopaedic implants—recent trends in development of implant coatings. Int. J. Mol. Sci. 15, 11878–11921 (2014).
Yao, Q. et al. Composition and bioactivity of calcium phosphate coatings on anodic oxide nanotubes formed on pure Ti and Ti-6Al-4V alloy substrates. Mater. Sci. Eng. C 110, 110687 (2020).
Kazemi, M., Ahangarani, S., Esmailian, M. & Shanaghi, A. Investigation on the corrosion behavior and biocompatibility of Ti-6Al-4V implant coated with HA/TiN dual layer for medical applications. Surf. Coatings Technol. 397, 126044 (2020).
Mohammadi Aref, S., Safarigezaz, M. & Khatamian, M. Synthesis of hydroxyapatite nanoparticles by the Sol-Gel method, investigation of its morphology and comparison of its structure with intact tooth. Iran. J. Phys. Res. 20, 445–453 (2020).
Sahu, T., Singh Bisht, S., Ranjan Das, K. & Kerkar, S. Nanoceria: Synthesis and biomedical applications. Curr. Nanosci. 9, 588–593 (2013).
Safari-Gezaz, M. & Parhizkar, M. Effect of ionic liquid as a surfactant in hydroxyapatite coatings for improvement corrosion resistance of Ti-6Al-4V substrates for implant applications. Heliyon 10 (2024).
Liu, L., Huang, R. & Zhang, L. Cobalt element doping for biomedical use: a review. In Materials Science Forum Vol. 993 (ed. Liu, L.) 811–819 (Trans Tech Publ., 2020).
Zhao, Q. et al. Ion incorporation into bone grafting materials. Periodontology 2000(94), 213–230 (2024).
Vindhya, P. S. & Kavitha, V. T. Effect of cobalt doping on antimicrobial, antioxidant and photocatalytic activities of CuO nanoparticles. Mater. Sci. Eng. B 289, 116258 (2023).
Azab, A. A., Esmail, S. A. & Abdelamksoud, M. K. Studying the effect of cobalt doping on optical and magnetic properties of zinc oxide nanoparticles. Silicon 11, 165–174 (2019).
Hafeez, M. et al. Synthesis of cobalt and sulphur doped titanium dioxide photocatalysts for environmental applications. J. King Saud Univ. 34, 102028 (2022).
Wu, X. et al. Preparation, characterization, and bioactivities of cobalt, strontium and fluorine co-doped oxide films on titanium surface for clinical application. J. Biomed. Nanotechnol. 20, 678–686 (2024).
Kermani, F. et al. Strontium-and cobalt-doped multicomponent mesoporous bioactive glasses (MBGS) for potential use in bone tissue engineering applications. Materials (Basel) 13, 1348 (2020).
Barrioni, B. R. et al. Evaluation of in vitro and in vivo biocompatibility and structure of cobalt-releasing sol-gel bioactive glass. Ceram. Int. 44, 20337–20347 (2018).
Kargozar, S., Baino, F., Hamzehlou, S., Hamblin, M. R. & Mozafari, M. Nanotechnology for angiogenesis: opportunities and challenges. Chem. Soc. Rev. 49, 5008–5057 (2020).
Kramer, E., Itzkowitz, E. & Wei, M. Synthesis and characterization of cobalt-substituted hydroxyapatite powders. Ceram. Int. 40, 13471–13480 (2014).
Wang, L. et al. Mild hyperthermia-mediated osteogenesis and angiogenesis play a critical role in magnetothermal composite-induced bone regeneration. Nano Today 43, 101401 (2022).
Yang, Y. et al. Effects of strontium and cobalt codoped nanotube implants on osseointegration in osteoporotic rats. Mater. Today Commun. 33, 104339 (2022).
Safari-Gezaz, M., Parhizkar, M. & Asghari, E. Investigation of the structural properties of Si4+-doped HAP coatings on Ti-6Al-4V substrate as a corrosion barrier in biomedical media. Colloids Surfaces A Physicochem. Eng. Asp. 699, 134742 (2024).
Mendhe, A. C. Spin coating: Easy technique for thin films. In Simple Chemical Methods for Thin Film Deposition: Synthesis and Applications (ed. Mendhe, A. C.) 387–424 (Springer, 2023).
Yin, Y. Advances and perspectives of spin coating techniques. Appl. Comput. Eng 7, 291–301 (2023).
Eliaz, N. et al. The effect of surface treatments on the adhesion of electrochemically deposited hydroxyapatite coating to titanium and on its interaction with cells and bacteria. J. Mater. Sci. Mater. Med. 22, 1741–1752 (2011).
Nisar, S. S. & Choe, H.-C. Mechanical hydroxyapatite coatings on PEO-treated Ti–6Al–4V alloy for enhancing implant’s surface bioactivity. Ceram. Int. 50, 17703–17719 (2024).
Prakash, V. C. A., Venda, I., Thamizharasi, V. & Sathya, E. A new attempt on synthesis of spherical nano hydroxyapatite powders prepared by dimethyl sulfoxide-poly vinyl alcohol assisted microemulsion method. Mater. Chem. Phys. 259, 124097 (2021).
Farshid, S., Ebrahimian-Hosseinabadi, M. & Rafienia, M. Electrophoretic deposition of biphasic calcium phosphate/graphene nanocomposite coatings on Ti6Al4V substrate for biomedical applications. J. Alloys Compd. 892, 162150 (2022).
Karunakaran, G. et al. Microwave-assisted hydrothermal synthesis of mesoporous carbonated hydroxyapatite with tunable nanoscale characteristics for biomedical applications. Ceram. Int. 45, 970–977 (2019).
Karunakaran, G. et al. Ascorbic acid-assisted microwave synthesis of mesoporous Ag-doped hydroxyapatite nanorods from biowaste seashells for implant applications. ACS Appl. Bio Mater. 2, 2280–2293 (2019).
Prakash, V. C. A., Venda, I. & Thamizharasi, V. Synthesis and characterization of surfactant assisted hydroxyapatite powder using microemulsion method. Mater. Today Proc. 51, 1788–1792 (2022).
Blakeslee, K. C. & Condrate, R. A. Sr. Vibrational spectra of hydrothermally prepared hydroxyapatites. J. Am. Ceram. Soc. 54, 559–563 (1971).
Mirzaei-Saatlo, M. et al. Phase change ionogel based choline formate as a green solid-state electrolyte with novel poly (aniline-co-4-nitroaniline) electrode material for flexible supercapacitors. J. Energy Storage 82, 110534 (2024).
Kumar, G. S. et al. One step method to synthesize flower-like hydroxyapatite architecture using mussel shell bio-waste as a calcium source. Ceram. Int. 43, 3457–3461 (2017).
de Ferri, L., Lorenzi, A. & Lottici, P. P. OctTES/TEOS system for hybrid coatings: real-time monitoring of the hydrolysis and condensation by Raman spectroscopy. J. Raman Spectrosc. 47, 699–705 (2016).
Prakash, V. C. A., Venda, I., Thamizharasi, V. & Sathya, E. A comparative study on microemulsion synthesis of hydroxyapatite powders by ionic and Non-Ionic surfactants. Mater. Today Proc. 51, 1701–1705 (2022).
Bhattacharjee, A. et al. Antibacterial and magnetic response of site-specific cobalt incorporated hydroxyapatite. Ceram. Int. 46, 513–522 (2020).
Nuswantoro, N. F. et al. Effect of hydroxyapatite coating thickness on inflammation and osseointegration of Ti–29Nb–13Ta-4.6 Zr (TNTZ) implants. J. Mater. Res. Technol. 30, 6210–6217 (2024).
de Paula, A. S. et al. Influence of organic coating thickness on electrochemical impedance spectroscopy response. Coatings 14, 285 (2024).
Mandracci, P., Mussano, F., Rivolo, P. & Carossa, S. Surface treatments and functional coatings for biocompatibility improvement and bacterial adhesion reduction in dental implantology. Coatings 6, 7 (2016).
Valadez-Martínez, M. A. et al. Influence of roughness on the early corrosion of API steels by atomic force microscopy (AFM): A real-time assessment. Mater. Today Commun. 36, 106519 (2023).
Alshemary, A. Z., Hussain, R., Dalgic, A. D. & Evis, Z. Bactericidal and in vitro osteogenic activity of nano sized cobalt-doped silicate hydroxyapatite. Ceram. Int. 48, 28231–28239 (2022).
Stango, S. A. X. & Vijayalakshmi, U. Synthesis and characterization of hydroxyapatite/carboxylic acid functionalized MWCNTS composites and its triple layer coatings for biomedical applications. Ceram. Int. 45, 69–81 (2019).
Stango, S. A. X., Karthick, D., Swaroop, S., Mudali, U. K. & Vijayalakshmi, U. Development of hydroxyapatite coatings on laser textured 316 LSS and Ti-6Al-4V and its electrochemical behavior in SBF solution for orthopedic applications. Ceram. Int. 44, 3149–3160 (2018).
Mirzaei-Saatlo, M. et al. 4-Ferrocenylbutyl-based corrosion inhibitors for mild steel in acidic solution. Mater. Chem. Phys. 293, 126895 (2023).
Jamali, H. et al. Prolonged corrosion protection via application of 4-ferrocenylbutyl saturated carboxylate ester derivatives with superior inhibition performance for mild steel. Sci. Rep. 14, 13847 (2024).
Gholivand, K. et al. Evaluation of newly synthesized phosphoramide derivatives as mild steel anti-corrosions using experimental and theoretical approaches. J. Mol. Struct. 1274, 134505 (2023).
Chkirate, K. et al. Corrosion inhibition potential of 2-[(5-methylpyrazol-3-yl) methyl] benzimidazole against carbon steel corrosion in 1 M HCl solution: Combining experimental and theoretical studies. J. Mol. Liq. 321, 114750 (2021).
Fakhraei, M., Mirzaei-Saatlo, M. & Asghari, E. A LiPF6 gel-polymer electrolyte for a solid-state supercapacitor with polyaniline/MnCo layered double hydroxide nanosheets as active electrode material. J. Energy Storage 101, 113930 (2024).
Farhadian, A. et al. A theoretical and experimental study of castor oil-based inhibitor for corrosion inhibition of mild steel in acidic medium at elevated temperatures. Corros. Sci. 175, 108871 (2020).
Perumal, P. et al. Investigation of TiN coating uniformity and its corrosion behaviour using image process. Mater. Res. Express 6, 46411 (2019).
Puig, M. et al. Adhesion enhancement of powder coatings on galvanised steel by addition of organo-modified silica particles. Prog. Org. Coatings 77, 1309–1315 (2014).
Mirzaei-Saatlo, M., Asghari, E. & Shekaari, H. Fabrication of solid state supercapacitors using phase change ionogel electrolytes based on 2-hydroxyethyl cellulose and protic ionic liquids with formate, acetate, and propionate as anions. J. Energy Storage 108, 115069 (2025).
Cristoforetti, A., Rossi, S., Deflorian, F. & Fedel, M. On the limits of the EIS low-frequency impedance modulus as a tool to describe the protection properties of organic coatings exposed to accelerated aging tests. Coatings 13, 598 (2023).
Sun, T., Wang, M., Shao, Y., Wang, L. & Zhu, Y. The effect and osteoblast signaling response of trace silicon doping hydroxyapatite. Biol. Trace Elem. Res. 181, 82–94 (2018).
Yan, L. et al. Cobalt-doped hydroxyapatite for bone tissue engineering: Synthesis, characterization and in vitro biocompatibility of real-time extract. Mater. Today Commun. 38, 108554 (2024).
Shanaghi, A., Mehrjou, B., Ahmadian, Z., Souri, A. R. & Chu, P. K. Enhanced corrosion resistance, antibacterial properties, and biocompatibility by hierarchical hydroxyapatite/ciprofloxacin-calcium phosphate coating on nitrided NiTi alloy. Mater. Sci. Eng. C 118, 111524 (2021).
Hernández, L. et al. Biomimetic hydroxyapatite (HAp) coatings on pure Mg and their physiological corrosion behavior. Ceram. Int. 48, 1208–1222 (2022).
Kim, E.-J., Jeong, Y.-H., Choe, H.-C. & Brantley, W. A. Electrochemical behavior of hydroxyapatite/TiN multi-layer coatings on Ti alloys. Thin Solid Films 572, 113–118 (2014).
Afshari, M., Mohammadloo, H. E., Sarabi, A. A. & Roshan, S. Modification of hydroxyapatite-based coating in the presence of polyvinylalcohol (PVA) for implant application: Corrosion, structure and surface study. Corros. Sci. 192, 109859 (2021).
Singh, S., Pandey, K. K. & Keshri, A. K. Effect of plasma power on corrosion behaviour of plasma sprayed hydroxyapatite coatings. Met. Mater. Int. 27, 4455–4462 (2021).
Acknowledgements
The financial support for this work from the University of Tabriz, Iran is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Meysam Safari-Gezaz: Conceptualization, acquisition of data, Formal analysis, Writing – original draft, Approval of the version of the manuscript to be published. Mojtaba Parhizkar: Conceptualization, acquisition of data, Formal analysis, Writing – original draft, Approval of the version of the manuscript to be published. Elnaz Asghari: Conceptualization, acquisition of data, Formal analysis, Writing – original draft, Approval of the version of the manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Safari-Gezaz, M., Parhizkar, M. & Asghari, E. Effect of cobalt ions doping on morphology and electrochemical properties of hydroxyapatite coatings for biomedical applications. Sci Rep 15, 149 (2025). https://doi.org/10.1038/s41598-024-84055-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-84055-2
Keywords
This article is cited by
-
Sol–gel-derived hydroxyapatite/Co3O4 hybrid nanostructures as advanced electrodes for energy storage
Journal of Materials Science: Materials in Electronics (2026)
-
Cobalt titanate nanocatalyst for enhanced photodegradation of atrazine: kinetics, degradation efficiency, and mechanistic analysis
BMC Chemistry (2025)
-
Performance evaluation and multi-objective optimization of EDM parameters for Ti6Al4V using different tool electrodes
Scientific Reports (2025)
-
Tailoring silicon/zinc co-doped hydroxyapatite coatings via sol–gel route: a multifunctional strategy for high-adhesion and cytocompatible load-bearing implants
Journal of Sol-Gel Science and Technology (2025)
-
Antibacterial Activity of Plasma Electrolytic Oxidation Coated Ti6Al7Nb for Biomedical Applications
Biomedical Materials & Devices (2025)