Abstract
Preeclampsia leads to high fetal morbidity and pregnancy-induced mortality. However, the detailed molecular pathology of PE is currently unknown. shp1 has been shown to be critical to the pathogenesis of several diseases, but their role in PE requires further validation. In this study, TPI-1 administration significantly worsened PE mice resulting in impaired spiral artery remodelling. According to Western blot results, TPI-1 administration down-regulated the protein expression of SHP1 and up-regulated the protein expression of p-P38, p-Src. YAP, SP1, and JAG-1 in PE mice. In addition, Shp1 OE promoted Shp1 and p-Shp1 expression and inhibited SMCs cellular NICD, c-Myc, CyclinD1, MMP- through inhibition of trophoblast p-P38, SP1, PI3K, YAP, JAG1 protein expression as determined by in vitro trophoblast cell lines and smooth muscle cells cultured with trophoblast cell serum. 9, MMP-2 expression inhibited the proliferation and migration of SMCs cells. The P38 activator metformin Hcl inhibited the action of Shp1 OE. The SP1 activator plicamycin inhibited the action of metformin hydrochloride. The PI3K activator 740 Y-P inhibited the action of SP1 activator. The YAP inhibitor CA3 (CIL56) inhibited the action of the action of SP1 activators. In summary, SHP1 affects preeclampsia by inhibiting the expression of P38/SP1/PI3K/YAPxd proteins in trophoblast cells, which in turn regulates the protein expression of NICD, c-Myc, CyclinD1, MMP-9, MMP-2 in SMCs cells.
Similar content being viewed by others
Introduction
Preeclampsia (PE) is one of the leading causes of maternal complications and mortality globally, affecting about 5–8% of pregnant women each year and leading to more than 70,000 maternal deaths and 500,000 foetal deaths worldwide each year1,2. PE is characterised by new-onset hypertension, usually occurring after 20 weeks of gestation, and end-organ dysfunction1,2,3. Depending on the time of onset, PE can be classified as early-onset PE (before 34 weeks of gestation) and late-onset PE (after 34 weeks of gestation), which are considered to have different characteristics in terms of clinical presentation and pathological mechanisms4. In addition, preeclampsia has been associated with adverse neonatal outcomes, including respiratory distress syndrome, bronchopulmonary dysplasia, neurodevelopmental delay, and fetal or neonatal death5. Although the clinical manifestations and effects of PE have been extensively studied, the detailed molecular pathological mechanisms remain unclear. In recent years, several studies have revealed that multiple signalling pathways play key roles in the pathogenesis of PE, including the PI3K-Akt pathway, the Wnt/β-catenin pathway and the mTOR pathway6,7,8. These pathways play important roles in cell proliferation, migration and survival and may be potential therapeutic targets for PE.
Shp1 (Src homology domain 2 tyrosine phosphatase 1) is an important protein tyrosine phosphatase, which is widely found in a variety of cell types, and has important regulatory roles in a variety of cellular signalling pathways9,10,11. shp1 is involved in a variety of physiological and pathological processes including cell proliferation, differentiation, migration and apoptosis through the modulation of the phosphorylation status of its substrates12. The function and expression levels of Shp1 have been extensively studied in a variety of diseases, e.g. in autoimmune diseases, Shp1 has been suggested to be a key regulator in the maintenance of immune tolerance11; in tumours, inactivation of Shp1 is associated with proliferation and invasion of cancer cells9; and in cardiovascular diseases, alterations in the activity of Shp1 are closely associated with vascular inflammation and vascular remodelling13. Although the role of Shp1 in a variety of diseases has been clarified, the specific role in PE requires further study.
In view of this, in this study, we explored the mechanism of Shp1 in PE pathogenesis by using the Shp1 inhibitor TPI-1 and Shp1 overexpression (Shp1 OE) experiments, and deeply analysed the potential regulatory role of the P38/SP1/PI3K/YAP signaling pathway in it. Our results not only help to understand the molecular pathological mechanism of PE, but also may provide new ideas and targets for the treatment of PE.
Methods
Raw letter analysis
Pre-eclampsia related dataset GSE74341 downloaded from the GEO database contained gene expression data from 7 EOPE placental tissues and 5 control placental tissues, which were background corrected, normalised and corrected for batch effects, and then differentially expressed using the limma package, with the screening criteria of |log2 Fold Change|> 1 and a p value of < 0.05. Differentially expressed genes GO and Kyoto Encyclopedia of Genes and Genome (KEGG) pathway enrichment analyses were performed by the ClusterProfiler package, and correlations between gene variables were analysed using Spearman statistics14,15.
Construction of a PE mouse model
The procedures were in accordance with the ARRIVE guidelines (https://arriveguidelines.org). All procedures involving animals in this study were performed in following the institutional ethical guidelines. The animal experiments in this study have been approved by the Laboratory Animal Welfare Ethics Committee of Hebei North University, and the ethics batch number is HBNU2023022720147. C57BL/6 J mice (8 weeks old, weighing 18–20 g) were purchased from Henan Skibbes Biotechnology Co. Mice were reared without restriction of diet and water under pathogen-free conditions in a 12-h light–dark cycle at a fixed temperature of 25 °C. PE mice were modelled16 as previously described, and pregnancies were obtained by overnight allocation of females to fertile males in a ratio of 2:1, with the presence of a vaginal plug defined as a gestational day (GD) of 0.5. The experimental PE model was performed by daily subcutaneous infusion of L-NAME (40 mg/kg body weight) and L-NAME (1 mg/kg body weight). NAME (40 mg/kg body weight, N5751, Sigma Aldrich, Allentown, PA, USA) was induced in pregnant mice by daily subcutaneous infusion of L-NAME (40 mg/kg body weight, N5751, Sigma Aldrich, Allentown, PA, USA), ranging from GD 9.5 to GD 17.5. The TPI-1 group was treated with oral TPI-1 (3 mg/kg, daily, 5 d/wk) on top of the PE mice17.
Histological analysis
For histological assessment, HE and immunohistochemical analyses were performed. Tissues were fixed in 4% paraformaldehyde and subsequently embedded in paraffin. Then, 4 μm thick transects were processed for morphological analysis.
HE staining: the sections were stained by hematoxylin solution for 5 min, 1% hydrochloric acid alcohol differentiation, and eosin solution for 30 s before sealing and photographing the sections by light optical microscopy, respectively.
Regarding the immunohistochemical analysis, the sections were incubated with primary antibody CD31 (1:100, ab29364, Abcam) at 4 °C overnight. Subsequently, these sections were incubated with fluorescent secondary antibody. Cell nuclei were stained with DAPI (Invitrogen, Thermo Fisher Scientific, Shanghai, China). The sections were photographed using a fluorescence microscope (Olympus BX53, Tokyo, Japan). At least 5 random images from three samples were analysed per group. Immunohistochemical statistical analysis of optical density (OD) was performed.
Western blot
Tissues or cells were lysed by RIPA lysis buffer (Beyotime, Shanghai, China), proteins were isolated and quantified by BCA kit. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis at 120 V, followed by transferring the proteins to a PVDF membrane. The membranes were closed by 5% skimmed milk powder for 1 h and then primary antibodies were co-incubated with the membranes at 4 °C overnight. The membrane is then incubated with the HRP-coupled secondary antibody for 1 h at room temperature. Detection by exposure to chemiluminescence (ECL) kit (Solarbio, Beijing, China). Primary antibodies: p-P38 (1:1000, ab178867, Abcam), SP1 (1:1000, ab231778, Abcam), PI3K (1:1000, ab302958, Abcam), YAP (1:1000, ab2205270, Abcam), JAG1 (1:1000, ab109536, Abcam). ab109536, Abcam), NICD (1:1000, ab8925, Abcam), c-Myc (1:1000, ab32072, Abcam), CyclinD1 (1:10,000, ab134175, Abcam), MMP-9 (1:1000, ab76003, Abcam) MMP-2 (1:1000, ab181286, Abcam).
Cell culture
Human chorionic trophoblast cells HTR8/SVneo and Human Uterine Smooth Muscle Cells were purchased from Pricella(Wuhan, China) and maintained in standard culture conditions (37 °C, 5% CO2) using RPMI-1640 medium (Sigma-Aldrich, Merck KGaA, Germany) containing 5% foetal bovine serum (FBS), 100 Units/ml penicillin and 100 μM streptomycin. RPMI-1640 medium (Sigma-Aldrich, Merck KGaA, Germany) containing 5% foetal bovine serum (FBS), 100 units/ml penicillin and 100 μM streptomycin. To establish a PE trophoblast model, HTR-8/SVneo cells were cultured in hypoxic low-serum low-glucose conditions (1% O2) at 37 °C. Lipofectamine 3000 reagent (Invitrogen, Thermo Fisher Scientific, Shanghai, China) was used for HTR-8/SVneo transfection. For Shp1 overexpression, cells were infected with lentivirus (HANBIO Shanghai, China) and then selected with 1 μg/ml puromycin (Sigma-Aldrich, Merck KGaA, Germany) . Transfection efficiency was determined by Western blot. Regarding drug treatments: P38 activator metformin Hcl (0.01 mM)18 SP1 inhibitor Plicamycin (100 nM, catalogue no. HY-A0122, MedChemExpress, ShanghaI, China)19, PI3K activator 740 Y-P (20 μM, catalogue no. HY-P0175, MedChemExpress, Shanghai, China)20, YAP inhibitor CA3 (CIL56) (1 μmol/L, catalogue number: S8661, Selleck, ShanghaI, China)21.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde and then permeabilised using 0.3% Triton X-100. Cells were closed with 10% normal goat serum (Thermo Fisher Scientific, Shanghai, China) and later immersed in targeted primary antibody JAG1 (1:200, ab7771, Abcam). Cells were then incubated with species-appropriate fluorescent antibodies (AlexaFluor, Thermo Fisher Scientific, Shanghai, China) and nuclei were restained using 4′,6-diamidino-2-phenylindole (DAPI, Thermo Fisher Scientific, Shanghai, China). Cells were imaged using a Zeiss Axio fluorescence microscope.
Cell proliferation
Cell counting kit 8 (CCK-8) assay: cells were inoculated in 96-well plates at a density of 1 × 103/well. Cells were then pre-treated with each set of HTR-8/SVneo cell culture supernatant in fresh medium for 24 h. Cells were then incubated with CCK-8 solution (Selleck, Shanghai, China) in fresh medium at 37 °C for 90 min and the absorbance was measured at 450 nm using an enzyme marker (Bio-Rad, Hercules, CA, USA). colony formation assay: Cells (1 × 103) pre-treated with supernatant were inoculated in 6-well plates and incubated in complete medium. Colony formation assay: Cells (1 × 103) pre-treated with supernatant were inoculated into 6-well plates and cultured in complete medium for 2 weeks, fixed with 4% paraformaldehyde for 20 min and stained with 0.25% crystal violet for 30 min.
Transwell migration assay
SMCs cells were suspended in 200 μL of serum-free medium and inoculated into the upper chamber (8 μm pore size, 3422, Corning, USA) at a density of 5 × 104 cells per well. HTR-8/SVneo cell culture supernatant from each group was added to the upper and lower chambers. The plates were incubated at 37 °C and 5% CO2 for 24 h. Afterwards, cells were fixed with 4% paraformaldehyde for 20 min and stained with 0.2% crystal violet. A microscope was used to capture and count the number of cells present in the three randomised areas.
Statistics
Statistical analyses were performed using SPSS 26.0 software (IBM SPSS 26.0, SPSS Inc.). Statistical graphs were constructed using the GraphPad Prism 8 software package (GraphPad Software, CA, USA). The t-test was used to analyse normally distributed continuous variables. All values are expressed as SD ± mean. p < 0.05 was considered statistically significant.
Results
GO and KEGG enrichment analysis of differentially expressed genes in preeclampsia
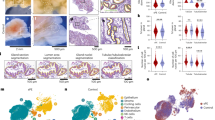
A total of 630 significant differentially expressed genes were screened, including 249 up-regulated genes and 381 down-regulated genes. The GO analysis revealed that these genes were differentially expressed in multiple cellular components (e.g., collagen-containing extracellular matrix, translation regulator complex and membrane microdomain) as well as molecular functions (e.g., signaling receptor activator activity, DNA-binding transcription factor binding and RNA microdomain). membrane microdomain) and molecular functions (e.g. signalling receptor activator activity, DNA-binding transcription factor binding and RNA polymerase II-specific DNA- binding transcription factor binding) showed significant enrichment. These results suggest that preeclampsia may be closely associated with remodelling of the extracellular matrix and alterations in transcriptional regulatory mechanisms. In terms of biological processes, DEGs were mainly enriched in processes related to response to changes in oxygen levels, such as response to oxygen levels, response to hypoxia and cellular response to decreased oxygen levels, suggesting that hypoxia may play an important role in the pathogenesis of pre-eclampsia. KEGG pathway analysis further revealed that DEGs were significantly enriched in PI3K-Akt, Wnt and mTOR signalling pathways, which have key roles in cell proliferation, migration and survival, and may provide us with new therapeutic targets. Spearman’s correlation analysis revealed a significant correlation between YAP and SP1, SP1 and SRC, YAP and JAG1, JAG1 and Notch. and JAG1 with Notch1, while SHP1 (PTPN6) showed a negative correlation with Src. These results suggest that transcription factors such as YAP and SP1 may play a mediating role in the pathogenesis of pre-eclampsia, and further functional experiments will help to elucidate the specific roles of these genes in pre-eclampsia (Fig. 1).
Raw letter analysis. (A) Sample-corrected box plots; (B) Sample differential PCA plots; (C) Differential gene volcano plots; (D) Differential gene sequencing plots; (E) GO enrichment analysis histograms; (F) KEGG enrichment analysis bubble plots; (G) KEGG enrichment analysis lollipop plots; (H) YAP and SP1 correlation scatterplots; (I) SP1 and SRC correlation scatterplots; (J) YAP and JAG1 correlation scatterplots; (K) JAG1 and Notch1 correlation scatterplot; (L) SHP1 (PTPN6) and Src correlation scatterplot.
TPI-1 affects uterine vascular remodelling via p-P38/p-Src/YAP/SP1/JAG1
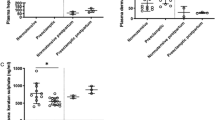
To investigate the effect of the SHP1 inhibitor TPI-1 on maternal arterial remodelling, uterine tissue staining was performed in PE mice and PE + TPI-1 mice to compare the lumen size of spiral arteries and replacement of trophoblast invasion and endothelial cells in mice. HE staining of tissue sections showed a reduction in the lumen size in PE + TPI-1 mice compared to PE mice (Fig. 2A).
TPI-1 affects uterine vascular remodelling via p-P38/p-Src/YAP/SP1/JAG1. (A) Hematoxylin and eosin (H&E) staining of mouse uterine tissue sections. (B) Staining of mouse uterine tissue sections with endothelial cell marker CD31. (C) Western blot detection of uterine tissues for p-Shp1, p-P38, p-Src, YAP, SP1 and JAG1.
In addition, the endothelial cell marker CD31, showed more intense staining in PE + TPI-1 mice compared to PE mice, indicating inadequate trophoblast invasion and poor remodelling (Fig. 2B). Western blot results showed decreased protein expression of p-Shp1 and significantly increased protein expression levels of p-P38, p-Src, YAP, SP1 and JAG1 in PE + TPI-1 mice compared to PE mice (Fig. 2C).
Shp1 regulates JAG1 by inhibiting P38 /SP1/ PI3K/YAP
Changes in cellular protein expression after intervention by hypoxia low serum low glucose, SHP1 overexpression, metformin Hcl, Plicamycin, 740 Y-P, CA3 (CIL56). An in vitro model of PE was constructed by hypoxia low serum low glucose. The protein expression of SHP1 and p-SHP1 in hypoxia low serum hypoglycemia was significantly lower than that in the NC group, and the protein expression of p-P38, SP1, p-PI3K, YAP and JAG1 was significantly higher than that in the NC group. Protein expression of Shp1 and p-SHP1 was increased and protein expression of p-P38, SP1, p-PI3K, YAP, and JAG1 was decreased in Shp1 OE cells transfected with the hypoxic low-serum low-glucose group cells. p-SHP1 protein expression was decreased in Shp1 OE cells transfected with hypoxic low-serum low-glucose group cells treated with the P38 agonist, metformin Hcl, and the protein expression of p -P38, SP1, p-PI3K, YAP and JAG1 protein expression was increased. protein expression of SP1, p-PI3K, YAP and JAG1 was decreased after treatment of hypoxic low serum low glucose + SHP1 OE + metformin Hcl with the SP1 inhibitor Plicamycin. Protein expression of p-PI3K, YAP and JAG1 was increased after treatment with the PI3K agonist 740 Y-P in hypoxic hypoxemic serum hypoglycaemia + SHP1 OE + metformin Hcl + plicamycin. protein expression of YAP and JAG1 was decreased after treatment with the YAP inhibitor CA3 (CIL56) (Fig. 3A). The results of immunofluorescence detection of JAG1 protein expression were consistent with the data in Fig. 3A, B
Shp1 affects cell proliferation by regulating NICD/c-Myc/ CyclinD1 expression in SMCs cells through inhibiting P38 /SP1/ PI3K/YAP in HTR-8/Svneo cells
NICD, c-Myc and CyclinD1 protein expression was significantly higher in the hypoxia low serum low glucose group than in the NC group. NICD, c-Myc and CyclinD1 protein expression was significantly reduced in hypoxic low serum low glucose group cells transfected with Shp1 OE. metformin Hcl treatment significantly increased NICD, c-Myc and CyclinD1 protein expression in hypoxic low serum low glucose group cells transfected with Shp1 OE cells. plicamycin treatment significantly increased NICD, c-Myc and CyclinD1 protein expression in hypoxic low serum low glucose + SHP1 OE + cells transfected with Plicamycin treatment. NICD, c-Myc and CyclinD1 protein expression was significantly reduced after SHP1 OE + metformin Hcl treatment.740 Y-P significantly increased NICD, c-Myc and CyclinD1 protein expression after hypoxic low serum low glucose + SHP1 OE + metformin Hcl + Plicamycin treatment.740 Y-P significantly increased NICD, c-Myc and CyclinD1 protein expression after CA3 (CIL56) treatment significantly decreased NICD, c-Myc and CyclinD1 protein expression (Fig. 4A).
Shp1 affects cell proliferation by regulating NICD/c-Myc/CyclinD1 expression in SMCs cells through inhibition of P38/SP1/ PI3K/YAP in SMCs cells. (A) Western blot for protein expression of NICD, c-Myc and CyclinD1 in SMCs cells. (B) CCK8 for cell viability of SMCs cells. c. Colony formation for cell proliferation of SMCs cells.
Cell proliferation of SMCs was analysed by CCK8 and colony formation assays. The results of CCK8 assay showed that cell viability increased after hypoxia low serum and low glucose, cell viability decreased after Shp1 OE, cell viability increased after metformin Hcl treatment, cell viability decreased after Plicamycin treatment, cell viability increased after 740 Y-P treatment, and cell viability decreased after CA3 (CIL56 ) treatment decreased cell viability (Fig. 4B). The results of colony formation assay also showed that hypoxic low serum oligosaccharides promoted cell proliferation. shp1 OE inhibited the effect of hypoxic low serum oligosaccharides, and metformin Hcl inhibited the inhibitory effect of shp1 OE and promoted cell proliferation. plicamycin inhibited metformin Hcl, which inhibited cell proliferation, and 740 Y-P inhibited the effect of metformin Hcl, which promotes cell proliferation. CA3 (CIL56) inhibits CA3 (CIL56) inhibits the action of 740 Y-P (Fig. 4C).
Shp1 affects cell migration by inhibiting P38/SP1/PI3K/YAP regulation of NICD/c-Myc/CyclinD1 expression in SMCs cells via inhibition of P38/SP1/PI3K/YAP in HTR-8/Svneo cells
MMP-9 and MMP-2 protein expression was significantly higher in the hypoxia low serum low glucose group than in the NC group. Expression of MMP-9 and MMP-2 proteins was significantly reduced in Shp1 OE cells transfected with hypoxic low-serum low-glucose group. metformin Hcl increased MMP-9 and MMP-2 protein expression in Shp1 OE cells transfected with hypoxic low-serum low-glucose group. plicamycin increased the expression of MMP-9 and MMP-2 proteins in Shp1 OE cells transfected with hypoxic low-serum low-glucose + Shp1 OE + metformin. MMP-9 and MMP-2 protein expression was significantly reduced after treatment with Plicamycin in hypoxic low serum oligosaccharide + SHP1 OE + metformin Hcl + Plicamycin.740 Y-P treatment significantly increased MMP-9 and MMP-2 protein expression in hypoxic low serum oligosaccharide + SHP1 OE + metformin Hcl + Plicamycin cells.740 Y-P treated MMP-9 and MMP-2 protein expression was significantly reduced by CA3 (CIL56) treatment (Fig. 5A).
SMCs cell q migration was analysed by Transwell. The results showed that hypoxia low serum hypoglycaemia promoted cell migration. shp1 OE inhibited hypoxia low serum hypoglycaemia. metformin Hcl inhibited the inhibitory effect of shp1 OE and promoted cell migration. plicamycin inhibited metformin Hcl and inhibited cell migration. 740 Y-P inhibited the effect of metformin Hcl and promotes cell migration. CA3 (CIL56) inhibits CA3 (CIL56) inhibits the action of 740 Y-P (Fig. 5B).
Discussion
Preeclampsia (PE) is a hypertensive disorder specific to pregnancy with a complex pathogenesis involving multiple factors including trophoblast dysfunction, poor vascular remodelling and inflammatory response22. Trophoblasts are important functional cells in the placenta and play a key role in maintaining placental function23. In patients with pre-eclampsia, dysfunction of trophoblast cells leads to poor remodelling of the uterine spiral arteries24. This process not only affects the blood flow supply at the maternal–fetal interface, but may also lead to placental dysplasia and fetal growth restriction.
During SpA remodelling, blood vessels lose their muscular elastic wall, which is replaced by a dense fibrin material containing intramural EVT, through a process involving the detachment and dedifferentiation (phenotypic switching) of vascular smooth muscle cells (VSMCs), as well as their eventual loss from the vascular wall through migration into the surrounding matrix and apoptosis25,26. In patients with preeclampsia, smooth muscle cell dysfunction may lead to poor vascular remodelling, further exacerbating gestational hypertension and other related complications. Lauren et al. demonstrated that preeclamptic mice exposed to postnatal hypertensive stimuli develop enhanced aortic stiffness, microvascular biotonia, and vasoconstriction, all of which were prevented in smooth muscle cell saline corticosteroid receptor (SMC-MR) knockout mice27. Jingyun Wang et al. demonstrated that alpha-2- macroglobulin (A2M) overexpression promotes the proliferation of human umbilical artery smooth muscle cells (HUASMCs) and inhibits apoptosis through TGFβ1 signalling, leading to remodelling of uterine spiral arteries and anomalous placental vascularisation28. Our results showed that NICD, c-Myc and CyclinD1 protein expression was significantly increased in smooth muscle cells under hypoxic low-serum low-glucose conditions, whereas the expression levels of these proteins were significantly reduced after Shp1 overexpression (Shp1 OE). This suggests that Shp1 overexpression inhibits smooth muscle cell proliferation under hypoxic conditions and contributes to improved vascular remodelling.
Oxygen is essential for the survival of most eukaryotes. In general, hypoxia puts severe stress on cells. There is growing evidence that hypoxia is a key factor in the pathogenesis of preeclampsia29,30. In hypoxic environments, intracellular levels of Reactive Oxygen Species (ROS), reactive molecules derived from O₂ molecules, are elevated; ROS are produced by a variety of sources such as the intracellular enzyme NADPH oxidase (NOX), xanthine oxidase, and others31. Normally, intracellular ROS are maintained at physiological levels to keep the antioxidant system in balance. Once there is an imbalance between ROS production and elimination, it accumulates and triggers oxidative stress. Oxidative stress is associated with numerous human diseases. In the pathology of preeclampsia, excessive ROS may be a causative factor for uteroplacental dysfunction and pregnancy complications32,33. Xiang-Qun Hu et al. demonstrated that ROS triggered vascular dysfunction in preeclampsia by mediating L-type voltage-gated Ca2 + (Cav1.2) in vascular smooth muscle cells34.
Oxidative stress affects cell function and fate by activating multiple signalling pathways, including the p38 mitogen-activated protein kinase (p38 MAPK) pathway35. Under normal conditions, p38 MAPK is relatively quiescent. During oxidative stress, changes in the cellular environment trigger a series of biochemical reactions that activate p38 MAPK by phosphorylation. The activated p38 MAPK can interact with the transcription factor SP1 to facilitate its translocation and localisation from the cytoplasm to the nucleus36. SP1, which enters the nucleus, binds to the YAP gene promoter region and recruits transcription-associated factors and enzymes to drive YAP gene transcription and translation, leading to YAP overexpression37. Overexpressed YAP binds to TEAD1 to form a complex, which binds to the SP1 gene promoter region to further promote SP1 expression, forming a positive feedback loop38. This loop amplifies the signal and significantly enhances YAP activity, which in turn affects vascular smooth muscle cell dysfunction and vascular homeostasis regulating intracellular gene expression and biological behaviours that drive disease progression39.
Overexpression of YAP not only directly regulates the transcription of multiple genes, but also affects cellular behaviour through a variety of mechanisms. Increased SP1 also promotes Src overexpression, in addition to oxidative stress that further promotes the phosphorylation of Src (p-Src)40, and Shp1 inhibits the activity of the Hippo signalling pathway by inhibiting the PI3K/AKT pathway41 (Hippo off)42. Inactivation of the Hippo signalling pathway reduces the phosphorylation of p-YAP and promotes the degradation of p-YAP, which in turn promotes the entry of YAP into the nucleus and enhances its transcriptional activity43.
After entering the nucleus, YAP can promote the transcription of JAG1, a Notch ligand44. The expression and release of JAG1 can activate the Notch-1 signaling pathway, which in turn promotes the proliferation and migration of smooth muscle cells. This process is critical for uterine vascular remodelling, but excessive activation may lead to poor vascular remodelling and further exacerbate pre-eclampsia. In addition to the effects in terms of vascular impairment, a study by Gudrun Meinhardt et al. demonstrated that YAP directly represses genes that promote trophoblast cell fusion, leading to failure of trophoblast cell differentiation in the human placenta resulting in severe pre-eclampsia and impaired intrauterine growth45.
Yuanhui Jia et al. demonstrated that Siglec-6 induced mitochondrial dysfunction by down-regulating SHP1 expression and recruiting SHP2, which promotes pre-eclampsia GPR20 expression, ultimately inducing a pre-eclampsia-like phenotype in pregnant mice46. To further validate the complexity of the Shp1 regulatory mechanism, we treated the cells with four different drugs to observe the changes in the expression of related proteins. Metformin Hcl: as a P38 MAPK agonist, treatment significantly increased the expression of p-P38, SP1, p-PI3K, YAP and JAG1. In addition Metformin Hcl co-administered SP1 inhibitor Plicamycin further inhibited the promotional effect of Metformin Hcl, resulting in a shift from elevated to decreased expression of the relevant proteins. p-PI3K agonist, 740 Y-P treatment significantly increased the expression of p-PI3K, YAP, and JAG1 inhibited the effect of Plicamycin. The YAP inhibitor CA3 (CIL56) significantly reduced the expression of YAP and JAG1, reversing the effects of 740 Y-P from elevated to significantly reduced protein expression.
In summary, Shp1, as an important negative regulator, can effectively reverse the dysfunction of trophoblast and smooth muscle cells and improve the remodelling of uterine vasculature in preeclampsia by inhibiting the activities of p38, SP1, PI3K, YAP and other signaling molecules. Therefore, intervention strategies targeting Shp1 and its downstream signalling pathways may provide new directions for the treatment of pre-eclampsia (Fig. 6). In addition, there is no doubt that the pathogenesis of preeclampsia (PE) still requires further in-depth studies in order to develop more targeted and efficient therapeutic interventions. In preclinical studies, using trophoblast cell lines and animal models of preeclampsia, the efficacy of Shp1 inhibitors can be initially assessed by detecting changes in blood pressure, urinary proteins, placental pathology, and signalling pathways, and multi-stage clinical trials can also be carried out, such as phase I trials to assess safety, and phase II trials to verify its effectiveness in improving blood pressure, urinary proteins, and maternal and infant complications in patients with moderate PE. We hope to provide a new direction for the treatment of pre-eclampsia.
Data availability
The datasets extracted and/or analysed during the current study are available in the Gene Expression Omnibus (GEO) repository (https://www.ncbi.nlm.nih.gov/geo/).
References
Rana, S., Lemoine, E., Granger, J. P. & Karumanchi, S. A. Preeclampsia: Pathophysiology, challenges, and perspectives. Circ. Res. 124(7), 1094–1112. https://doi.org/10.1161/circresaha.118.313276 (2019).
Ives, C. W., Sinkey, R., Rajapreyar, I., Tita, A. T. N. & Oparil, S. Preeclampsia-pathophysiology and clinical presentations: JACC state-of-the-art review. J. Am. Coll. Cardiol. 76(14), 1690–1702. https://doi.org/10.1016/j.jacc.2020.08.014 (2020).
ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol. 133(1), 1 (2019). https://doi.org/10.1097/aog.0000000000003018.
Lisonkova, S. & Joseph, K. S. Incidence of preeclampsia: Risk factors and outcomes associated with early- versus late-onset disease. Am. J. Obstet Gynecol. 209(6):544.e1–.e12 (2013). https://doi.org/10.1016/j.ajog.2013.08.019.
Backes, C. H. et al. Maternal preeclampsia and neonatal outcomes. J. Pregnancy. 2011, 214365. https://doi.org/10.1155/2011/214365 (2011).
Xu, Y. et al. ANXA4 promotes trophoblast invasion via the PI3K/Akt/eNOS pathway in preeclampsia. Am. J. Physiol. Cell Physiol. 316(4), C481–C491. https://doi.org/10.1152/ajpcell.00404.2018 (2019).
Zhang, Z. et al. Wnt/β-catenin signaling pathway in trophoblasts and abnormal activation in preeclampsia (Review). Mol. Med. Rep. 16(2), 1007–1013. https://doi.org/10.3892/mmr.2017.6718 (2017).
Tanaka, K. et al. Tadalafil treatment of mice with fetal growth restriction and preeclampsia improves placental mTOR signaling. Int. J. Mol. Sci. 23(3), 1474. https://doi.org/10.3390/ijms23031474 (2022).
Lim, S., Lee, K. W., Kim, J. Y. & Kim, K. D. Consideration of SHP-1 as a molecular target for tumor therapy. Int. J. Mol. Sci. 25(1), 331. https://doi.org/10.3390/ijms25010331 (2023).
Hao, F., Wang, C., Sholy, C., Cao, M. & Kang, X. Strategy for leukemia treatment targeting SHP-1,2 and SHIP. Front. Cell Dev. Biol. 9, 730400. https://doi.org/10.3389/fcell.2021.730400 (2021).
Sharma, Y., Bashir, S., Bhardwaj, P., Ahmad, A. & Khan, F. Protein tyrosine phosphatase SHP-1: Resurgence as new drug target for human autoimmune disorders. Immunol. Res. 64(4), 804–819. https://doi.org/10.1007/s12026-016-8805-y (2016).
Jiang, L. et al. SHP-1 regulates hematopoietic stem cell quiescence by coordinating TGF-β signaling. J. Exp. Med. 215(5), 1337–1347. https://doi.org/10.1084/jem.20171477 (2018).
Koch, E. et al. The endothelial tyrosine phosphatase SHP-1 plays an important role for vascular haemostasis in TNFα -induced inflammation in vivo. Mediators Inflamm. 2013, 279781. https://doi.org/10.1155/2013/279781 (2013).
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44(D1), D457–D462. https://doi.org/10.1093/nar/gkv1070 (2016).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28(1), 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Li, B. et al. Limosilactobacillus reuteri ameliorates preeclampsia in mice via improving gut dysbiosis and endothelial dysfunction. Biomed. Pharmacother. 161, 114429. https://doi.org/10.1016/j.biopha.2023.114429 (2023).
Kundu, S. et al. Novel SHP-1 inhibitors tyrosine phosphatase inhibitor-1 and analogs with preclinical anti-tumor activities as tolerated oral agents. J. Immunol. 184(11), 6529–6536. https://doi.org/10.4049/jimmunol.0903562 (2010).
Tarry-Adkins, J. L. et al. Impact of metformin treatment on human placental energy production and oxidative stress. Front Cell Dev. Biol. 10, 935403. https://doi.org/10.3389/fcell.2022.935403 (2022).
Deng, J. et al. Everolimus and plicamycin specifically target chemoresistant colorectal cancer cells of the CMS4 subtype. Cell Death Dis. 12(11), 978. https://doi.org/10.1038/s41419-021-04270-x (2021).
Lin, F. et al. Hydrogen sulfide protects against high glucose-induced human umbilical vein endothelial cell injury through activating PI3K/Akt/eNOS Pathway. Drug Des. Dev. Ther. 14, 621–633. https://doi.org/10.2147/dddt.S242521 (2020).
Zou, R. et al. Yes-associated protein promotes endothelial-to-mesenchymal transition of endothelial cells in choroidal neovascularization fibrosis. Int. J. Ophthalmol. 15(5), 701–710. https://doi.org/10.18240/ijo.2022.05.03 (2022).
Phipps, E., Prasanna, D., Brima, W. & Jim, B. Preeclampsia: Updates in pathogenesis, definitions, and guidelines. Clin. J. Am. Soc. Nephrol. 11(6), 1102–1113. https://doi.org/10.2215/cjn.12081115 (2016).
Zhou, W. et al. Trophoblast cell subtypes and dysfunction in the placenta of individuals with preeclampsia revealed by single-cell RNA sequencing. Mol. Cells 45(5), 317–328. https://doi.org/10.14348/molcells.2021.0211 (2022).
Hirano, H., Imai, Y. & Ito, H. Spiral artery of placenta: Development and pathology-immunohistochemical, microscopical, and electron-microscopic study. Kobe J. Med. Sci. 48(1–2), 13–23 (2002).
Harris, L. K. et al. Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a fas ligand-dependent mechanism. Am. J. Pathol. 169(5), 1863–1874. https://doi.org/10.2353/ajpath.2006.060265 (2006).
Zhang, J. Y. et al. Vitamin D promotes trophoblast cell induced separation of vascular smooth muscle cells in vascular remodeling via induction of G-CSF. Front Cell Dev. Biol. 8, 601043. https://doi.org/10.3389/fcell.2020.601043 (2020).
Biwer, L. A. et al. Smooth muscle mineralocorticoid receptor promotes hypertension after preeclampsia. Circ. Res. 132(6), 674–689. https://doi.org/10.1161/circresaha.122.321228 (2023).
Wang, J. et al. Alpha-2-macroglobulin is involved in the occurrence of early-onset pre-eclampsia via its negative impact on uterine spiral artery remodeling and placental angiogenesis. BMC Med. 21(1), 90. https://doi.org/10.1186/s12916-023-02807-9 (2023).
Hu, X. Q. & Zhang, L. Hypoxia and mitochondrial dysfunction in pregnancy complications. Antioxidants (Basel) 10(3), 405. https://doi.org/10.3390/antiox10030405 (2021).
Tong, W. & Giussani, D. A. Preeclampsia link to gestational hypoxia. J. Dev. Origins Health Dis. 10(3), 322–333. https://doi.org/10.1017/s204017441900014x (2019).
Zorov, D. B., Juhaszova, M. & Sollott, S. J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 94(3), 909–950. https://doi.org/10.1152/physrev.00026.2013 (2014).
Sun, Y. et al. PINK1-mediated mitophagy induction protects against preeclampsia by decreasing ROS and trophoblast pyroptosis. Placenta 143, 1–11. https://doi.org/10.1016/j.placenta.2023.09.010 (2023).
Hu, X. Q. & Zhang, L. Hypoxia and the integrated stress response promote pulmonary hypertension and preeclampsia: Implications in drug development. Drug Discov. Today. 26(11), 2754–2773. https://doi.org/10.1016/j.drudis.2021.07.011 (2021).
Hu, X. Q. & Zhang, L. Oxidative regulation of vascular Ca(v)1.2 channels triggers vascular dysfunction in hypertension-related disorders. Antioxidants (Basel) 11(12), 2432. https://doi.org/10.3390/antiox11122432 (2022).
Liu, J. et al. Chenodeoxycholic acid suppresses AML progression through promoting lipid peroxidation via ROS/p38 MAPK/DGAT1 pathway and inhibiting M2 macrophage polarization. Redox Biol. 56, 102452. https://doi.org/10.1016/j.redox.2022.102452 (2022).
Hu, H. et al. Elevated COX-2 expression promotes angiogenesis through EGFR/p38-MAPK/Sp1-dependent signalling in pancreatic cancer. Sci. Rep. 7(1), 470. https://doi.org/10.1038/s41598-017-00288-4 (2017).
Shi, Q. et al. GRP94 promotes anoikis resistance and peritoneal metastasis through YAP/TEAD1 pathway in gastric cancer. iScience 27(9), 110638. https://doi.org/10.1016/j.isci.2024.110638 (2024).
Huang, P. Y., Shih, I. A., Liao, Y. C., You, H. L. & Lee, M. J. A novel HDAC11 inhibitor potentiates the tumoricidal effects of cordycepin against malignant peripheral nerve sheath tumor through the Hippo signaling pathway. Am. J. Cancer Res. 12(2), 873–892 (2022).
Liu, H. et al. TGF-β/Smads signaling pathway, Hippo-YAP/TAZ signaling pathway, and VEGF: Their mechanisms and roles in vascular remodeling related diseases. Immun. Inflamm. Dis. 11(11), e1060. https://doi.org/10.1002/iid3.1060 (2023).
Gao, X. et al. Arsenic suppresses GDF1 expression via ROS-dependent downregulation of specificity protein 1. Environ. Pollut. 271, 116302. https://doi.org/10.1016/j.envpol.2020.116302 (2021).
Chi, D. et al. CLEC12B suppresses lung cancer progression by inducing SHP-1 expression and inactivating the PI3K/AKT signaling pathway. Exp. Cell Res. 409(2), 112914. https://doi.org/10.1016/j.yexcr.2021.112914 (2021).
Watkins, R. D. et al. SHP2 inhibition enhances Yes-associated protein-mediated liver regeneration in murine partial hepatectomy models. JCI Insight 7(15), e159930. https://doi.org/10.1172/jci.insight.159930 (2022).
Ibar, C. & Irvine, K. D. Integration of hippo-YAP signaling with metabolism. Dev. Cell 54(2), 256–267. https://doi.org/10.1016/j.devcel.2020.06.025 (2020).
Yang, Y. et al. Targeting Notch1-YAP circuit reprograms macrophage polarization and alleviates acute liver injury in mice. Cell Mol. Gastroenterol. Hepatol. 15(5), 1085–1104. https://doi.org/10.1016/j.jcmgh.2023.01.002 (2023).
Meinhardt, G. et al. Pivotal role of the transcriptional co-activator YAP in trophoblast stemness of the developing human placenta. Proc. Natl. Acad. Sci. USA 117(24), 13562–13570. https://doi.org/10.1073/pnas.2002630117 (2020).
Jia, Y. et al. Upregulation of Siglec-6 induces mitochondrial dysfunction by promoting GPR20 expression in early-onset preeclampsia. J. Transl. Med. 22(1), 674. https://doi.org/10.1186/s12967-024-05505-z (2024).
Acknowledgements
The authors thank all the participants of the present study for their contribution.
Funding
This study was funded by the Medical Science Research Project of Hebei (No. 20220634).
Author information
Authors and Affiliations
Contributions
Yan An and Chenyuan Cao contributed equally to this work and should be considered co-first authors. Both were involved in the conceptualization, methodology, investigation, data curation, and formal analysis. They also contributed to the writing of the original draft. Shaosong Sun contributed to the validation and supervision of the research. Hongli Wu and Jinzhi Zhang participated in the investigation and data curation. Rui Li contributed to the methodology and data analysis. Yakun Zhao played a significant role in the supervision, project administration, and funding acquisition. Yakun Zhao also reviewed and edited the manuscript extensively. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The animal experiments in this study have been approved by the Laboratory Animal Welfare Ethics Committee of Hebei North University, and the ethics batch number is HBNU2023022720147.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
An, Y., Cao, C., Sun, S. et al. SHP1 and its downstream p38/SP1/PI3K/YAP/Notch-1 signaling in trophoblast cells suppressed the progression of Preeclampsia via inhibiting proliferation of SMCs. Sci Rep 15, 16205 (2025). https://doi.org/10.1038/s41598-025-00164-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00164-6
Keywords
This article is cited by
-
Integrated bioinformatic identification and translational validation of key biomarkers and therapeutic candidates for preeclampsia-related acute kidney injury
Archives of Gynecology and Obstetrics (2026)
-
Zinc and animal health: an in-depth exploration of its role in physiological functions and regulatory molecular mechanisms
Journal of Animal Science and Biotechnology (2025)
-
Targeting SHP-1 to alleviate testicular inflammation and apoptosis in a Poly(I:C)-induced orchitis model
European Journal of Medical Research (2025)