Abstract
The concentration of heavy metals in 120 green tea samples grown in Hangzhou area were investigated by inductively coupled plasma mass spectrometry (ICP-MS). Except for mercury (Hg) (69.2%), Sb (98.3%), Se (93.3%) and Sn (94.2%), the detection rate of the other 11 elements were all 100.0%. The mean concentration of heavy metals in tea showed the following order: manganese (Mn) > aluminum (Al) > barium (Ba) > copper (Cu) > nickel (Ni) > chromium (Cr) > lead (Pb) > tin (Sn) > lithium (Li) > vanadium (V) > selenium (Se) > arsenic (As),> cadmium (Cd) > antimony (Sb) > mercury (Hg). Mn was the highest element found in the tea samples with a concentration ranging from 202.00 to 2010.00 mg/kg and a mean of 836.00 mg/kg. The lowest element was Hg, with a concentration ranging from ND to 0.017 mg/kg and the mean concentration was 0.0033 mg/kg. And all the 120 samples did not exceed the standard limit value of heavy metal. The pollution level of heavy metals indicated that the single pollution index and comprehensive pollution index of green tea produced in Hangzhou area are far below 0.7, which belong to the clean and safety level. Health risk of inhabitant via consuming green tea was conducted and the HI value was 0.42, less than 1, which indicates that these chemicals pose no risk to human health in Hangzhou area.
Similar content being viewed by others
Introduction
Tea, the second most popular drink in the world1,2 and the most popular in China, is globally known for its health benefits. Indeed, tea is recognized as the best health beverage in the world1,3. Over the last decades, the global production of this plant has grown at a rate greater than 1.8%, and global tea consumption has increased by more than 2% per year4. Today, 18–20 billion cups of this drink are consumed by about 3 billion people on a daily basis5,6,7. The health benefits of tea are mainly attributed to polyphenols, polysaccharides, and other active ingredients, which endow the beverage with antioxidant properties. However, the presence of toxic elements such as heavy metals and pesticide residues in tea leaves and infusions poses a serious health risk8,9,10,11,12.
Heavy metals are persistent inorganic pollutants that can bioaccumulate in animals and plants. In tea, the metal content is attributed to the tea processing procedure2 as well as to the acidic soils in which the tea plant is cultivated13. Other sources such as rainfall, atmospheric dust, plant protective agents, and fertilizers also contribute to metal contamination in tea14. Although some heavy metals, such as Mn, Cu, Zn, and Fe, play an essential role in metabolism, others, such as Hg, Cd, Pb, and As, are non-essential and can cause adverse health effects, even in trace quantities15,16,17. Dietary intake constitutes the main route of human exposure to heavy metals18. Continued exposure to these metals may lead to nervous, bone, and kidney diseases, as well as to cardiovascular and chronic accumulation19.
After entering the human body, heavy metals such as Pb, Cr, Hg, Cd, As, and Ni will have an adverse effect on physiological function and metabolism. For example, mercury and lead can damage the nervous system, leading to slow reactions and dementia2. Meanwhile, cadmium and nickel can cause bone pain necrosis and damaged liver function, respectively. Chromium poisoning of the human body is systemic and may cause dermatitis, eczema, tracheitis, and rhinitis, as well as genetic defects that may lead to metamorphosis and cancer. Mercury, cadmium, arsenic, and lead are also carcinogenic. Unfortunately, their incubation period is long, and thus they can accumulate in the human body to levels that cause irreversible damage, especially since they cannot be easily detected in seemingly healthy individuals20.
In this study, we analyze the contents of Al, As, Ba, Cd, Cr, Cu, Hg, Li, Mn, Ni, Pb, Sb, Se, Sn, and V metals in 120 tea samples produced in Hangzhou, using inductively coupled plasma mass spectrometry (ICP-MS). The ratio of the dose of human pollutant intake to the standard reference dose (Standard reference dose, RfD) is used as the evaluation criterion21, as recommended by the Environmental Protection Agency (USEPA). The health risk is assessed based on the calculated hazard coefficient (hazard Quotients, HQ) and risk index (HI) to ensure the drinking safety of the product and help develop it further.
Materials and methods
Chemicals and equipment
The standard stock solution of mixed Al, As, B, Ba, Be, Bi, Cd, Co, Cr, Cu, Fe, Ga, Li, Mg, Mn, Ni, Pb, Sb, Sn, Sr, Ti, Tl, V, and Zn metals (100 µg/mL) was purchased from Bruker and the National Center of Analysis and Testing for Nonferrous Metals and Electronic Materials. Meanwhile, the standard stock solutions of Hg and Se (100 µg/mL) were bought from TM Standard and NCS Testing Technology Co., Ltd, respectively. The standard working solutions of the metal mixture and Se (0.0, 1.0, 2.0, 4.0, 8.0, 20.0, and 40.0 µg/L) were prepared by step dilution of standard stock solutions with 1% HNO3, while those of Hg (0.0, 0.2, 0.4, 1.0, 1.5, and 2.0 µg/L) were prepared with 1% HCl. Trace metal grade HNO3 and Suprapur HCl were obtained from Thermo Fisher and Merck, respectively. High-purity deionized water (18.2 MΩ cm) was used in all preparations. To eliminate the blank of the trace analytes and other contaminants, all containers were dipped in 20% HNO3 over 24 h and rinsed with high-purity deionized water prior to use. Sample digestion and metal analyses were achieved using a microwave digestion system bought from CEM, USA, and an 8900 ICP-MS system obtained from Agilent, USA.
Sample collection
A total of 120 green tea samples were collected from supermarkets (67 of 120), local markets (43 of 120), and planting link (10 of 120) in Hangzhou. They were planted in Xihu District (33 of 120), Yuhang District (26 of 120), Fuyang District (18 of 120), Linan District (13 of 120), Chunan County (12 of 120), Jiande city (9 of 120), and Tonglu County (9 of 120) of Hangzhou area. The experiment complied with relevant institutional, national, and international guidelines and legislation.
Pretreatment of samples
Samples of tea leaves (0.3 g, accurate to 0.0001 g) were placed in a microwave digestion tube and mixed with 5 mL HNO3. Two hours later, the capped tubes were moved to the microwave digestion instrument and heated as per the following program: step 1: heating time: 15 min, holding temperature 140 °C, holding time 2 min; step 2: heating time: 6 min, holding temperature 180 °C, holding time 30 min). Afterward, the samples were cooled to room temperature, and the solution was eluted with ultrapure water to 25.00 mL. The obtained solutions were analyzed on the quadrupole inductively coupled plasma-mass spectrometer (ICP-MS), and the concentrations of different metals in each solution were determined based on the calibration curve constructed using standard multi-element solutions of different concentrations. The element indium (In) (3 µg/L) was used as an internal standard. All instrument operating parameters are presented in Table 1.
Statistical analysis
As recommended by the World Health Organization22, all non-detected (ND) values were assumed to be anywhere between zero and the limit of detection (LOD), with zero being the lower bound (LB) and LOD being the upper bound (UB). middle bound (MB) is defined as LOD/2. Herein, the ND level of a heavy metal was considered to be 0 when more than 80% of results were less than the LOD, and it was considered to be LOD when more than 60% but less than 80% of results were less than the LOD.
The correlation between seven heavy metal elements was analyzed using the SPSS19.0 (https://spss.en.softonic.com/) statistical analysis software.
Evaluation method of heavy metal pollution level
The comprehensive pollution index, also known as the Numero integrative pollution index, is proposed by American scholar named Numero in his book《Scientific Analysis of River Pollution》. It is one of the most commonly used methods to calculate the comprehensive pollution index at home and abroad. The comprehensive pollution level of heavy metals in tea was evaluated by calculating the comprehensive pollution index (P), as per Eqs. (1) and (2)23:
where Pi is the single factor pollution index of heavy metal i in tea, Ci is the measured content of heavy metal i in tea (mg/kg), Si is the evaluation limit standard of heavy metal i, max is the maximum value, and ave is the average value. Table 2 summarizes the standard limit values of specific metals, and Table 3 shows comprehensive pollution index classification criteria.
Where C is the metal content (mg/kg), ED is the exposure duration, EF is the exposure frequency (365 days/year), FIR is the tea ingestion rate (g/person/day), TA is the average exposure time (EF × ED), and bw is the consumer body weight in kg. According to “world health statistics 2023: monitoring health for the SDGs, sustainable development goals”, life expectancy in China is 77.4 years (ED)26, and based on the fifth China total diet study performed by Wu et al.27, the citizens of Zhejiang Province consume 0.73 g of green tea per day. As for the mean weight of the Hangzhou citizens aged between 20 and 59 years (23,868 citizens), it is 64.36 kg, as per the Hangzhou Citizen Physical Fitness Test Data Analysis Report published in 201928. The calculated EDI values were compared with the established RfD values of the corresponding metals to assess the risk.
The hazard quotient (HQ) was used to assess long-term exposure to heavy metals, and it was calculated using the following formula29:
According to the EPA Integrated Risk Information Database (IRIS) and International The RfDs of Cu, As, V, Al, Mn, Sn, Hg, Cd, Ni, Cr, Ba, Li, Se and Sb are 0.04, 0.0003, 0.007, 1, 0.024, 0.6, 0.0003, 0.0018, 0.02, 1.5, 0.2, 0.002, 0.005 and 0.0004 mg/(kg day) respectively30,31,32,33,34,35,36,37,38,39; Tolerable daily intake (TDI) for Pb is 3.6 µg/(kg day)40,41. HQ values greater than 1 signify that the exposed consumers are unlikely to experience obvious toxic effects.
The risk assessment of cumulative exposure to the detected heavy metals was performed by calculating the hazard index (HI), which is equivalent to the sum of HQs of the heavy metals that a consumer is exposed to:
HI values greater than 1 indicate that green tea consumption poses a risk to human health.
Results and discussion
Method validation
To validate the analytical method applied in this study, the precision, accuracy, linearity, LOD, and uncertainty values were calculated for each element according to the according to the European Commission’s SANCO/3103/2000 guidelines42,43. For all analytes, the correlation coefficients (R) of the calibration curves were greater than 0.9995–0.9999, and the LOD values were between 0.0003 and 0.03 mg/kg. To test the feasibility of the method, heavy metals were added to the matrices. The accuracy of the method was validated using standard material for biocomposition analysis of green tea [GBW10052(GSS-30)], and the recoveries were between 86.4% and 97.5%.
Sample analysis
Previous studies indicate that when the levels of heavy metals in tea are very high, the absorption of these elements into the human body may cause poisoning, resulting in several health problems. Table 4 summarizes the concentrations of heavy metals in the tea samples analyzed herein. Of the 15 analytes, 11 have a detection rate of 100%. The detection rates of Hg, Sb, Se, and Sn are 69.2%, 98.3%, 93.3%, and 94.2%, respectively. The mean concentrations of heavy metals in tea decrease in the following order: Mn > Al > Ba > Cu > Ni > Cr > Pb > Sn > Li > V > Se > As > Cd > Sb > Hg, which agrees well with the data reported by Fatemeh Pourramezani44.
The mean concentration of heavy metals in green tea plant soil in west lake scenic area was in the following order: Zn > Cr > Ni > Pb > Cu > Co > As > Cd > Hg45, except Cu, the mean concentration trend of heavy metals in green tea plant soil and in green tea were similar. According to Peng et al.46, the mean concentration of Cu in Indian and Sri Lankan black tea is higher than those of Pb and Cd, and the concentrations of heavy metals decrease in the following order: Cu > Pb > As > Cd > Hg, which is consistent with our results. Why the mean concentration of Cu in tea plant soil was lower than Cr, Ni and Pb, but in tea it was higher than Cr, Ni and Pb. This maybe for the reason that the heavy metal enrichment capacity of soil-tea tree follows the following order: Cu > Cd > Zn > Ni > Hg > As > Cr > Pb47, the enrichment capacity of Cu is the highest, meanwhile, green tea contains Cu itself.
The elements with the highest and lowest concentrations detected in tea are Mn and Hg, and their concentrations range from 202.00 to 2010.00 mg/kg (mean of 836.00 mg/kg) and from ND (not detected) to 0.017 mg/kg (mean of 0.0033 mg/kg), respectively. Notably, none of the 120 samples analyzed herein show heavy metal levels exceeding the standard limit value (Table 2). Jia et al.48 studied the heavy metal pollution in different types of soil in Hangzhou city, the results revealed that the mean concentration of Mn was the highest in all types of soil studied.
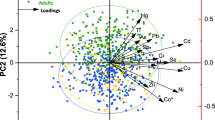
The correlation between the concentrations of seven heavy metals in green tea was assessed. Probability (p) results were in the bracket in Table 5, where p < 0.05 indicates correlation reached a significant level, and p < 0.01 indicates that correlation reached a highly significant level. The results demonstrate that Pb concentration is positively correlated with the concentrations of Cd, Cr, Mn, and As. Meanwhile, Cd has positive correlations with Hg and As; Cr with Mn and As; Hg with Mn and As; and Mn with As. The correlation analysis shows that with the exception of Cu, the heavy metal concentrations are correlated, and thus there may be synergy between these elements.
Heavy metal pollution level
The Numero integrative pollution index was adopted to evaluate the level of heavy metal pollution in tea samples. Considering that the single and comprehensive pollution index values listed in Table 6 are far below 0.7, the green tea produced in Hangzhou area is clean and safe to drink.
The calculated EDI, HQ, and HI values of the metals ingested into the human body through tea consumption are presented in Table 7. Among the investigated metals, Mn and Sn have the highest (0.40) and lowest (3.4 × 10− 6) HQ values, respectively. Considering that the HI value of the heavy metals (0.42) is well below 1, it is not likely that green tea consumption may pose a risk to human health.
Conclusions
The concentrations of heavy metals in green tea samples produced in Hangzhou were determined by ICP-MS. All the 120 samples did not exceed the standard limit value of heavy metal. The pollution level of heavy metals indicated that the single pollution index and comprehensive pollution index of green tea produced in Hangzhou area are far below 0.7, which belong to the clean and safety level. Health risk of inhabitant via consuming green tea was conducted and the HI value was 0.42, less than 1 indicate that green tea consumption are unlikely pose risk to human health.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request. All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors.
References
Das, S., de Oliveira, L. M., da Silva, E., Liu, Y. & Ma, L. Q. Fluoride concentrations in traditional and herbal teas: Health risk assessment. Environ. Pollut. 231, 779–784. https://doi.org/10.1016/j.envpol.2017.08.083 (2017).
Qin, F. & Chen, W. Lead and copper levels in tea samples marketed in Beijing, China. Bull. Environ Contam. Toxicol. 78(2), 118–121. https://doi.org/10.1007/s00128-007-9052-7 (2007).
Mehra, A. & Baker, C. Leaching and bioavailability of aluminium, copper and manganese from tea (Camellia sinensis). Food Chem. 100(4), 1456–1463. https://doi.org/10.1016/j.foodchem.2005.11.038 (2007).
Karimzadeh, L. et al. Evaluation of lead, cadmium and copper in black tea leaves in Mazandaran factories, spring and summer 2011. J. Mazandaran Univ. Med. Sci. 23(99), 2–10 (2013). https://api.semanticscholar.org/CorpusID:127040897
Miao, D. R., Li, X. & Yang, W. Q. Health risk assessment of Cu, Pb, Zn, Cd, Cr and as in tea leaves from Fengqing county in Yunnan J. Kunming Univ. 41(3), 56–60. https://doi.org/10.14091/j.cnki.kmxyxb.2019.03.012 (2019).
Yang, Y. F. & Jia, Y. Development of composite beverage of rose and black tea. J. Inner Mongolia Agri Univ. 32(4), 202–205 (2011).
Ni On the historical node of Chinese tea development. Fujiang Tea. 42(6), 320–321 (2020).
Salahinejad, M. & Aflaki, F. Toxic and essential mineral elements content of black tea leaves and their tea infusions consumed in Iran. Biol. Trace Elem. Res. 134, 109–117 https://doi.org/10.1007/s12011-009-8449-z (2010).
Seenivasan, S., Manikandan, N., Muraleedharan, N. N. & Selvasundaram, R. Heavy metal content of black teas from south India. Food Control. 19, 746–749. https://doi.org/10.1016/j.foodcont.2007.07.012 (2008).
Fernandez, P. L., Pablos, F., Martin, M. J. & Gonzalez, A. G. Multi-element analysis of tea beverages by inductively coupled plasma atomic emission spectrometry. Food Chem. 76, 483–489. https://doi.org/10.1016/S0308-8146(01)00312-0 (2002).
Matsuura, H., Hokura, A., Katsuki, F., Itoh, A. & Haraguchi, H. Multielement determination and speciation of major-to-trace elements in black tea leaves by ICP-AES and ICP-MS with the aid of size exclusion chromatography. Anal. Sci. 17, 391–398. https://doi.org/10.2116/analsci.17.391 (2001).
Fung, K. F., Zhang, Z. Q., Wong, J. W. C. & Wong, M. H. Fluoride contents in tea and soil from tea plantations and the release of fluoride into tea liquor during infusion. Environ. Pollut. 104, 197–205. https://doi.org/10.1016/S0269-7491(98)00187-0 (1999).
Karak, T. & Bhagat, R. M. Trace elements in tea leaves, made tea and tea infusion: a review. Food Res. Int. 43, 2234–2252. https://doi.org/10.1016/j.foodres.2010.08.010 (2010).
Nookabkaew, S., Rangkadilok, N. & Satayavivad, J. Determination of trace elements in herbal tea products and their infusions consumed in Thailand. J. Agricultural Food Chem. 54, 6939–6944. https://doi.org/10.1021/jf060571w (2006).
Marti-Cid, R., Llobet, J. M., Castell, V. & Domingo, J. L. Dietary intake of arsenic, cadmium, mercury, and lead by the population of Catalonia, Spain. Biol. Trace Elem. Res. 125, 120–132. https://doi.org/10.1007/s12011-008-8162-3 (2008).
Ferre-Huguet, N., Marti-Cid, R., Schuhmacher, M. & Domingo, J. L. Risk assessment of metals from consuming vegetables, fruits and rice grown on soils irrigated with waters of the Ebro River in Catalonia, Spain. Biol. Trace Elem. Res. 123, 1–14. https://doi.org/10.1007/s12011-008-8113-z (2008).
Martorell, I. et al. Human exposure to arsenic, cadmium, mercury, and lead from foods in Catalonia, Spain: temporal trend. Biol. Trace Elem. Res. 142, 309–322. https://doi.org/10.1007/s12011-010-8787-x (2011).
Sharma, R. K., Agarwal, M. & Marshall, F. M. Heavy metal (Cu, Zn, cd and pb) contamination of vegetables in urban India: a case study in Varanasi. Environ. Pollut. 154, 254–263. https://doi.org/10.1016/j.envpol.2007.10.010 (2008).
WHO. World Health Organization. Cadmium: Environmental Health Criteria, (Vol.134) (WHO, 1992).
Ma, M. H. & Li, X. H. Heavy metal contamination in the condiment raw materials. Jiangsu Seasoning non-staple food. 132(1), 38–41. https://doi.org/10.16782/j.cnki.32-1235/ts.2013.01.011 (2013).
Fu, Q. L. et al. A survey on the heavy metal contents in Chinese traditional egg products and their potential health risk assessment. Food Addit. Contaminants:Part B. 7(2), 99–105. https://doi.org/10.1080/19393210.2013.853106 (2014).
WHO. World Health Organization. Second Workshop on Reliable Evaluation of low-level Contamination of food (1995).
He, Y. S., Liao, X. J., Ni, Q., Wang, F. & Fu, X. L. Distribution characteristics and ebaluation of heavy metals in soils and vegetables of vegetable bases in Haikou city. Chin. J. Soil. Sci. 46(3), 721–727. https://doi.org/10.19336/j.cnki.trtb.2015.03.034 (2015).
Zheng, N., Wang, Q. & Zheng, D. Health risk of hg, pb, cd, zn, and Cu to the inhabitants around Huludao zinc plant in China via consumption of vegetables. Sci. Total Environ. 383, 81–89. https://doi.org/10.1016/j.scitotenv.2007.05.002 (2007).
Zhuang, P., McBride, M. B., Xia, H., Li, N. & Lia, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 407, 1551–1561. https://doi.org/10.1016/j.scitotenv.2008.10.061 (2009).
WHO. World Health Organization. World health statistics 2023: monitoring health for the SDGs, sustainable development goals, (2023).
Wu, Y. N., Zhao, Y. F. & Li, J. G. The Fifth China Total diet Study (The Science Press, 2017).
Hangzhou, C. Physical Fitness Test Data Analysis Report, (2019).
Cao, H., Qiao, L., Zhang, H. & Chen, J. Exposure and risk assessment for aluminium and heavy metals in Puerh Tea. Sci. Total Environ. 408(14), 2777–2784. https://doi.org/10.1016/j.scitotenv.2010.03.019 (2010).
Li, L., Fu, Q. L., Achal, V. & Liu, Y. A comparison of the potential health risk of aluminum and heavy metals in tea leaves and tea infusion of commercially available green tea in Jiangxi. China. Environ. Monit. Assess. 187(5), 228–232. https://doi.org/10.1007/s10661-015-4445-2 (2015).
Betha, R. et al. Chemical speciation of trace metals emitted from Indonesian peat fires for health risk assessment. Atmos. Res. 122, 571–578. https://doi.org/10.1016/j.atmosres.2018.01.020 (2013).
Gao, Y. et al. Characteristics of PM2.5 in Miyun, the northeastern suburb of Beijing: chemical composition and evaluation of health risk. Environ. Sci. Pollut. Res. 22(21), 16688–16699. https://doi.org/10.1007/s11356-015-4851-6 (2015).
Mei, L. & Wang, Q. Advances in using nanotechnology structuring approaches for improving food packaging. Annu. Rev. Food Sci. Technol. 11, 339–364. https://doi.org/10.1146/annurev-food-032519-051804 (2020).
Kraśniewska, K., Galus, S. & Gniewosz, M. Biopolymers-based materials containing silver nanoparticles as active packaging for food applications–a review. Int. J. Mol. Sci. 21(3), 698. https://doi.org/10.3390/ijms21030698 (2020).
EPA & U.S. Environmental Protection Agency. Chemical Assessment Summary National Center for Environmental Assessment, (1987).
EPA, U. S. & Environmental Protection Agency National Center for Environmental Assessment, (1991).
EPA & U.S. Environmental Protection Agency. Chemical Assessment Summary National Center for Environmental Assessment, (2005).
EPA & U.S. Environmental Protection Agency Chemical Assessment Summary National Center for Environmental Assessment., Provisional Peer Reviewed Toxicity Values for Lithium, (2008).
EPA & U.S. Environmental Protection Agency Chemical Assessment Summary National Center for Environmental Assessment., IRIS Toxicological Review of Hexavalent Chromium [Cr(VI)], (2024).
Barnes, D. G. et al. Reference dose (RFD)-description and use in health risk assessment. Regul. Toxicol. Pharmacol. 8(4), 471–486. https://doi.org/10.1016/0273-2300(88)90047-5 (1988).
EPA, J. & Philadelphia, P. A. United States Environmental Protection Agency, Washington DC, (2000).
European Union. Quality control procedure for pesticide residue analysis. Guidelines for residues monitoring in the European Union, 2nd ed. 1999/2000. SANCO/3103/2000. Prepared on behalf of the European Commission by Alan Hill (UK) and the organizing committee. http://europa.eu.int/comm/food/fs/ph_ps/pest/qualcontrol_en.pdf. Accessed 19 July 2015. (2000).
European Union. Opinion of the commission pursuant to article 251 (2), third subparagraph, point (c) of the EC Treaty, on the European Parliament’s amendments to the council’s common position regarding the proposal for a regulation of the European Parliament and of the council on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending council directive 91/414/EEC (COM/2003/117/F-2003/0052/COD). (2005). Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri¼CELEX%3A52005PC0022&from¼EN. Accessed 12 November 2016.
Pourramezani, F., Mohajeri, F. A., Salmani, M. H., Tafti, A. D. & Sadrabad, E. K. Evaluation of heavy metal concentration in imported black tea in Iran and consumer risk assessments. Food Sci. Nutr. 7, 4021–4026. https://doi.org/10.1002/fsn3.1267 (2019).
Zhang, Z. X. & Tao, X. M. Heavy metal content detection and pollution evaluation in green tea gardens around West Lake in Hangzhou. South. Forum. 53(8), 42–44 (2022).
Peng, C. Y. et al. Aluminum and heavy metal accumulation in tea leaves: an interplay of environmental and plant factors and an assessment of exposure risks to consumers. J. Food Sci. 83(4), 1165–1172. https://doi.org/10.1111/1750-3841.14093 (2018).
Zeng, Q. G., Peng, P. H., Luo, F., Wang, Y. & Hu, Y. W. Study on relation of geological background and high-quality tea producing in Qionglai mountains. Southwest. China J. Agricultural Sci. 23(06), 1969–1975. https://doi.org/10.16213/j.cnki.scjas.2010.06.058 (2010).
Jia, H. Q. & Zhang, M. K. Accumulation release potential and spatial variation of heavy metals in urban soils of Hangzhou City. J. Zhejiang Univ.. 33(6), 677–684. (2007).
Acknowledgements
This work was supported by Hangzhou science and technology development plan project (A20220609). The authors have declared no conflict of interest.We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by Hangzhou science and technology development plan project (A20220609).
Author information
Authors and Affiliations
Contributions
Wang S.T. and Fan J.C. wrote the main manuscript text, these authors have contributed equally to this work; Gong L. K.and Ren R. prepared tables; Jin Q. overall planning. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fan, J., Wang, S., Gong, L. et al. Occurrence, exposure and health risk assessment of heavy metals in green tea samples cultivated in Hangzhou area. Sci Rep 15, 19405 (2025). https://doi.org/10.1038/s41598-024-84287-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-84287-2
Keywords
This article is cited by
-
Elemental profile and health risk assessment of South African rooibos cultivated under ecological conditions
Journal of Radioanalytical and Nuclear Chemistry (2025)