Abstract
Postoperative infections are a significant challenge in orthopedic surgeries, particularly after procedures involving femoral shaft fractures treated with intramedullary nailing. Antibiotic-impregnated bone cement (AIBC) offers a promising solution by providing targeted antimicrobial delivery to the surgical site, potentially enhancing infection control and bone healing. A retrospective analysis was conducted from January 2020 to January 2023, involving 40 patients who developed postoperative infections after femoral shaft fracture treatment with intramedullary nailing. All patients underwent surgical debridement, removal of the original intramedullary nail, reaming of the medullary canal, and implantation of antibiotic-impregnated bone cement rods tailored with vancomycin. Patients were followed for 12 months to assess infection recurrence, bone healing, changes in inflammatory markers (white blood cell count, erythrocyte sedimentation rate, C-reactive protein, and procalcitonin), and functional outcomes including lower limb motor function and daily living activities. All 40 patients showed no signs of infection recurrence, achieving a 100% success rate in managing postoperative infections. Radiological assessments confirmed complete osseous union in all cases. Inflammatory markers significantly decreased post-surgery, indicating effective inflammation control. Significant improvements were also noted in motor functions and daily living activities, as measured by the Fugl-Meyer Assessment and the Activities of Daily Living scales. The use of antibiotic-impregnated bone cement in the treatment of postoperative infections following femoral shaft fracture treatment with intramedullary nailing is highly effective. AIBC not only prevents infection recurrence but also supports robust bone healing and functional recovery. This treatment approach holds promise for broader application in orthopedic surgery.
Similar content being viewed by others
Introduction
Femoral shaft fractures are a common and serious injury, often resulting from high-energy trauma such as motor vehicle accidents or falls from significant heights. These injuries frequently require surgical intervention to achieve optimal alignment and facilitate bone healing1. Intramedullary nailing, a well-established technique in orthopedic surgery, involves the insertion of a metal rod into the marrow canal of the femur to stabilize the bone2,3. While this method is effective for mechanical stabilization, it introduces certain risks, notably postoperative infections. Postoperative infections following femoral shaft fracture management with intramedullary nailing can have devastating consequences. Once an infection develops within the medullary cavity, it can severely impede fracture healing, prolong the duration of treatment, and necessitate additional surgeries4,5. These infections often lead to poor functional outcomes of the affected limb due to the invasive nature of the bacteria, which predominantly colonize along the implant, spreading throughout much of the medullary cavity. Complications such as localized infections around the fracture ends, formation of sinuses, and sequestration of bone (osteomyelitis) further complicate the clinical management, making treatment challenging and often resulting in long-term morbidity6,7.
Antibiotic-impregnated bone cement (AIBC) has emerged as a promising intervention to address these issues. AIBC not only serves as a physical spacer providing structural support but also delivers high local concentrations of antibiotics directly to the site of infection, which can be crucial in eradicating bacterial colonies that are otherwise difficult to reach with systemic antibiotic therapy. This targeted approach allows for sustained release of antibiotics, ensuring prolonged exposure at the site of infection, which is vital for preventing the development of resistant bacterial strains and enhancing the efficacy of the treatment8,9. The use of AIBC in the setting of femoral shaft fractures treated with intramedullary nailing has been the subject of ongoing research and debate. Clinical studies have demonstrated varied outcomes, with some showing significant reductions in the rate of postoperative infections, while others suggest only modest benefits. The effectiveness of AIBC depends on several factors, including the type and duration of antibiotic release, the antibiotic’s spectrum of activity, and the pathogen involved in the infection10,11,12. Furthermore, the physical properties of the cement itself can affect the mechanical stability of the fixation and the overall healing process. In our study, we specifically used an antibiotic-impregnated bone cement-coated pin implanted into the reamed intramedullary canal after removal of the original nail and thorough debridement. This technique provides both local antibiotic delivery and temporary mechanical support, functioning as a local antimicrobial spacer to manage infection and maintain canal patency during the healing process.
Given the severe impact of postoperative infections on patient outcomes, there is a critical need to further explore and optimize the use of antibiotic-impregnated bone cement in this context. This paper aims to examine the effectiveness of AIBC in treating postoperative infections following femoral shaft fractures managed with intramedary nailing.
Methods
Study design
An in-depth retrospective analysis was conducted at our healthcare facility to evaluate the effectiveness of AIBC in managing postoperative infections following femoral shaft fracture treatment with intramedullary nailing. The timeframe for this analysis extended from January 2020 to January 2023. During this period, 40 patients who received a comprehensive surgical and pharmacological treatment protocol—including implant removal, extensive debridement, medullary canal reaming, systemic antibiotics, and implantation of antibiotic-impregnated bone cement (AIBC)—were included in the study to evaluate the effectiveness of a combined treatment protocol—including debridement, nail removal, medullary canal reaming, and implantation of AIBC—in managing postoperative infections. Informed consent was secured from all subjects, ensuring they were adequately briefed about the study’s goals, methods, and potential consequences, in line with ethical standards. Informed consent was obtained from all subjects and/or their legal guardian(s). The study design, purpose, and procedures underwent rigorous scrutiny by the ethics committee of our institution. All procedures were executed following applicable guidelines and regulations. This study adhered to the ethical norms of the Declaration of Helsinki regarding medical research involving human subjects. We maintained confidentiality throughout the data handling process by anonymizing all personal identifiers before analysis to safeguard participant privacy.
Inclusion and exclusion criteria
Inclusion criteria
-
(1)
Age and Health: Patients aged 18 years or older, of any gender or ethnicity.
-
(2)
Diagnosis: Patients who have undergone femoral shaft fracture treatment with intramedullary nailing.
-
(3)
Postoperative Infection: Patients who developed a clinically diagnosed postoperative infection within six weeks following surgery.
-
(4)
Treatment with AIBC: Patients treated with antibiotic-impregnated bone cement for the postoperative infection.
-
(5)
Consent: Patients who provided written informed consent to participate in the study.
Exclusion criteria
-
(1)
Prior Leg Surgery: Patients with previous surgical interventions on the same femur, which could predispose them to altered surgical outcomes or infection risks.
-
(2)
Systemic Infections: Patients presenting with active systemic infections at the time of surgery.
-
(3)
Chronic Bone Diseases: Patients with chronic bone diseases such as osteoporosis or osteoarthritis that might interfere with the outcomes of the surgery or use of bone cement.
-
(4)
Allergy or Adverse Reactions: Patients with a known allergy or history of adverse reactions to the antibiotics used in the bone cement.
-
(5)
Immunocompromised Status: Patients with immunocompromised conditions, including those on chronic immunosuppressive therapy.
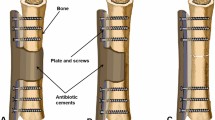
Protocol for medullary reaming and antibiotic-impregnated bone cement implantation therapy
The treatment strategy adopted in this study was multimodal, comprising surgical removal of infected hardware, aggressive debridement, medullary canal preparation, and local antibiotic delivery using AIBC.
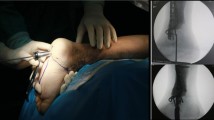
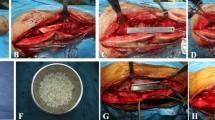
Following removal of the intramedullary nail, an effective management strategy for infected bone tissue is initiated by creating a cortical window at the distal femur or tibia to ensure adequate visualization and operative space. Subsequently, the medullary canal is reamed with a drill of slightly greater diameter than the removed nail, ensuring complete debridement of infected tissue. During reaming, the cavity is irrigated with 3.0–5.0 L of saline using a pulsatile lavage system to effectively remove debris and reduce the bacterial load. Tissue samples obtained from the infected sites are sent for bacterial culture and sensitivity testing, thereby guiding the selection of appropriate postoperative antibiotic therapy.
After debridement, the surgical field is re-sterilized and the instruments are replaced in preparation for further intervention. If preoperative antibiotic sensitivity testing results are available, the antibiotic to be implanted is selected based on these findings. In the absence of preoperative data, antibiotic-impregnated bone cement rods are fabricated by mixing 40.0 g of bone cement with 4.0 g of vancomycin until a non-sticky, paste-like consistency is achieved. This paste is then introduced into a custom mold that features dual semi-tubular grooves. A 3.5 mm Kirschner wire is inserted into one groove to provide reinforcement, while a 1.0 mm diameter wire is twisted into the other groove with its end extending beyond the mold. Once the paste is in place, both half-tubular grooves are closed until the bone cement hardens. Thereafter, the mold is opened and the formed bone cement rod is extracted; any residual irregular regions and excess cement are subsequently removed to obtain a smooth, uniform rod. The finalized antibiotic-impregnated bone cement rod is then implanted into the medullary canal.
Postoperative management involves the initial administration of intravenous antibiotics, guided by culture results, with a subsequent transition to oral antibiotics after three weeks. This regimen is designed to continue effective infection control, facilitate healing, and reduce the risk of reinfection, ultimately supporting the recovery of bone integrity.
Data collection and outcome measures evaluation
Twelve-Month Follow-Up for Bone Healing and Infection Recurrence: Patients will be monitored for a period of 12 months to assess bone healing and the recurrence of infection. Bone healing will be evaluated through radiological examinations indicating bridging at the fracture ends, callus formation, absence of or minimal tenderness, and the ability for independent movement, which are considered criteria for osseous union. The criteria for infection resolution include complete disappearance of symptoms and normalization of laboratory indices. Conversely, signs of infection recurrence include fever, redness, swelling, pain, sinus formation, and abnormal laboratory indices.
Measurement of Inflammatory Markers Before and Six Weeks After Treatment: Inflammatory markers will be assessed at baseline (pre-treatment) and six weeks post-treatment. The specific markers to be measured include white blood cell count (WBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and procalcitonin (PCT). These markers are pivotal for monitoring the inflammatory response and evaluating the effectiveness of the treatment in reducing systemic inflammation associated with infection.
Evaluation of Lower Limb Motor Function and Daily Living Activities Before and Twelve Months After Treatment: Lower limb motor function will be assessed using the Fugl-Meyer Assessment of Lower Extremity Motor Function and the Brunnstrom Approach Stroke Motor Function Assessment. Both scales provide a quantitative measure of motor function, with higher scores indicating better functionality. Additionally, the capability to perform daily living activities will be evaluated using the Activities of Daily Living (ADL) scales, which include the Physical Self-Maintenance Scale and the Instrumental Activities of Daily Living Scale. In these assessments, lower scores represent better performance and greater independence in daily activities. These evaluations will be conducted both before treatment and at the 12-month follow-up to assess improvements in motor function and daily life activities, providing insight into the long-term benefits of the treatment protocol.
Statistical analysis
Statistical evaluations were conducted utilizing SPSS software (Version 27.0) with a comprehensive approach to data categorization and analysis. Normality testing was performed to determine the distribution of the data. Quantitative datasets adhering to a normal distribution were analyzed using independent sample t-tests to evaluate inter-group differences, with results expressed as mean ± standard deviation. For quantitative data that did not follow a normal distribution, values were represented by the median and interquartile ranges (M[P25, P75]), and inter-group comparisons were made using the Mann–Whitney U test. Hypothesis testing was bi-directional, and statistical significance was determined by a p-value of less than 0.05.
Results
Participant demographics and clinical characteristics
The study encompassed a cohort of 40 patients, consisting of 23 males and 17 females. The age of participants ranged from 21 to 77 years, with a mean age of 43.36 ± 2.98 years. The etiology of injuries included 21 cases from traffic accidents, 6 from high falls, and 13 from slips or falls. Among these, 23 were open fractures and 17 were closed fractures. The body mass index (BMI) of patients varied from 19.25 to 28.56 kg/m2, with an average of 22.18 ± 1.28 kg/m2. The clinical classification of osteomyelitis according to the Cierny–Mader system was detailed as follows: 33 patients were categorized as Type A hosts (uncomplicated), while 7 patients, who had conditions such as hypertension, diabetes, or anemia, were classified as Type B hosts. In terms of anatomical classification, 22 cases were identified as Type I (medullary), 11 as Type I combined with Type III (localized), and 7 as Type I combined with Type IV (diffuse). Chronic sinus formation was noted in 19 cases (Table 1).
Analysis of bone healing and infection recurrence
This study monitored 40 patients over a 12-month period following surgery to evaluate the efficacy of a comprehensive treatment protocol for managing postoperative infections in femoral shaft fractures. Notably, none of the patients exhibited signs of infection recurrence during the follow-up period, reflecting a 100% infection control rate. This outcome underscores the effectiveness of a multimodal approach that included surgical debridement, hardware removal, systemic antibiotic therapy, and local antibiotic delivery via antibiotic-impregnated bone cement. The AIBC component played a critical adjunctive role in maintaining local antimicrobial activity and promoting a sterile environment within the medullary canal. Regarding bone healing, all patients achieved complete osseous union within 12 months, corresponding to a 100% bone healing rate. This finding suggests that the treatment strategy—particularly the local implantation of AIBC—did not interfere with the physiological bone regeneration process. Instead, it likely contributed to an environment conducive to bone consolidation and recovery.
Significant reductions in inflammatory markers post-surgery
Postoperative evaluation of inflammatory markers in a cohort of 40 patients revealed substantial reductions across all measured parameters, underscoring the effectiveness of the surgical intervention in mitigating inflammation. The WBC, a key indicator of systemic inflammation and immune response, demonstrated a significant decrease from a preoperative mean of 14.09, with a range of 11.70 to 16.66, to a postoperative mean of 8.36, ranging from 5.27 to 11.79. This shift suggests a robust resolution of acute inflammatory responses following surgery. Similarly, the ESR, which reflects longer-term inflammation status, showed a considerable reduction. Preoperatively, the mean ESR was 64.60, with values spanning from 39.39 to 89.76, which decreased to a postoperative mean of 26.36, with a range of 19.14 to 34.56. CRP, a sensitive and fast-responding biomarker for inflammation, also saw a dramatic decline. The preoperative mean was 104.75, ranging from 87.68 to 129.41, which fell to a postoperative mean of 11.62, with values between 4.95 and 21.84. This marked reduction highlights the effective management of the acute phase inflammatory response after surgical treatment. PCT, specifically useful for assessing the risk of bacterial infection, decreased from a preoperative mean of 1.81, with a range from 1.25 to 2.21, to a postoperative mean of 0.21, ranging from 0.11 to 0.34 (Table 2). This substantial drop indicates a significantly lowered risk of postoperative bacterial infections. These results collectively demonstrate that the multimodal treatment strategy—combining surgical and pharmacological interventions—was highly effective in reducing both the immediate and sustained markers of inflammation. The local antibiotic delivery via bone cement complemented the systemic antibiotic regimen and thorough debridement efforts (Fig. 1).
Significant improvements in lower limb motor functions and daily living activities
This study’s findings provide compelling evidence of significant improvements in lower limb motor functions and daily living activities after 12 months of targeted intervention. The results demonstrate marked enhancement in both functional mobility and the ability to perform daily activities, critical components for improving the quality of life in patients. The Fugl-Meyer Assessment, which evaluates the motor function of lower limbs, showed a noteworthy improvement from a pre-treatment mean score of 13.80 to 30.24 twelve months post-treatment. This increase indicates substantial gains in motor recovery, supporting the efficacy of the therapeutic approaches used. Similarly, the Brunnstrom Scale, used to measure the recovery stages in limb movement following neurological damage, reflected significant progress. Scores rose from an initial mean of 2.02–4.11, underscoring enhanced voluntary control over limb movements and a decrease in reflexive dependency. In terms of daily living activities, both the Physical Self-Maintenance Scale and the Instrumental Activities of Daily Living Scale recorded substantial improvements. The Physical Self-Maintenance Scale, which assesses basic self-care activities, showed a reduction in scores from 20.03 to 8.23, indicating a higher degree of independence in personal care tasks. Likewise, the Instrumental Activities of Daily Living Scale, which evaluates more complex daily activities, saw scores decrease from 30.67 to 9.32, reflecting improved functionality in managing day-to-day responsibilities (Table 3, Fig. 2).
Discussion
Postoperative infections remain a formidable challenge in orthopedic surgery, particularly following procedures such as intramedullary nailing for femoral shaft fractures. These infections can significantly hinder recovery, prolong hospital stays, and increase the need for additional surgeries, thereby escalating healthcare costs and impacting patient quality of life13,14. Traditional methods of systemic antibiotic administration often fall short in effectively managing these infections due to inadequate local drug concentrations and the potential for systemic toxicity. AIBC has emerged as a strategic innovation aimed at addressing these challenges. By delivering high concentrations of antibiotics directly to the site of surgery, AIBC provides targeted antimicrobial therapy without the systemic side effects associated with high-dose antibiotic regimens15,16. This localized delivery is particularly crucial in orthopedic applications, where blood supply may be compromised by the nature of the injury or the surgical intervention itself, rendering systemic antibiotics less effective.
The introduction of AIBC has revolutionized the approach to preventing and treating postoperative infections in orthopedic patients. Its use in femoral shaft fracture treatment with intramedullary nailing exemplifies a proactive measure against the colonization of surgical implants and the surrounding tissue by infectious agents, thus safeguarding the healing process and enhancing overall surgical outcomes. The findings of this study illuminate the substantial benefits of employing AIBC in the treatment of femoral shaft fractures, particularly with respect to preventing postoperative infections, facilitating bone healing, and reducing systemic inflammation17,18. These outcomes highlight the multifaceted role of AIBC in enhancing patient recovery post-surgery. The absence of infection recurrence across all patients in this study underscores the potent antibacterial properties of AIBC. The sustained release of antibiotics directly at the site of injury is likely responsible for this effect. AIBC acts as a local depot, continuously releasing antibiotics into the surrounding tissue, which maintains therapeutic levels of the drug without the systemic side effects often associated with high-dose intravenous antibiotic therapies. This localized, high-concentration delivery system targets pathogens more effectively and reduces the likelihood of developing antibiotic-resistant strains19. Moreover, the physical presence of the cement may serve as a barrier, preventing bacterial colonization along the implanted hardware.
The complete osseous union observed in all patients within 12 months post-surgery suggests that AIBC does not impede bone healing; rather, it may facilitate this process. The local antibiotic delivery might reduce the microbial burden and mitigate inflammatory responses that can otherwise impair bone regeneration and repair. Furthermore, the properties of the bone cement itself may provide structural support to the healing bone, effectively acting as a scaffold that encourages osteoconduction and potentially osteoinduction20,21. The integration of bone growth into the cement could further stabilize the fracture and enhance healing kinetics. The significant reductions in inflammatory markers post-surgery, including WBC, ESR, CRP, and PCT, indicate an effective control of both acute and chronic inflammatory responses. This reduction is likely multifactorial; primarily, the effective eradication of infection reduces the inflammatory burden22,23. Additionally, the use of AIBC may limit the local inflammatory response to the foreign body (i.e., the metal nail and the bone cement). By controlling infection and inflammation, AIBC minimizes the systemic inflammatory response, which is often a precursor to complications and slower recovery in orthopedic surgeries24,25.
The improvements in lower limb motor functions and daily living activities are particularly noteworthy. These enhancements may be partially attributed to the successful prevention of infection and effective management of inflammation, which can contribute to better overall outcomes in physical rehabilitation. The absence of infection allows for uninterrupted and more aggressive rehabilitation protocols, which are crucial for recovery of motor function following severe injuries like femoral shaft fractures26,27. Improved motor function, as assessed by the Fugl-Meyer and Brunnstrom scales, reflects successful neuromuscular recovery and adaptation post-injury. Similarly, improvements in daily living activities suggest that patients were able to regain independence and functionality, which are important factors in enhancing quality of life post-surgery.
The antibiotic-impregnated PMMA rod used in our study, though not designed for traditional mechanical fixation, may have provided supportive mechanical contributions during the healing process. Its presence within the medullary canal likely maintained the structural space, minimized dead space, and prevented hematoma formation—conditions that favor infection control and bone regeneration. Additionally, it may have offered limited internal support to facilitate early stabilization and biological responses post-debridement. While these mechanical effects were secondary, they should be considered as part of the overall therapeutic outcome. Further biomechanical research is recommended to better quantify these contributions. Previous studies have reported favorable outcomes using antibiotic cement-coated nails for the management of chronic post-traumatic osteomyelitis or infected nonunion of the femur and tibia, with success rates approaching 94–97% 28,29. However, these studies primarily focused on nonunion cases or chronic infections and often involved comparisons between custom-made and commercially available antibiotic-coated implants. In contrast, our study specifically targets early postoperative infections following intramedullary nailing for acute femoral shaft fractures, a clinical scenario with different pathophysiological characteristics, treatment goals, and timing of intervention. Moreover, we incorporated not only infection control and bone union, but also functional recovery and inflammatory marker trends over a 12-month follow-up. To our knowledge, few studies have comprehensively evaluated the role of AIBC as part of a combined surgical and antimicrobial protocol in this specific context. Our findings contribute to a more nuanced understanding of AIBC’s utility beyond chronic osteomyelitis, particularly in acute implant-associated infections in long bone fractures.
This study has several limitations that should be acknowledged. First, the relatively small sample size and the absence of a control group without AIBC limit the generalizability and strength of causal inferences. Second, being a single-center retrospective study, potential institutional biases in surgical technique and postoperative management cannot be excluded. Additionally, while no major complications were observed during the 12-month follow-up, the limited duration may have precluded detection of rare or delayed adverse events. Although minor postoperative symptoms such as transient pain and swelling were noted in a subset of patients and resolved conservatively, long-term surveillance is necessary to assess late-onset complications. From a material perspective, PMMA is non-biodegradable, and in certain clinical scenarios, retained cement may require secondary surgical removal due to mechanical irritation or foreign body response. Furthermore, variability in antibiotic selection, mixing technique, and elution kinetics may affect both therapeutic efficacy and safety. There is also a theoretical risk of bacterial resistance if subtherapeutic local antibiotic levels persist over time. Future large-scale, multicenter randomized controlled trials with extended follow-up are needed to confirm the efficacy of AIBC, optimize antibiotic formulations, and evaluate long-term outcomes, including resistance patterns and cement-related complications.
Conclusions
The combined treatment of medullary reaming and antibiotic-impregnated bone cement implantation following intramedullary nailing shows promising results. This approach effectively reduces inflammatory markers, suppresses inflammation, prevents infection recurrence, and enhances lower limb motor function and daily living activities. Its efficacy and benefits suggest that it is a valuable treatment strategy worth broader adoption in orthopedic surgery.
Data availability
The data sets generated and analyzed during this study are not public, but under reasonable requirements, the correspondence author can provide.
References
Nino, S., Courington, R., Brooks, P., Langford, J. & Haidukewych, G. Retrograde nailing for extremely proximal fractures of the femoral shaft. J. Orthop. Trauma 37(7), 346–350 (2023).
Poirot Seynaeve, B. et al. Intramedullary nailing of femoral shaft fractures: an analysis of rotational malunions using 3D EOS. Eur. J. Orthop. Surg. Traumatol. 34(4), 1893–1899 (2024).
Chen, W. et al. Minimally invasive treatment of displaced femoral shaft fractures with a rapid reductor and intramedullary nail fixation. Int. Orthop. 40(1), 167–172 (2016).
Panteli, M. et al. Subtrochanteric femoral fractures and intramedullary nailing complications: a comparison of two implants. J. Orthop. Traumatol. 23(1), 27 (2022).
Rathod, P. M., Kumar, P., Aggarwal, S., Rajnish, R. K. & Jindal, K. Is early intramedullary interlocked nailing an effective treatment option for open grade III femoral shaft fractures: A systematic review of literature and pooled analysis of 176 cases. Int. J. Burns Trauma 11(5), 357–364 (2021).
Tahir, M., Ahmed, N., Faraz, A., Shafiq, H. & Khan, M. N. Comparison of open and closed nailing for femoral shaft fractures: A retrospective analysis. Cureus 13(6), e16030 (2021).
Sadic, S. et al. Complications and functional recovery in treatment of femoral shaft fractures with unreamed intramedullary nailing. Med. Arch. 68(1), 30–33 (2014).
Inzana, J. A., Schwarz, E. M., Kates, S. L. & Awad, H. A. A novel murine model of established Staphylococcal bone infection in the presence of a fracture fixation plate to study therapies utilizing antibiotic-laden spacers after revision surgery. Bone 72, 128–136 (2015).
Zalikha, A. K., Sayeed, Z., Stine, S. A., Bray, R. & Vaidya, R. Antibiotic cement-coated interlocked intramedullary nails for the treatment of infected nonunion after intramedullary nailing. J. Orthop. Trauma 37(1), e1–e6 (2023).
Paley, D. & Herzenberg, J. E. Intramedullary infections treated with antibiotic cement rods: Preliminary results in nine cases. J. Orthop. Trauma 16(10), 723–729 (2002).
Solanki, T., Maurya, M. K. & Singh, P. K. Results of antibiotic-impregnated cement/polymer-coated intramedullary nails in the management of infected nonunion and open fractures of long bones. Cureus 15(8), e43421 (2023).
Lo Presti, M. et al. Küntscher nails with static cement spacer: A simple technique in periprosthetic knee infections with massive bone loss and instability. Knee 29, 580–588 (2021).
Walter, N. et al. Femoral shaft fractures in eldery patients: An epidemiological risk analysis of incidence, mortality and complications. Injury 54(7), 110822 (2023).
Tsai, M. C. et al. Reconstruction intramedullary nailing for ipsilateral femoral neck and shaft fractures: Main factors determining prognosis. Chang Gung Med. J. 32(5), 563–573 (2009).
Dall’Oca, C., Maluta, T., Moscolo, A., Lavini, F. & Bartolozzi, P. Cement augmentation of intertrochanteric fractures stabilised with intramedullary nailing. Injury 41(11), 1150–1155 (2010).
Cho, J. W. et al. Antibiotic coated hinged threaded rods in the treatment of infected nonunions and intramedullary long bone infections. Injury 49(10), 1912–1921 (2018).
Sharma, P. & Baghel, A. Outcome of intramedullary nail coated with antibiotic-impregnated cement in chronic osteomyelitis. Ann. Afr. Med. 22(4), 434–439 (2023).
Bu, Z. Y., Hu, L. J., Li, C. & Li, A. J. Clinical analysis of application of antibiotic bone cement spacer combined with membrane induction technology in treatment of osteomyelitis after femoral intramedullary nail operation: A case series. J. Pak. Med. Assoc. 70(2), 360–362 (2020).
Wasko, M. K. & Borens, O. Antibiotic cement nail for the treatment of posttraumatic intramedullary infections of the tibia: Midterm results in 10 cases. Injury 44(8), 1057–1060 (2013).
Qiang, Z. et al. Use of antibiotic cement rod to treat intramedullary infection after nailing: Preliminary study in 19 patients. Arch. Orthop. Trauma Surg. 127(10), 945–951 (2007).
Thonse, R. & Conway, J. Antibiotic cement-coated interlocking nail for the treatment of infected nonunions and segmental bone defects. J. Orthop. Trauma 21(4), 258–268 (2007).
Wang, G. et al. Custom-made antibiotic cement-coated nail for the treatment of infected bone defect. Biomed. Res. Int. 2021, 6693906 (2021).
Nizegorodcew, T., Palmieri, G. & Marzetti, E. Antibiotic-coated nails in orthopedic and trauma surgery: State of the art. Int. J. Immunopathol. Pharmacol. 24(1 Suppl 2), 125–128 (2011).
Steflik, M. J., Griswold, B. G., Patel, D. V., Blair, J. A. & Davis, J. M. Antibiotic cement-coated intramedullary nail is cost-effective for the initial treatment of GAIII open tibia fractures. Injury 53(10), 3471–3474 (2022).
Pradhan, C. et al. Can antibiotic impregnated cement nail achieve both infection control and bony union in infected diaphyseal femoral non-unions?. Injury 48(Suppl 2), S66-s71 (2017).
Shyam, A. K. et al. Use of antibiotic cement-impregnated intramedullary nail in treatment of infected non-union of long bones. Indian J. Orthop. 43(4), 396–402 (2009).
Dar, T. A. et al. Antibiotic impregnated cement coated ilizarov rod for the management of infected non union of long bone. Acta Orthop Belg 83(4), 521–526 (2017).
Garabano, G. et al. Antibiotic cement-coated rigid locked nails in infected femoral and tibial non-union. Reoperation rates of commercial versus custom-made nails. Injury 54(Suppl 6), 110650 (2023).
Garabano, G., Del Sel, H., Rodriguez, J. A., Perez Alamino, L. & Pesciallo, C. A. The effectiveness of antibiotic cement-coated nails in post-traumatic femoral and tibial osteomyelitis: Comparative analysis of custom-made versus commercially available nails. J. Bone Jt. Infect. 6(9), 457–466 (2021).
Acknowledgements
Thank you to all the staff involved in this study and to every patient who participated.
Funding
This research was supported by the Medical Science Research Project of Hebei (Project No. 20220691).
Author information
Authors and Affiliations
Contributions
Le-Cai Gao initiated the study and, together with Jia-Nan Chen, made substantial contributions to the literature review, data extraction, quality assessment, data analysis, and manuscript preparation. Additionally, Le-Cai Gao and Mu-Gang Li were instrumental in enhancing the article’s language, style, and protocol development. Mu-Gang Li also facilitated the analysis through constructive discussions. Finally, Fu-Bin Li meticulously revised the manuscript and provided the final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Hebei Cangzhou Hospital of Integrated Traditional Chinese Medicine and Western Medicine (2023-08-19). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants involved in the study.
Consent for publication
Written informed consent for publication was obtained from all patients included in this retrospective analysis.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, LC., Chen, JN., Li, MG. et al. Effectiveness of antibiotic-impregnated bone cement in treating postoperative infections after femoral shaft fracture treatment with intramedullary nailing. Sci Rep 15, 16992 (2025). https://doi.org/10.1038/s41598-025-00934-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00934-2