Abstract
Brain and central nervous system (CNS) cancers are a major cause of cancer-related mortality and morbidity among adolescents and young adults (AYAs) aged 15–39, presenting significant global health challenges despite advances in treatment. This study assesses the global burden and future trends of CNS cancers in AYAs using data from the Global Burden of Disease (GBD) 2021 database. Data on incidence, mortality, and disability-adjusted life years (DALYs) from 1990 to 2021 were analyzed for 204 countries and territories. Age-standardized rates for incidence (ASIR), mortality (ASMR), and DALYs (ASDR) were calculated, with temporal trends assessed using Joinpoint regression and future projections estimated using the Bayesian Age-Period-Cohort (BAPC) model. Disparities were evaluated using the Socio-Demographic Index (SDI), a composite measure of income, education, and fertility rates. In 2021, global CNS cancer incidence among AYAs was 57,645 cases, with a prevalence of 271,770. The ASIR was 1.92 per 100,000, the ASMR 0.95 per 100,000, and DALYs totaled 1,744,650. High-SDI regions reported higher ASIR but lower ASMR and ASDR. By 2040, case numbers are projected to rise, while age-standardized rates may stabilize or decline. This study highlights significant global disparities in CNS cancer burden, calling for investments in cancer registries, equitable healthcare access, and tailored prevention and treatment strategies.
Similar content being viewed by others
Introduction
Brain and central nervous system (CNS) cancers represent a significant and growing public health concern worldwide, particularly among adolescents and young adults (AYAs) aged 15–39 years1. These cancers, which include malignant neoplasms of the brain, meninges, spinal cord, and other CNS components, are often associated with high morbidity and mortality, leading to a substantial burden of disease. CNS cancers are the leading cause of cancer-related deaths in children and young adults, with unique biological, clinical, and psychosocial challenges that differentiate them from cancers in older populations2.
The Global Burden of Disease (GBD) study provides a comprehensive framework for understanding the incidence, mortality, and disability-adjusted life years (DALYs) associated with a wide range of diseases, including CNS cancers3. Using data from 204 countries and territories, the GBD 2021 database offers an opportunity to explore global trends in CNS cancer burden, assess disparities across socio-demographic contexts, and predict future trends. In particular, this study focuses on the AYA population, a demographic that is often underrepresented in cancer research, despite the distinct challenges they face in terms of diagnosis, treatment, and long-term outcomes. One reason for this underrepresentation is the transitional nature of the AYA age group, which falls between pediatric and adult oncology4. As a result, AYAs often do not fit neatly into either pediatric or adult cancer research frameworks, leading to gaps in data collection, research focus, and clinical guidelines. Additionally, while CNS cancers in children have been extensively studied due to their early onset and aggressive nature, and cancers in older adults are a focus due to their high prevalence, the lower overall incidence of CNS cancers in AYAs has historically resulted in less research and fewer clinical trials targeting this group5. This underrepresentation has significant implications, as AYAs face unique biological, psychosocial, and treatment-related challenges that differ markedly from those of younger or older patients. Addressing these gaps is critical for improving health equity and developing targeted interventions for this vulnerable population.
CNS cancers in AYAs are influenced by a complex interplay of genetic, environmental, and lifestyle factors. Genetic predisposition, such as familial cancer syndromes, plays a critical role in some cases, while environmental factors, including exposure to ionizing radiation and occupational hazards, are increasingly recognized as significant contributors to the rising incidence in certain regions6. Additionally, lifestyle factors, such as obesity, sedentary behavior, and dietary changes, may exacerbate the risk of developing CNS cancers, particularly in high-income countries undergoing rapid industrialization and urbanization7.
Despite advances in medical technology and treatment options, CNS cancers remain challenging to manage due to their location, complexity, and often aggressive nature. Early diagnosis is critical for improving survival rates, yet many low- and middle-income countries (LMICs) lack the necessary healthcare infrastructure for timely detection and treatment, leading to significant disparities in outcomes8. Furthermore, the long-term impacts of CNS cancers—ranging from cognitive impairment to physical disabilities—contribute to a heavy burden on patients, families, and healthcare systems9.
This study aims to provide a comprehensive analysis of the global burden of CNS cancers in AYAs using data from the GBD 2021 database. By examining incidence, mortality, and DALYs across different socio-demographic regions, we seek to identify key trends and disparities in CNS cancer burden. Additionally, the study utilizes the Bayesian Age-Period-Cohort (BAPC) model to predict future trends in CNS cancer incidence and mortality through 2040. Understanding these patterns is crucial for guiding public health strategies, improving early detection and treatment, and addressing the growing global burden of CNS cancers among AYAs.
Results
Global and National burden
In 2021, CNS cancers among AYAs aged 15–39 years posed a significant global health burden. The age-standardized incidence rate (ASIR) was 1.92 (95% uncertainty intervals (UI): 1.62–2.22) per 100,000 population, the age-standardized prevalence rate (ASPR) was 9.05 (UI: 7.75–10.45) per 100,000 population, the age-standardized mortality rate (ASMR) was 0.95 (UI: 0.79–1.12) per 100,000 population, and the age-standardized disability rate (ASDR) was 58.06 (UI: 48.42–68.81) per 100,000 population. These rates corresponded to approximately 57,645.1 (UI: 49,001.9–66,916.8) new cases globally, with a total prevalence of 271,770.1 (UI: 232,723.8–313,870.6). Mortality was estimated at 28,589.7 deaths (UI: 23,897.8–33,927.1), and DALYs reached 1,744,649.8 (UI: 1,455,357.1–2,068,272.4). Males exhibited slightly higher incidence rates (ASIR: 2.05, UI: 1.52–2.58) compared to females (ASIR: 1.78, UI: 1.54–2.06), as well as higher mortality and disability rates. Specifically, the estimated number of new cases for males was 31,243.0 (UI: 23,149.5–39,469.2), while females accounted for approximately 26,402.1 cases (UI: 22,855.5–30,604.1) (Table 1). The 21 regions defined within the GBD framework were also presented in the table, highlighting regional variations in the burden of CNS cancers.
Substantial disparities in CNS cancer burden were observed across countries and regions with varying levels of development. Countries with a High Socio-Demographic Index (SDI) exhibited the highest ASPR and ASIR, while High-middle SDI countries had the highest ASMR and ASDR. In contrast, Low SDI countries demonstrated the lowest burden across all metrics. Regionally, the High-income Asia Pacific had the highest ASPR, while Western Europe displayed the highest ASIR. Central Asia experienced the highest ASMR and ASDR, reflecting the severe health burden in this region (Supplementary Table S1).
At the national level, Monaco, San Marino, and Norway exhibited the highest ASIRs, while Gambia, Mali, and Nigeria had the lowest. The ASDR were highest in Turkmenistan, Tajikistan, and Georgia, and lowest in Gambia, Mali, and the Cook Islands. Temporal trends revealed that Ecuador, Turkmenistan, and Kyrgyzstan experienced the fastest increases in ASIR and ASDR, while Greenland, Luxembourg, and Fiji showed the most significant declines (Supplementary Tables S2 and S3).
Joinpoint regression analysis
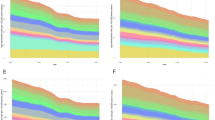
From 1990 to 2021, the global burden of CNS cancers demonstrated mixed trends in incidence, mortality, and disability rates. The ASIR increased overall, with an Average Annual Percentage Change (AAPC) of 0.5, particularly during 1990–1997 and 2012–2019. However, a slight decline was observed in recent years (2019–2021). The ASMR showed a steady decline (AAPC = − 0.29), with significant reductions during 2001–2013 and 2018–2021. This decline was more pronounced in males (AAPC = − 0.33) compared to females (AAPC = − 0.24), reflecting greater improvements in reducing mortality and disability rates among males. Similarly, the ASDR decreased slightly (AAPC = − 0.3), driven by reductions in 2001–2006 and 2018–2021. When stratified by sex, females exhibited a higher increase in incidence (AAPC = 0.55) compared to males (AAPC = 0.45). However, mortality and disability reductions were more pronounced in males, suggesting differential progress in treatment outcomes between sexes (Fig. 1, Supplementary Table S4).
Global trends in CNS cancer burden among AYAs from 1990 to 2021 using joinpoint regression analysis. This figure illustrates the ASIR, ASMR and ASDR of brain and CNS cancers among AYAs globally from 1990 to 2021. Joinpoint regression analysis was used to identify significant shifts in trends. A Global ASIR of CNS cancers for both sexes. B Global ASIR of CNS cancers for males. C Global ASIR of CNS cancers for females. D Global ASMR of CNS cancers for both sexes. E Global ASMR of CNS cancers for males. F Global ASMR of CNS cancers for females. G: Global ASDR of CNS cancers for both sexes. H Global ASDR of CNS cancers for males. I Global ASDR of CNS cancers for females.
Cross-national inequality
The analysis of 204 countries reveals significant absolute and relative inequalities related to SDI in the AYA population for CNS cancers. From 1990 to 2021, the Slope Index of Inequality (SII) for ASIR increased from 2.24 (95% CI: 1.85–2.62) to 2.90 (95% CI: 2.48–3.32), indicating a growing disparity in incidence rates between low- and high-SDI countries. In contrast, the SII for ASDR and ASMR showed a decline, with ASDR decreasing from 68.89 (95% CI: 56.16–81.62) to 53.20 (95% CI: 41.41-65.00), and ASMR falling from 1.12 (95% CI: 0.91–1.33) to 0.86 (95% CI: 0.66–1.05), suggesting improvements in reducing mortality and disability disparities, particularly in lower-SDI countries. The Concentration index (CI) for ASIR increased slightly, from 0.27 in 1990 to 0.31 in 2021, indicating that incidence has become more concentrated in high-SDI countries. Meanwhile, the CI for ASDR and ASMR remained stable at 0.16 from 1990 to 2021, reflecting that the burden of mortality and disability has not shifted significantly between SDI groups. These trends suggest ongoing improvements in cancer treatment globally (Fig. 2, Supplementary Fig. S1, Supplementary Fig. S2).
Global inequality in CNS cancer burden among AYAs from 1990 to 2021 using concentration index analysis. This figure presents the global inequality in the burden of CNS cancers among AYAs from 1990 to 2021, based on the SDI. Concentration indices are provided to quantify inequality in CNS cancer burden across countries with different SDI levels. A ASDR by SDI in 1990 and 2021. B Cumulative distribution of DALYs by SDI in 1990 and 2021. C ASIR by SDI in 1990 and 2021. D Cumulative distribution of CNS cancer incidence by SDI in 1990 and 2021. E ASMR by SDI in 1990 and 2021. F Cumulative distribution of CNS cancer mortality by SDI in 1990 and 2021.
SDI correlation analysis
From 1990 to 2021, a correlation analysis between SDI and CNS cancer burden (measured by ASIR, ASMR, and ASDR) revealed a strong positive relationship. Higher SDI was consistently associated with higher CNS cancer incidence, with a strong correlation for both the total population (R = 0.85) and across genders (R = 0.84 for males and R = 0.85 for females). Mortality rates also showed a moderate correlation with SDI (R = 0.58 for the total population, R = 0.61 for males, and R = 0.54 for females), while disability rates followed a similar trend with moderate correlations (R = 0.59, R = 0.61, and R = 0.55, respectively). However, some regions deviated from these trends. Sub-Saharan Africa and parts of South Asia exhibited lower CNS cancer burdens despite rising SDI, while Eastern Europe and Central Asia showed disproportionately higher burdens relative to their SDI levels, suggesting the influence of regional factors beyond socioeconomic development. Overall, the analysis highlights a global pattern of increasing CNS cancer burden with higher socioeconomic development, with notable regional exceptions (Fig. 3, Supplementary Fig. S3).
Correlation between SDI and CNS cancer burden in AYAs from 1990 to 2021. This figure demonstrates the correlation between the SDI and the ASIR, ASMR, and ASDR of CNS cancers among AYAs, with separate analyses for both sexes, males, and females. Pearson correlation coefficients (R) and p-values are provided to assess the strength and significance of the relationships. A Correlation between SDI and ASIR of CNS cancers for both sexes. B Correlation between SDI and ASIR of CNS cancers for males. C Correlation between SDI and ASIR of CNS cancers for females. D Correlation between SDI and ASMR of CNS cancers for both sexes. E Correlation between SDI and ASMR of CNS cancers for males. F Correlation between SDI and ASMR of CNS cancers for females. G Correlation between SDI and ASDR of CNS cancers for both sexes. H Correlation between SDI and ASDR of CNS cancers for males. I Correlation between SDI and ASDR of CNS cancers for females.
Bayesian Age-Period-Cohort model for prediction
Based on the BAPC model, the global burden of CNS cancers in AYAs is projected to rise by 2040, with the total number of prevalent cases expected to reach 306429.13 (UI: 136474.36-476383.88) and the annual number of new cases increasing slightly from 2021, reaching 61458.91 (UI: 28839.56-94078.27). Despite this rise in prevalence and incidence, the ASIR, ASMR, and ASDR are all projected to decline. By 2040, ASIR is expected to decrease to 1.81(UI: 0.91–2.71) per 100,000 population, ASMR to 0.80 (UI: 0.42–1.17) per 100,000 population, and ASDR to 49.16 (UI: 26.41–71.90) per 100,000 population. Notably, for males, these rates are projected to continue their steady decline over the next 20 years (Fig. 4, Supplementary Table S5, Supplementary Fig. S4).
Projected global trends in incidence, mortality, and DALYs for CNS cancers in AYAs, 1990–2040. This figure illustrates the observed and predicted trends in the age-standardized rates and total numbers for incidence, mortality, and DALYs of CNS cancers among AYAs. Predictions are based on the Bayesian Age-Period-Cohort (BAPC) model, forecasting trends through 2040. A ASDR for CNS cancers, including observed data from 1990 to 2021 and predicted trends through 2040. B Total number of DALYs due to CNS cancers from 1990 to 2040, with observed and projected data. C ASMR for CNS cancers, including observed rates from 1990 to 2021 and predicted rates through 2040. D Total number of deaths due to CNS cancers from 1990 to 2040, including both observed and predicted figures. E ASIR for CNS cancers, including observed data from 1990 to 2021 and projected trends through 2040. F Total number of new CNS cancer cases from 1990 to 2040, based on observed data and future projections.
Discussion
This study, through a comprehensive analysis of the GBD 2021 database, provides a multi-dimensional perspective on the global burden of the CNS cancers in AYAs. The burden of CNS cancers among AYAs is increasing globally, and by 2040, the number of cases is projected to rise significantly. Despite this increase in total global cases, ASIR, ASMR, and ASDR are expected to decline, reflecting advancements in medical technology and improvements in cancer diagnosis and treatment. However, from an etiological standpoint, the trends observed are driven by a complex interplay of factors that vary significantly across different countries and regions.
The pathogenesis of the CNS cancers is multifaceted, involving genetic, environmental, lifestyle, and immunological factors. One of the critical drivers of CNS cancer is genetic susceptibility and gene mutations. Familial cancer syndromes, such as Li-Fraumeni syndrome, neurofibromatosis, and retinoblastoma, significantly increase the risk of CNS tumors10. These syndromes are often associated with mutations in tumor suppressor genes, including TP53, NF1, NF2, and RB1, which play crucial roles in cell cycle regulation and DNA repair11. High-income countries have advanced genetic screening and mutation detection capabilities, allowing them to identify high-risk individuals at earlier stages, which may partly explain the higher incidence rates observed in these regions12. In contrast, low-income countries, lacking the necessary infrastructure for genetic testing, may underestimate the actual incidence of CNS cancers due to undetected genetic susceptibility13. Additionally, the interaction between genetic predisposition and environmental factors likely plays a significant role in CNS cancer development, particularly in more industrialized nations where environmental pollution may accelerate the carcinogenic process14. While this study explores several potential contributors to CNS cancer burden, it is worth noting that the GBD 2021 dataset does not include CNS-specific risk factor modeling. Factors such as genetic predisposition, environmental exposures (e.g., ionizing radiation, air pollution), and lifestyle changes remain underrepresented in current global estimates4. Future iterations of the GBD framework should incorporate these data to provide a more comprehensive understanding of the global burden of CNS cancers and their geographical variations.
Environmental exposure is another key factor contributing to CNS cancer risk, particularly ionizing radiation. Research has consistently shown that ionizing radiation is a well-established risk factor for brain tumors, and cancer survivors who have undergone radiation therapy are at increased risk of developing secondary CNS malignancies15. While high-income countries are equipped with advanced medical technologies, radiation therapy remains a significant contributor to secondary brain tumors16. Moreover, long-term exposure to air pollution, particularly fine particulate matter (PM2.5) and chemical pollutants (e.g., formaldehyde and benzene), has been linked to an increased risk of CNS cancers. The carcinogenic mechanism of air pollution likely involves chronic inflammation and oxidative stress, which affect the nervous system and may promote tumor development17. The industrialization and urbanization of high-income countries exacerbate environmental pollution, which could explain the rising incidence of CNS cancers in these regions. Occupational exposure to harmful substances, such as organic solvents, pesticides, and petrochemicals, further increases the risk of CNS cancers, particularly in highly industrialized areas18.
Viral infections and immune suppression are also closely related to the development of CNS cancers. Epstein-Barr virus (EBV) has been implicated in primary CNS lymphomas, and individuals with compromised immune function, such as those with HIV, are at a significantly higher risk of developing CNS tumors19,20. This association is particularly pronounced in sub-Saharan Africa, where the HIV/AIDS epidemic has led to increased immune suppression and, consequently, a higher burden of CNS cancers. The immune system plays a crucial role in recognizing and eliminating abnormal cells, including cancer cells. When immune function is impaired—whether due to HIV infection, organ transplantation, or long-term immunosuppressive therapy—the body’s ability to clear cancerous cells diminishes, thereby increasing the risk of CNS cancer21. In low-income countries, where medical resources are limited and HIV prevalence is high, the burden of immune-related CNS cancers is likely underreported22.
Changes in lifestyle, particularly in high-income countries, may also act as driving factors in CNS cancer incidence23. Obesity and metabolic syndrome have been associated with various cancers, and while the direct link between obesity and CNS cancer remains unclear, obesity may indirectly increase cancer risk through chronic low-grade inflammation, insulin resistance, and oxidative stress. Dietary changes, particularly the increased consumption of high-fat and high-sugar foods, may further exacerbate these metabolic disruptions, contributing to the risk of CNS cancers24. Additionally, sedentary lifestyles have been identified as an independent risk factor for cancer. Lack of physical activity leads to metabolic dysregulation, fat accumulation, and an increased risk of chronic diseases, which may indirectly affect CNS cancer development. Residents of high-income countries are more exposed to these lifestyle risks, which could explain the rising cancer burden in these regions.
Socioeconomic and cultural differences also significantly influence the burden of CNS cancers. Residents of high-income countries typically have better access to healthcare services, including early screening, precise diagnostics, and advanced treatment options, resulting in higher incidence rates but lower mortality25. In contrast, low-income countries, with limited healthcare resources, often diagnose patients at later stages, leading to higher mortality rates26. In these regions, a lack of awareness about cancer further exacerbates the burden. For instance, in some cultural contexts, cancer may be perceived as an incurable disease, leading patients to delay seeking medical help or refuse treatment altogether27,28. Gender disparities also play a role in the burden of CNS cancers. In certain cultures, women may not receive adequate medical attention, which can result in delayed diagnosis and treatment, further increasing their disease burden29.
According to the BAPC model, although the total number of CNS cancer cases is expected to continue rising in the future, incidence rates, mortality, and disability rates are projected to decline. This trend reflects the progress in medical technology and the strengthening of preventive measures. In high-income countries, the widespread application of precision medicine, genetic testing, immunotherapy, and targeted treatments will likely further improve CNS cancer outcomes. Future prevention strategies should focus on reducing environmental exposure, promoting healthy lifestyles, and enhancing the control of viral infections, particularly in low-income countries where additional technological support and resource allocation are needed to address the growing health inequities associated with CNS cancers30,31,32.
Despite the significant advantages of this study, several limitations must be considered. First, the cancer registration systems in some low-income countries are incomplete, leading to underreporting or inaccuracies in the data, which may underestimate the true burden of CNS cancers in these regions33. Second, this study does not differentiate between the various types of CNS cancers, which have distinct etiologies, prognoses, and treatment responses. Additionally, while the BAPC model provides valuable predictions for future trends, it is based on historical data and may not fully account for future social, economic, and technological changes. For instance, the broad adoption of emerging therapies, such as gene therapy or more advanced immunotherapies, could significantly alter CNS cancer treatment outcomes34. This study also did not explicitly adjust for potential confounders such as healthcare access or population growth, which could influence the observed patterns of CNS cancer burden. However, the GBD framework inherently accounts for some of these factors through its modeling processes (e.g., SDI as a composite index and DALYs adjusted for age structure), and future studies could incorporate more granular adjustments, such as the HAQ Index, to refine these analyses35. Finally, this study lacks a detailed analysis of individual risk factors, such as genetic predisposition, lifestyle, and environmental exposure. Future research should adopt a multidisciplinary approach, incorporating genetics, environmental science, and epidemiology to further explore the causes of CNS cancers, particularly the interaction between genetic susceptibility and environmental exposure.
In conclusion, this study fills a significant gap in the literature by offering a global, multi-dimensional analysis of the burden of CNS cancers and predicting future trends. However, the limitations related to data quality, lack of differentiation between CNS cancer types, and uncertainties in predictive modeling remain challenges. Further research should focus on improving data collection, refining the classification of CNS cancers, and enhancing etiological studies to better understand the global burden of CNS cancers and support the development of more targeted public health policies.
Methods
Data source
This descriptive epidemiological study is based on a secondary analysis of data from the GBD 2021 database, which provides standardized estimates of health outcomes globally. The GBD study integrates data from diverse sources, including vital registration systems, household surveys, hospital records, and disease registries, using advanced statistical modeling techniques to address data gaps and ensure comparability3. Key health metrics such as incidence, mortality, and DALYs are estimated through this rigorous process. The study focuses on malignant CNS cancers among AYAs aged 15–39 years across 204 countries and territories from 1990 to 2021. This age range was selected to capture the unique biological, clinical, and psychosocial characteristics of AYAs. CNS cancers were defined using ICD-10 codes C70 (malignant neoplasm of meninges), C71 (malignant neoplasm of brain), and C72 (malignant neoplasm of spinal cord, cranial nerves, and other parts of the CNS)36. Inclusion criteria were cases meeting these definitions with complete data on incidence, mortality, and DALYs for the 15–39 age group. Exclusion criteria included individuals outside this age range, non-CNS cancers, and countries or territories with incomplete or inconsistent data. By applying standardized definitions, age-standardization, and quality control, the GBD study ensures robust and comparable estimates across locations and time periods, forming the foundation for analyzing trends and disparities in CNS cancer burden.
Data standardization
Ensuring comparability across regions and populations required the use of age-standardized metrics, calculated using the World Health Organization Standard Population37. These metrics include the ASIR, ASPR, ASDR, and ASMR. Age standardization adjusts for differences in population age structures, allowing for meaningful comparisons over time and between regions. The calculations involved applying age-specific rates to the WHO standard population distribution, with population data sourced from the GBD 2021 dataset38.
Joinpoint regression analyss
Joinpoint regression analysis was used to assess trends in the incidence and mortality of CNS cancers in the 15-39-year-old population from 1990 to 2021. This method identifies points where significant changes in trend occur and calculates the Annual Percentage Change (APC) for each segment, reflecting the rate of change over time. The number of joinpoints was determined using permutation tests, with statistical significance set at p < 0.05. Furthermore, the AAPC was calculated to summarize the overall trend across the study period. The application of joinpoint regression allowed for the identification of distinct periods of increase or decrease in CNS cancer burden39.
Health inequality analysis
To quantify disparities in the burden of CNS cancers across countries, multiple measures of inequality were employed, including the SII, the Relative Index of Inequality (RII), and the CI. The SII represents the absolute difference in burden (e.g., age-standardized rates of incidence, mortality, or disability) between the highest and lowest ends of the SDI spectrum, calculated using a population-weighted ordinary least squares (OLS) regression model40. The RII captures relative inequality, estimating proportional disparities using a generalized linear model (GLM) with a log-link function, where the dependent variable is the natural logarithm of the age-standardized rate. Both indices were weighted by population size to ensure robust and unbiased estimates, with the SII reflecting absolute gradients and the RII representing rate ratios across the SDI continuum41. The CI was used to further assess inequalities by quantifying the extent to which a health outcome, such as incidence, mortality, or DALYs, is disproportionately concentrated among populations ranked by SDI. The CI ranges from − 1 to 1, where negative values indicate concentration of the burden in populations with lower SDI, positive values indicate concentration in higher SDI populations, and a value of 0 reflects perfect equality42. These complementary measures enabled a comprehensive evaluation of absolute and relative disparities, as well as the directional concentration of CNS cancer burden, providing a standardized framework to assess global health inequalities.
SDI correlation analysis
The SDI is a composite measure ranging from 0 to 1, based on total fertility rate, mean years of education among individuals aged 15 and older, and lag-distributed income per capita. In this study, SDI was treated as a continuous variable to assess its association with CNS cancer burden. For subgroup analysis, SDI was divided into five quintiles: low (0–0.35), low-middle (0.36–0.50), middle (0.51–0.65), high-middle (0.66–0.80), and high (0.81–1). These quintiles were used to explore disparities in disease burden across different levels of socio-demographic development30. A correlation analysis wasonducted to explore the relationship between SDI and the burden of brain and CNS cancers, using key metrics: ASIR, ASDR, and ASMR. The analysis included 204 countries and territories and 21 regions. Pearson’s correlation coefficients were calculated to assess the strength and direction of the relationship between SDI and CNS cancer burden. Positive values indicate a direct relationship, while negative values indicate an inverse relationship. The statistical significance was tested with a p-value < 0.05.
Bayesian Age-Period-Cohort model for prediction
We applied the BAPC model to analyze CNS cancer trends, which decomposes temporal variations into age, period, and cohort effects while addressing the identifiability problem of traditional APC models. Using Bayesian hierarchical methods, the model employs random walk priors for smooth transitions and hyperpriors to control variability, ensuring robust estimates even with sparse data43. To achieve computational efficiency, we used Integrated Nested Laplace Approximations (INLA), which provides accurate and scalable posterior inference. This framework allows for disentangling temporal effects, projecting future trends44, and quantifying uncertainty, making it a reliable approach for large-scale analyses like the GBD study.
Statistical software and statistical analysis
The following software tools were used for data extraction, analysis, and modeling: R (version 4.2.0): For data analysis, trend estimation, and BAPC modeling. Joinpoint Regression Program (Version 4.9.1.0) from the National Cancer Institute (NCI):.For joinpoint analysis. GBD Results Tool & GBD Compare Visualization Tool: For data extraction and visualization of CNS cancer burden metrics. Statistical significance was determined at a threshold of p < 0.05. For estimates derived from the GBD 2021 database, UIs were used to assess the precision of the estimates, with non-overlapping UIs considered indicative of statistically significant differences.
The GBD framework commonly adopts linear assumptions, particularly for inequality measures like SII and RII, which are based on linear gradients across population groups. The association between SDI and inequality measures is theoretically supported, as SDI typically exhibits a consistent linear relationship with health disparities in large-scale aggregated data45.
Data availability
The authors confirm that the datasets analyzed in this study are publicly available via the Global Health Data Exchange at https://vizhub.healthdata.org/gbd-results/ and https://ghdx.healthdata.org/ihme_data. This study is a secondary analysis of publicly accessible data from the Global Burden of Disease Study 2021. Processed data supporting the findings are provided in the article and supplementary materials.
References
Miller, K. D. et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 71, 381–406 (2021).
Miranda-Filho, A., Piñeros, M., Soerjomataram, I., Deltour, I. & Bray, F. Cancers of the brain and CNS: global patterns and trends in incidence. Neuro Oncol. 19, 270–280 (2017).
Murray, C. J. L. Findings from the global burden of disease study 2021. Lancet 403, 2259–2262 (2024).
Li, W. et al. Global cancer statistics for adolescents and young adults: population based study. J. Hematol. Oncol. 17, 99 (2024).
Ferrari, A. et al. Adolescents and young adults (AYA) with cancer: a position paper from the AYA working group of the European society for medical oncology (ESMO) and the European society for paediatric oncology (SIOPE). ESMO Open. 6, 100096 (2021).
Toss, A. et al. Cancer predisposition genes in adolescents and young adults (AYAs): a review paper from the Italian AYA working group. Curr. Oncol. Rep. 24, 843–860 (2022).
Liu, X. et al. Associations between adiposity, diabetes, lifestyle factors and the risk of gliomas. Front. Med. (Lausanne). 10, 1207223 (2023).
Suh, J. H. et al. Current approaches to the management of brain metastases. Nat. Rev. Clin. Oncol. 17, 279–299 (2020).
Vizer, L. M., Mikles, S. P. & Piepmeier, A. T. Cancer-related cognitive impairment in survivors of adolescent and young adult non-central nervous system cancer: A scoping review. Psychooncology 31, 1275–1285 (2022).
Wang, C. et al. Genetic and clinical characteristics of genetic tumor syndromes in the central nervous system cancers: implications for clinical practice. iScience 27, 111073 (2024).
Wang, H., Guo, M., Wei, H. & Chen, Y. Targeting p53 pathways: mechanisms, structures, and advances in therapy. Signal. Transduct. Target. Ther. 8, 92 (2023).
Ginsburg, O., Ashton-Prolla, P., Cantor, A., Mariosa, D. & Brennan, P. The role of genomics in global cancer prevention. Nat. Rev. Clin. Oncol. 18, 116–128 (2021).
Anandasabapathy, S., Asirwa, C., Grover, S. & Mungo, C. Cancer burden in low-income and middle-income countries. Nat. Rev. Cancer. 24, 167–170 (2024).
Carbone, M. et al. Tumour predisposition and cancer syndromes as models to study gene-environment interactions. Nat. Rev. Cancer. 20, 533–549 (2020).
Braganza, M. Z. et al. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol. 14, 1316–1324 (2012).
Fujii, M. et al. Secondary brain tumors after cranial radiation therapy: A single-institution study. Rep. Pract. Oncol. Radiother. 25, 245–249 (2020).
Hvidtfeldt, U. A. et al. Long-term air pollution exposure and malignant intracranial tumours of the central nervous system: a pooled analysis of six European cohorts. Br. J. Cancer. 129, 656–664 (2023).
Huang, J. et al. Disease burden, risk factors, and trends of primary central nervous system (CNS) cancer: A global study of registries data. Neuro Oncol. 25, 995–1005 (2023).
Lurain, K. et al. Treatment of HIV-associated primary CNS lymphoma with antiretroviral therapy, rituximab, and high-dose methotrexate. Blood 136, 2229–2232 (2020).
Gandhi, M. K. et al. EBV-associated primary CNS lymphoma occurring after immunosuppression is a distinct Immunobiological entity. Blood 137, 1468–1477 (2021).
Desland, F. A. & Hormigo, A. The CNS and the brain tumor microenvironment: implications for glioblastoma immunotherapy. Int. J. Mol. Sci. 21, 7358 (2020).
Montaño, M. A. et al. Impact of antiretroviral therapy on Cancer treatment outcomes among people living with HIV in Low- and Middle-Income countries: a systematic review. Curr. HIV/AIDS Rep. 18, 105–116 (2021).
Zhang, Y. B. et al. Combined lifestyle factors, incident cancer, and cancer mortality: a systematic review and meta-analysis of prospective cohort studies. Br. J. Cancer. 122, 1085–1093 (2020).
Sergentanis, T. N. et al. Obesity and risk for brain/cns tumors, gliomas and meningiomas: A Meta-Analysis. PLoS One. 10, e0136974 (2015).
Fan, Y. et al. Burden and trends of brain and central nervous system cancer from 1990 to 2019 at the global, regional, and country levels. Arch. Public. Health. 80, 209 (2022).
Bamodu, O. A. & Chung, C. C. Cancer care disparities: overcoming barriers to Cancer control in Low- and Middle-Income countries. JCO Glob Oncol. 10, e2300439 (2024).
Malope, S. D., Norris, S. A. & Joffe, M. Culture, community, and cancer: Understandings of breast cancer from a non-lived experience among women living in Soweto. BMC Womens Health. 24, 594 (2024).
Afaya, A. et al. Socio-cultural beliefs and perceptions influencing diagnosis and treatment of breast cancer among women in Ghana: a systematic review. BMC Womens Health. 24, 288 (2024).
Brach, C. & Fraser, I. Reducing disparities through culturally competent health care: an analysis of the business case. Qual. Manag Health Care. 10, 15–28 (2002).
Kassebaum, N. J. et al. Global, regional, and National disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet 388, 1603–1658 (2016).
Kuehn, J. C. et al. Comprehensive genetic profiling and molecularly guided treatment for patients with primary CNS tumors. NPJ Precis Oncol. 8, 180 (2024).
Fountzilas, E., Tsimberidou, A. M., Vo, H. H. & Kurzrock, R. Clinical trial design in the era of precision medicine. Genome Med. 14, 101 (2022).
Jena, S., Sahoo, K. C., Samantaray, K., Satpathy, N. & Epari, V. Operational feasibility of Hospital-Based Cancer registries in Low- and Middle-Income countries: A systematic review. Cureus 15, e42126 (2023).
Trächsel, B., Rousson, V., Bulliard, J. L. & Locatelli, I. Comparison of statistical models to predict age-standardized cancer incidence in Switzerland. Biom J. 65, e2200046 (2023).
GBD 2015 Healthcare Access and Quality Collaborators. Electronic address: Cjlm@uw.edu & GBD 2015 healthcare access and quality collaborators. healthcare access and quality index based on mortality from causes amenable to personal health care in 195 countries and territories, 1990–2015: a novel analysis from the global burden of disease study 2015. Lancet 390, 231–266 (2017).
Liu, X., Cheng, L. C., Gao, T. Y., Luo, J. & Zhang, C. The burden of brain and central nervous system cancers in Asia from 1990 to 2019 and its predicted level in the next twenty-five years: burden and prediction model of CNS cancers in Asia. BMC Public. Health. 23, 2522 (2023).
Chen, W. Q., Zeng, H. M., Zheng, R. S., Zhang, S. W. & He, J. Cancer incidence and mortality in China, 2007. Chin. J. Cancer Res. 24, 1–8 (2012).
Talbot, D., Duchesne, T., Brisson, J. & Vandal, N. Variance Estimation and confidence intervals for the standardized mortality ratio with application to the assessment of a cancer screening program. Stat. Med. 30, 3024–3037 (2011).
Kim, H. J., Fay, M. P., Feuer, E. J. & Midthune, D. N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 19, 335–351 (2000).
Mackenbach, J. P. & Kunst, A. E. Measuring the magnitude of socio-economic inequalities in health: an overview of available measures illustrated with two examples from Europe. Soc. Sci. Med. 44, 757–771 (1997).
Regidor, E. Measures of health inequalities: part 2. J. Epidemiol. Community Health. 58, 900–903 (2004).
O’Donnell, O., O’Neill, S., Van Ourti, T. & Walsh, B. Conindex: Estimation of concentration indices. Stata J. 16, 112–138 (2016).
Riebler, A. & Held, L. Projecting the future burden of cancer: bayesian age-period-cohort analysis with integrated nested Laplace approximations. Biom J. 59, 531–549 (2017).
Paul, M., Riebler, A., Bachmann, L. M., Rue, H. & Held, L. Bayesian bivariate meta-analysis of diagnostic test studies using integrated nested Laplace approximations. Stat. Med. 29, 1325–1339 (2010).
GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 396, 1223–1249 (2020).
Acknowledgements
We sincerely thank the collaborators of the Global Burden of Diseases, Injuries, and Risk Factors Study 2021 for their outstanding efforts. Special thanks to Jieni Wu for her insightful suggestions on the data formatting in this manuscript.
Funding
This work was supported by grants from the 2021 Basic Research Plan of Shanxi Province (Grant No. 202103021224386 and 20210302124380) and the 2024 Graduate Education Innovation Program of the Shanxi Provincial Department of Education (Grant No. 2024SJ177 and 2024SJ178 ).
Author information
Authors and Affiliations
Contributions
Conceptualization: Bin Huangfu, Xuanchen Liu; Methodology: Xuanchen Liu, Xiao Wang, Xiaochen Niu; Data Collection: Chunhong Wang; Data Curation: Rui Cheng; Data Analysis: Xuanchen Liu, Hongming Ji; Statistical Analysis: Xuanchen Liu, Xiaochen Niu; Investigation: Bin Huangfu; Formal Analysis: Bin Huangfu, Rui Cheng; Validation: Xiao Wang; Visualization: Xuanchen Liu; Resources: Xiaochen Niu; Data Interpretation: Chunhong Wang; Project Administration: Bin Huangfu; Supervision: Hongming Ji, Chunhong Wang; Funding Acquisition: Xuanchen Liu, Xiao Wang, Chunhong Wang, Hongming Ji; Writing—Original Draft: Xuanchen Liu, Bin Huangfu; Writing—Review & Editing: Bin Huangfu, Xuanchen Liu, Xiao Wang, Xiaochen Niu, Chunhong Wang, Rui Cheng, Hongming Ji.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
This study used anonymized, aggregated data from the Global Burden of Disease 2021 database, managed by the Institute for Health Metrics and Evaluation. As the data contains no personally identifiable information, the study qualifies for exemption from full ethical review based on guidelines from the Shanxi Provincial People’s Hospital Ethics Committee and the University of Washington’s IRB, which oversees the GBD project.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huangfu, B., Liu, X., Wang, X. et al. Global trends and burden of brain and central nervous system cancers in adolescents and young adults GBD 2021 study. Sci Rep 15, 17049 (2025). https://doi.org/10.1038/s41598-025-01368-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01368-6