Abstract
Aneurysmal subarachnoid hemorrhage (aSAH) is a severe cerebrovascular disease.This retrospective two-center cohort study aimed to construct a nomogram model for predicting delayed cerebral ischemia (DCI) in aSAH patients using LASSO- logistic regression. A total of 604 aSAH patients were included. We collected serological indicators of patients at admission. Lasso and multivariate logistic regression analysis and was performed to screen variables and constructed the independent predictors into a nomogram using R language. After LASSO and multivariate logistic regression, Alcoholism, PLT, Na, and APTT were identified as independent risk factors for DCI. A nomogram model was then developed based on these factors. The model showed good predictive performance in both the training set (AUC = 0.703) and the validation set (AUC = 0.633), along with stable calibration and favorable clinical benefits. Alcoholism, PLT, Na, and APTT may be independent predictors of DCI in aSAH patients. This nomogram can potentially help clinicians assess the risk of DCI in aSAH patients at an early stage and implement timely preventive and treatment measures.
Similar content being viewed by others
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is an extremely severe cerebrovascular disease characterized by high incidence, high disability rate, and high mortality1. Globally, the annual incidence of aSAH is approximately 6–9 per 100,000 population, with a mortality rate as high as 35%2. Although significant progress has been made in the diagnosis and treatment of aSAH in recent years, such as the development of neuroimaging and the widespread application of surgical clipping and endovascular interventional therapy, which have improved the survival rate of patients in the acute phase, the complications after aSAH still seriously affect the prognosis of patients3,4,5.
Delayed cerebral ischemia (DCI) is one of the most common and severe complications of aSAH, usually occurring 4–14 days after hemorrhage6. The pathogenesis of DCI is relatively complex, mainly involving aspects such as cerebral vasospasm, microcirculation disorders, impaired cerebral blood flow autoregulation, and inflammatory responses7,8,9. Statistically, the incidence of DCI in aSAH patients is as high as 30-70%. Patients with DCI often have a poor prognosis, and a considerable proportion of surviving patients suffer from severe neurological deficits, imposing a heavy burden on patients’ families and society10.
Currently, the clinical diagnosis of DCI mainly relies on clinical symptoms, neuroimaging examinations (such as CT perfusion imaging, magnetic resonance perfusion-weighted imaging, Doppler ultrasound, etc.), and cerebral angiography11,12. However, these diagnostic methods often can only detect DCI when it has already occurred or obvious neurological deficits have emerged, thus missing the optimal intervention time13,14. Therefore, identifying effective predictive indicators and constructing an accurate prediction model are of great significance for early recognition of the risk of DCI in aSAH patients, enabling the implementation of targeted preventive and treatment measures, and improving patient prognosis15.
In recent years, numerous studies have been dedicated to exploring the risk factors associated with the occurrence of DCI, including patient-related factors such as age, gender, disease severity at admission (e.g., Hunt-Hess grade, Fisher grade, etc.), characteristics of aneurysms (e.g., location, size, shape, etc.), blood biomarkers (e.g., C-reactive protein, neuron-specific enolase, etc.), and genetic factors16,17,18,19. Although some progress has been made, the accuracy of predicting DCI using a single factor is limited. Constructing a prediction model by integrating multiple factors may improve the accuracy and reliability of prediction. However, the existing prediction models vary among different studies, and some models are insufficiently validated, and their clinical applicability still needs to be further improved20. Therefore, this study aims to construct a more accurate and practical nomogram prediction model for DCI through Lasso regression analysis and multivariate analysis of aSAH patients, providing strong support for early clinical intervention.
Materials and methods
Patient section
This was a retrospective study. A total of patients diagnosed with aneurysmal subarachnoid hemorrhage in Huizhou Central People’s Hospital and Gaozhou People’s Hospital from January 2020 to January 2024 were collected.
The inclusion criteria were as follows: (1) Definite diagnosis of aneurysmal subarachnoid hemorrhage: Subarachnoid hemorrhage was confirmed by cranial CT, and it was determined to be caused by the rupture of an intracranial aneurysm through imaging examinations such as digital subtraction angiography (DSA), CT angiography (CTA), or magnetic resonance angiography (MRA).
The exclusion criteria were as follows: (1) History of cerebrovascular diseases: Such as a history of cerebral infarction, intracerebral hemorrhage (not caused by the current aSAH), cerebrovascular malformations, moyamoya disease, etc. (2) Complicated with severe systemic diseases: Such as severe cardiopulmonary insufficiency, liver and kidney failure, advanced malignant tumors, hematological and immune system diseases, etc. (3) Presence of mental illness or cognitive impairment, unable to cooperate with the study-related assessments. (4) Receiving special treatments that may affect cerebral vasospasm or the occurrence of DCI: For example, long-term use of anticoagulant or anti-platelet drugs before the onset and inability to discontinue the drugs, or having received drug treatments that may affect the cerebrovascular state before admission (such as the use of calcium channel blockers at supra-normal doses, etc.). (5) Pregnant or lactating women.
All patients received standardized DCI prevention measures, including nimodipine by intravenous pump or oral(60 mg/4 h), maintenance of systolic blood pressure > 120 mmHg, volume management, and glycemic control. All included patients with coronary artery disease or atrial fibrillation were not receiving regular or chronic antiplatelet/anticoagulant therapy. Based on the exclusion criteria, patients taking these drugs chronically were excluded.
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Huizhou Central People’s Hospital Ethics Committee. All research was performed in accordance with relevant guidelines/regulations. Since this is a retrospective study, the Huizhou Central People’s Hospital Ethics Committee approved the waiver of informed consent in accordance with national regulations and institutional requirements. This study only collected clinical information and test data of patients, and no informed consent was required from patients and their families. To protect patient privacy, all patient identity information has been anonymized.
Data collection and definition
In this study, the baseline clinical data of patients at admission, as well as the test results of blood routine, liver and kidney function, blood biochemistry, and coagulation function, were collected. The baseline clinical data included information such as gender, age, Glasgow Coma Scale (GCS) score, history of hypertension, diabetes, hyperlipidemia, coronary heart disease, atrial fibrillation, alcoholism, and smoking.
Meanwhile, the test results of patients at admission were collected, including the following data: white blood cell count (WBC), hemoglobin count (HGB), platelet count (PLT), mean corpuscular hemoglobin concentration (MCH), mean corpuscular volume (MCV), absolute neutrophil count (NEU), lymphocyte count (LYM), monocyte count (MONO), eosinophil count (EOS), basophil count (BASO), hematocrit (HCT), total bilirubin (TBIL), serum albumin (ALB), gamma-glutamyl transpeptidase (GGT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine (Cr), uric acid (UA), bicarbonate ion (HCO3-), potassium ion (K), sodium ion (Na), chloride ion (Cl), calcium ion (Ca), magnesium ion (Mg), prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen (FIB), etc.
Outcome
After aneurysmal subarachnoid hemorrhage, delayed cerebral ischemia (DCI) usually occurs 4–14 days after hemorrhage6. DCI was diagnosed if both of the following conditions were met: (1) New focal neurological deficits (e.g., hemiplegia, aphasia, altered consciousness) occurring 4–14 days post-hemorrhage; (2) Imaging confirmation of cerebral ischemia (CT perfusion or diffusion-weighted MRI) with exclusion of alternative causes (e.g., rebleeding, cerebral edema). (3) CT perfusion parameters: cerebral blood flow (CBF) < 30 mL/100 g/min and mean transit time (MTT) prolongation > 145% were used as ischemic thresholds21. The occurrence of DCI after aSAH was defined as a positive outcome event, and the non-occurrence of DCI was defined as a negative outcome event.
Statistical analysis
Baseline data comparison and comparison between groups with and without DCI
The patient data from Huizhou Central People’s Hospital were used as the training set, and the patient data from Gaozhou People’s Hospital were used as the validation set. First, the baseline data of the training set and the validation set were compared to verify their comparability. Second, the data of the training set were grouped according to the presence or absence of DCI, and the clinical data and test results were compared between the groups. For continuous data, the Shapiro normality test was used to determine normality. If the data conformed to a normal distribution, they were expressed as mean ± standard deviation, and one-way analysis of variance was used for comparison between groups. If the data did not conform to a normal distribution, they were expressed as median (25th percentile, 75th percentile), and the Kruskal-Wallis test was used for comparison between groups. Categorical data were statistically described using frequency (percentage), and the chi-square test or Fisher’s exact test was used for comparison between groups. A two-sided p-value less than 0.05 was considered statistically significant.
Least absolute shrinkage and selection operator (LASSO)
As the study involved numerous potential influencing factors such as patient basic information, clinical symptoms, and laboratory test indicators, there may be multicollinearity among variables, which could interfere with the accuracy and stability of the model. To address this issue, Lasso analysis was used for variable selection to construct a more reliable prediction model22. Before performing Lasso analysis, continuous variables were standardized to eliminate the influence of dimensions. The Lasso regression method was used to analyze the above-mentioned data. The optimal regularization parameter λ was selected through cross-validation to balance the goodness-of-fit of the model and the variable selection effect. The specific operation was implemented in R software using the ‘glmnet’ package. During the Lasso regression process, by continuously adjusting the λ value, some regression coefficients were shrunk to zero, thereby screening out the variables that had a significant impact on the occurrence of delayed cerebral ischemia. We performed LASSO regression analyses using the “glmnet” package (version 4.1-7, URL: https://cran.r-project.org/web/ packages/glmnet/index.html) in R (version 4.2.2, URL: https://www.r-project.org).
Binary logistic regression analysis
We performed binary logistic regression analyses using SPSS 21.0 (IBM Corp., Armonk, NY, USA; URL: https://www.ibm.com/products/spss-statistics). SPSS 21.0 software was used to calculate the optimal cut-off value for each clinical factor in the training set. The clinical factors were classified based on the optimal cut-off value, and then binary logistic regression analysis was performed to evaluate the independent predictors of DCI22. First, univariate binary logistic regression analysis was performed, and the odds ratio (OR) and its 95% confidence interval (CI) of each independent variable for DCI were calculated respectively. Variables with a p-value less than 0.1 were selected for multivariate binary logistic regression analysis. The enter method was used in the multivariate analysis to eliminate the multicollinearity among independent variables, and finally, a binary logistic regression model containing significant influencing factors was constructed.
Construction of nomogram
Based on the results of Lasso analysis and multivariate binary logistic regression, the data of the training set were used in R studio (version 4.2.2) to draw the nomogram of independent predictors. The predictive efficacy of the model was evaluated by the receiver operating characteristic (ROC) curve and the area under the curve (AUC). The mean error of the model was calculated through the calibration plot, and the clinical benefit of the model was analyzed by the decision curve analysis (DCA). In addition, the data of the validation set were also subjected to relevant analyses and graph-drawing to verify the predictive benefit of the nomogram.
Result
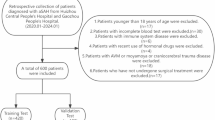
A total of 671 patients with aneurysmal subarachnoid hemorrhage were collected in this study. A total of 67 patients were excluded, and finally, 604 patients were included in the retrospective study. The process is shown in Fig. 1. Among them, there were 369 patients in the training set and 235 patients in the validation set.
Baseline data comparison
The baseline data of the two groups were compared between groups. In the training set, there were 213 male patients (57.72%), with an age of 54 (45, 63) years old, a GCS score of 13 (12, 15) points. The number of patients with a history of hypertension, coronary heart disease, hyperlipidemia, atrial fibrillation, diabetes, smoking, and alcoholism was 154 (41.73%), 27 (7.32%), 47 (12.74%), 58 (15.72%), 53 (14.36%), 68 (18.43%), and 54 (14.63%), respectively. In the validation set, there were 119 male patients (50.64%), with an age of 54 (45, 61) years old, a GCS score of 12 (11, 14) points. The number of patients with a history of hypertension, coronary heart disease, hyperlipidemia, atrial fibrillation, diabetes, smoking, and alcoholism was 84 (35.74%), 13 (5.53%), 43 (18.3%), 51 (21.7%), 36 (15.32%), 40 (17.02%), and 27 (11.49%), respectively. There was no significant difference in the baseline data between the two groups (p > 0.05), indicating that the data were comparable (Table 1).
Comparison between groups with and without DCI
The data of the training set were grouped according to the presence or absence of DCI. The group with DCI was defined as the DCI group, and the group without DCI was defined as the No DCI group. The comparison between the two groups showed that atrial fibrillation, diabetes, alcoholism, WBC, NEUT, PLT, AST, ALT, Cr, Mg, and APTT had statistically significant differences among the groups (p < 0.05) (Table 2).
Lasso analysis
After Lasso analysis, a total of 4 variables significantly related to the occurrence of delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage were screened out, namely alcoholism, PLT, Na, and APTT(Fig. 2A). The regression coefficients of these variables were non-zero in the Lasso model, indicating their important role in predicting the occurrence of delayed cerebral ischemia. The area under the receiver operating characteristic curve (AUC) of the prediction model constructed based on the selected variables in the training set was 0.715, indicating that the model had good predictive ability(Fig. 2B). In the cross-validation set, the AUC of the model was 0.639, further verifying the stability and generalization ability of the model(Fig. 2C).
Univariate and multivariate logistic regression analysis
Each clinical factor was included in the univariate logistic regression analysis. The results showed that Age (4.7 [1.97, 11.23], p < 0.001), Atrial fibrillation (1.92 [1.03, 3.59], p = 0.040), Diabetes (2.01 [1.06, 3.83], p = 0.032), Alcoholism (2.17 [1.15, 4.08], p = 0.016), WBC (2.81 [1.48, 5.33], p = 0.002), NEUT (2.62 [1.5, 4.59], p = 0.001), MONO (1.86 [1.08, 3.19], p = 0.025), PLT (5.04 [2.53, 10.05], p < 0.001), EOS (3.19 [1.23, 8.29], p = 0.017), HCT (1.9 [1.03, 3.52], p = 0.041), MCV (0.41 [0.19, 0.86], p = 0.019), MCH (0.42 [0.21, 0.86], p = 0.018), TBIL (0.38 [0.18, 0.81], p = 0.011), GGT (0.52 [0.31, 0.87], p = 0.012), AST (0.43 [0.26, 0.73], p = 0.002), ALT (0.49 [0.29, 0.81], p = 0.006), Ca (2.21 [1.05, 4.66], p = 0.038), P (1.82 [1.05, 3.14], p = 0.032), FIB (2.62 [1, 6.85], p = 0.049), and APTT (3.21 [1.53, 6.71], p = 0.002) were all risk factors for DCI. The factors with p < 0.1 in the univariate analysis were included in the multivariate logistic regression analysis. The results showed that Alcoholism (2.13 [1.01, 4.48], p = 0.047), PLT (1 [1, 1.01], p = 0.003), Na (0.89 [0.79, 0.99], p = 0.030), and APTT (1.1 [1.01, 1.21], p = 0.034) were independent predictors (Table 3). Variables with non-significant group differences in Table 2 (e.g., WBC, NEUT) showed ORs with 95% CIs crossing 1 in univariate analysis, suggesting potential confounding. LASSO regression prioritized variables with stronger independent contributions (alcoholism, PLT, Na, APTT).
Nomogram construction
Based on the results of LASSO regression and multivariate analysis (Alcoholism, PLT, Na, APTT), a nomogram model was constructed using R studio software. To calculate DCI risk using the nomogram: Assign points based on alcoholism (yes = 30, no = 0), PLT (10 points per 50 × 10⁹/L increase), Na (5 points per 1 mmol/L decrease), and APTT (5 points per 1-second prolongation). Sum the points and align the total with the risk axis to estimate the probability. For example, 100 total points correspond to ~ 35% DCI risk. We constructed the nomogram with the data from the training group(Fig. 3). The receiver operating characteristic (ROC) curve of the training set was plotted to verify the predictive efficiency of this nomogram (Fig. 4A). The area under the curve (AUC) was 0.703 (95%CI: 0.637–0.769), indicating good predictive performance. At the same time, the sensitivity, specificity and accuracy of the training model are 0.701, 0.630, 0.645 (Table 4). The calibration curve of the training set was drawn (Fig. 5A), and it was observed that the ideal curve and the actual curve were basically coincident, with an average error of 0.016, suggesting the stable predictive performance of the model. The decision curve analysis (DCA) of the training set was plotted (Fig. 6A), which indicated that there was a good clinical benefit when the threshold was between 0.01 and 0.65. Meanwhile, the data from the validation set were used for verification. The ROC curve was plotted (Fig. 4B), with an AUC of 0.633 (95%CI: 0.555–0.712). At the same time, the sensitivity, specificity and accuracy of the training model are 0.592, 0.673, 0.660 (Table 4). The calibration curve was drawn (Fig. 5B), showing that the ideal curve and the actual curve were basically coincident, with an average error of 0.03, also indicating the stable predictive performance of the model. The DCA of the validation set was plotted (Fig. 6B), suggesting a good clinical benefit when the threshold was between 0.01 and 0.65. Evidently, this nomogram demonstrated good predictive efficiency in both the training set and the validation set, with a small error, and it could benefit most clinical patients.
Discussion
This study proposed that Alcoholism, PLT, Na, and APTT might be independent predictors of DCI in aSAH patients. Alcoholism may affect the cerebrovascular system through multiple mechanisms. Long-term alcohol abuse can lead to vascular endothelial dysfunction, altering the reactivity of blood vessels to various stimuli and increasing the risk of cerebral vasospasm23,24. Meanwhile, the study by Stettler GR et al.25 suggested that a high alcohol content in the blood was associated with an increased incidence of fibrinolytic arrest. Alcohol may interfere with the coagulation mechanism, resulting in a hypercoagulable state of the blood and further affecting cerebral blood flow perfusion26. An excessively high platelet count may increase blood viscosity, making it easy to form micro-thrombi that block the cerebral microcirculation and thus trigger cerebral ischemia27. On the other hand, various bioactive substances released by platelets, such as thromboxane A2, can cause cerebral vasoconstriction, further reducing cerebral blood flow28,29. Hyponatremia is relatively common in aSAH patients, which can lead to cellular edema and affect the normal metabolism and function of brain tissue30. In addition, hyponatremia may also reduce the tolerance of cerebral blood vessels to ischemia by affecting vascular tone and the cerebral blood flow autoregulation mechanism31. APTT reflects the coagulation function of the intrinsic coagulation pathway. Prolonged APTT may indicate coagulation dysfunction, increasing the risk of bleeding. The hematoma and coagulation products after bleeding may exert mechanical compression and chemical stimulation on cerebral blood vessels, leading to cerebral vasospasm and cerebral ischemia32,33. Chen Hao et al.34 proposed that abnormal APTT and fibrinogen had independent predictive effects on the occurrence of cerebral infarction in patients with moderate to severe craniocerebral injuries.
Currently, numerous studies have been conducted on exploring the risk factors and prediction models for DCI in aSAH patients11,12,13,14,15,16,17,18,19,20. Ma Xinlong et al.17 proposed that an early increase in blood CRP after SAH was associated with DCI. Liu Haonan et al.20 proposed that age > 65 years old, modified Fisher grade 3–4, rupture of anterior-circulation aneurysms, Hunt-Hess grade 4–5, high blood pressure at admission, and plasma homocysteine level ≥ 10 µmol/L were independent risk factors for DCI after aSAH. Compared with traditional single-factor prediction methods, the nomogram proposed in this study integrates information from multiple variables, enabling a more comprehensive and accurate assessment of patients’ risk status. Our study focused more on the serological indicators of patients, and wanted to construct a nomogram related to serological indicators to predict the occurrence of DCI in patients. Our study can serve as a supplement to the classic prediction model. Classic indicators such as Fisher grading and Hunt-Hess grading have been confirmed by too many scholars and widely used in clinical practice, so we did not consider including these indicators in the study. Additionally, the low proportion of elderly patients (> 65 years, median age 54) in our cohort may have limited the statistical power of age-related effects. It is worth mentioning that the lower AUC compared to some studies (AUC = 0.799, 95%CI: 0.737–0.861)35 may stem from the exclusion of imaging grades or genetic markers. According to a recent systematic review36, high AUC prediction models usually integrate imaging grading (such as modified Fisher grading), dynamic biomarkers (such as GFAP, S100B) and machine learning algorithms (such as XGBoost, random forest). For example, Liu et al. [20] combined age, aneurysm location and plasma homocysteine to construct a model (AUC = 0.82) using support vector machine (SVM) to optimize feature weights. In contrast, this study focused on easily accessible serological indicators, which sacrificed some prediction accuracy but enhanced clinical practicality. Future work can integrate imaging features with dynamic biomarkers and try to integrate learning algorithms to further improve performance.
The advantages of this study are as follows: (1) This study used a combination of lasso regression analysis and multivariate logistic regression analysis for variable screening, addressing the issue of multicollinearity among variables and ensuring that the screened factors are independent and significant. (2) The nomogram we proposed is based on the most common medical history data and test results in clinical practice. It does not require complex statistical calculations and has high clinical practicability. The limitations of this study are as follows: (1) This was a retrospective study, which may be subject to information bias and selection bias. Future research will consider conducting multi-center, prospective studies to externally validate the nomogram and improve its clinical promotion value. (2) Although this study identified some important independent risk factors through a comprehensive variable-screening method, other potential influencing factors may still have been overlooked. For example, gene polymorphisms, inflammatory factors, etc., may play important roles in the occurrence of DCI after aSAH. Follow-up studies can further expand the research scope and include more potential factors for analysis.(3) This study did not include some classic predictive indicators such as Fisher grade and Hunt-Hess grade. Our study can serve as a supplement to the widely used predictive indicators in clinical practice.
Conclusion
Alcoholism, PLT, Na, and APTT may be independent predictors of DCI in aSAH patients. The nomogram constructed in this study can assist clinicians in early assessment of the probability of DCI occurrence, achieving the goals of early prevention and early treatment, and benefiting more patients.The modest validation AUC (0.633) may reflect limitations in generalizability. Future studies should optimize variable weights using larger multicenter datasets or integrate dynamic biomarkers (e.g., inflammatory cytokines) to enhance predictive performance.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- aSAH:
-
Aneurysmal subarachnoid hemorrhage
- DCI:
-
Delayed cerebral ischemia
- DSA:
-
Digital subtraction angiography
- CTA:
-
Computed tomography angiography
- MRA:
-
Magnetic resonance angiography
- Lasso:
-
Least absolute shrinkage and selection operator
- WBC:
-
White blood cell
- RBC:
-
Red blood cell
- HGB:
-
Hemoglobin
- MCV:
-
Mean corpuscular volume
- MCH:
-
Mean corpuscular hemoglobin
- PLT:
-
Platelet
- HCT:
-
Hematocrit
- NEU:
-
Neutrophils
- LYM:
-
Lymphocyte
- MONO:
-
Monocyte
- EOS:
-
Eosinophils
- BASO:
-
Basophil
- ALB:
-
Albumin
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- TBIL:
-
Total bilirubin
- GGT:
-
Gamma glutamyl transferase
- PT:
-
Prothrombin time
- APTT:
-
Activated partial thrombin time
- FIB:
-
Fibrinogen
- Cr:
-
Creatinine
- UA:
-
Uric acid
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- AUC:
-
The area under the curve
- DCA:
-
Decision curve analysis
- ROC:
-
Receiver operating characteristic
References
Hoh, B. L. et al. 2023 Guideline for the Management of Patients With Aneurysmal Subarachnoid Hemorrhage: A Guideline From the American Heart Association/American Stroke Association [published correction appears in Stroke. ;54(12):e516. doi: 10.1161/STR.0000000000000449]. Stroke. 2023;54(7):e314-e370. (2023). https://doi.org/10.1161/STR.0000000000000436
Neifert, S. N. et al. Aneurysmal subarachnoid hemorrhage: the last decade. Transl Stroke Res. 12 (3), 428–446. https://doi.org/10.1007/s12975-020-00867-0 (2021).
Petridis, A. K. et al. Aneurysmal subarachnoid hemorrhage. Dtsch. Arztebl Int. 114 (13), 226–236. https://doi.org/10.3238/arztebl.2017.0226 (2017).
Rouanet, C. & Silva, G. S. Aneurysmal subarachnoid hemorrhage: current concepts and updates. Arq. Neuropsiquiatr. 77 (11), 806–814. https://doi.org/10.1590/0004-282X20190112 (2019).
Spears, W. E., Greer, D. M. & Nguyen, T. N. Comment on the 2023 guidelines for the management of patients with aneurysmal subarachnoid hemorrhage. Stroke 54 (10), 2708–2712. https://doi.org/10.1161/STROKEAHA.123.043541 (2023).
Abdulazim, A., Heilig, M., Rinkel, G. & Etminan, N. Diagnosis of delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage and triggers for intervention. Neurocrit Care. 39 (2), 311–319. https://doi.org/10.1007/s12028-023-01812-3 (2023).
Balança, B., Bouchier, B. & Ritzenthaler, T. The management of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Rev. Neurol. (Paris). 178 (1–2), 64–73. https://doi.org/10.1016/j.neurol.2021.11.006 (2022).
Suzuki, H., Kawakita, F. & Asada, R. Neuroelectric mechanisms of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Int. J. Mol. Sci. 23 (6), 3102. https://doi.org/10.3390/ijms23063102 (2022). Published 2022 Mar 13.
Suzuki, H. et al. Cerebrovascular pathophysiology of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Histol. Histopathol. 36 (2), 143–158. https://doi.org/10.14670/HH-18-253 (2021).
Ikram, A. et al. Delayed cerebral ischemia after subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 30 (11), 106064. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.106064 (2021).
Han, H. et al. The value of early CT perfusion parameters for predicting delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Neurosurg. Rev. 45 (4), 2517–2531. https://doi.org/10.1007/s10143-022-01779-3 (2022).
Mir, D. I. et al. CT perfusion for detection of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. AJNR Am. J. Neuroradiol. 35 (5), 866–871. https://doi.org/10.3174/ajnr.A3787 (2014).
Kim, Y. et al. The TCD hyperemia index to detect vasospasm and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. J. Neuroimaging. 33 (5), 725–730. https://doi.org/10.1111/jon.13132 (2023).
Chang, J. J. et al. Transcranial doppler velocity and associations with delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. J. Neurol. Sci. 415, 116934. https://doi.org/10.1016/j.jns.2020.116934 (2020).
Rautalin, I., Juvela, S., Martini, M. L., Macdonald, R. L. & Korja, M. Risk factors for delayed cerebral ischemia in Good-Grade patients with aneurysmal subarachnoid hemorrhage. J. Am. Heart Assoc. 11 (23), e027453. https://doi.org/10.1161/JAHA.122.027453 (2022).
van Os, H. J. A. et al. Delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage in young patients with a history of migraine. Stroke 53 (6), 2075–2077. https://doi.org/10.1161/STROKEAHA.121.038350 (2022).
Ma, X., Lan, F. & Zhang, Y. Associations between C-reactive protein and white blood cell count, occurrence of delayed cerebral ischemia and poor outcome following aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Acta Neurol. Belg. 121 (5), 1311–1324. https://doi.org/10.1007/s13760-020-01496-y (2021).
Spantler, D. et al. Biomarker associations in delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Int. J. Mol. Sci. 23 (15), 8789. https://doi.org/10.3390/ijms23158789 (2022). Published 2022 Aug 7.
Zhang, X. et al. Neutrophil-to-Albumin ratio as a biomarker of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. World Neurosurg. 147, e453–e458. https://doi.org/10.1016/j.wneu.2020.12.084 (2021).
Liu, H., Xu, Q. & Li, A. Nomogram for predicting delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage in the Chinese population. J. Stroke Cerebrovasc. Dis. 29 (9), 105005. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105005 (2020).
Cremers, C. H. et al. CT perfusion and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J. Cereb. Blood Flow. Metab. 34 (2), 200–207. https://doi.org/10.1038/jcbfm.2013.208 (2014).
Tang, G. et al. Evaluation and analysis of incidence and risk factors of lower extremity venous thrombosis after urologic surgeries: A prospective two-center cohort study using LASSO-logistic regression. Int. J. Surg. 89, 105948. https://doi.org/10.1016/j.ijsu.2021.105948 (2021).
Grogan, J. R. & Kochar, M. S. Alcohol and hypertension. Arch. Fam Med. 3 (2), 150–154. https://doi.org/10.1001/archfami.3.2.150 (1994).
Cahill, P. A. & Redmond, E. M. Alcohol and cardiovascular disease–modulation of vascular cell function. Nutrients 4 (4), 297–318. https://doi.org/10.3390/nu4040297 (2012).
Stettler, G. R. et al. Do not drink and Lyse: alcohol intoxication increases fibrinolysis shutdown in injured patients. Eur. J. Trauma. Emerg. Surg. 47 (6), 1827–1835. https://doi.org/10.1007/s00068-020-01328-x (2021).
Tousoulis, D. et al. Acute effects of different alcoholic beverages on vascular endothelium, inflammatory markers and thrombosis fibrinolysis system. Clin. Nutr. 27 (4), 594–600. https://doi.org/10.1016/j.clnu.2008.01.002 (2008).
Wei, K., Huang, H., Liu, M., Shi, D. & Ma, X. Platelet-Derived exosomes and atherothrombosis. Front. Cardiovasc. Med. 9, 886132. https://doi.org/10.3389/fcvm.2022.886132 (2022). Published 2022 Apr 15.
Smith, J. B., Araki, H. & Lefer, A. M. Thromboxane A2, Prostacyclin and aspirin: effects on vascular tone and platelet aggregation. Circulation 62 (6 Pt 2), V19–V25 (1980).
Drouin, A. et al. Up-regulation of thromboxane A₂ impairs cerebrovascular eNOS function in aging atherosclerotic mice. Pflugers Arch. 462 (3), 371–383. https://doi.org/10.1007/s00424-011-0973-y (2011).
Rondon-Berrios, H. Diagnostic and therapeutic strategies to severe hyponatremia in the intensive care unit. J. Intensive Care Med. 39 (11), 1039–1054. https://doi.org/10.1177/08850666231207334 (2024).
Kleindienst, A., Hannon, M. J., Buchfelder, M. & Verbalis, J. G. Hyponatremia in neurotrauma: the role of vasopressin. J. Neurotrauma. 33 (7), 615–624. https://doi.org/10.1089/neu.2015.3981 (2016).
Ji, X., Meng, R., Zhou, J., Ling, F. & Jia, J. Dynamic change of coagulation and anticoagulation markers of patients with acute cerebral infarction during intravenous urokinase thrombolysis. Neurol. Res. 28 (1), 46–49. https://doi.org/10.1179/016164106X91861 (2006).
Ho, C. H., Hu, H. H. & Wong, W. J. The serial hemostasis-related changes in patients with cerebral infarction: comparison between progressing and non-progressing stroke. Thromb. Res. 56 (5), 635–647. https://doi.org/10.1016/0049-3848(89)90271-5 (1989).
Chen, H. et al. The influence of hemocoagulation disorders on the development of posttraumatic cerebral infarction and outcome in patients with moderate or severe head trauma. Biomed. Res. Int. 2013, 685174. https://doi.org/10.1155/2013/685174 (2013).
Zhao, L. et al. Development and validation of an early predictive nomogram for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Ann. Transl Med. 9 (22), 1664. https://doi.org/10.21037/atm-21-5200 (2021).
Mohammadzadeh, I. et al. Leveraging machine learning algorithms to forecast delayed cerebral ischemia following subarachnoid hemorrhage: a systematic review and meta-analysis of 5,115 participants. Neurosurg Rev. 48(1) 26. https://doi.org/10.1007/s10143-024-03175-5 (2025).
Author information
Authors and Affiliations
Contributions
Wencai Li designed this study. Minyun Mao and Rimei Zhou are responsible for writing articles, conducting statistical analysis, reviewing articles, and creating images.Yishu Chen, Jiajun Wei and Mingjian Lin are responsible for collecting data and conducting statistical analysisAll authors reviewed the manuscript.All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Huizhou Central People’s Hospital Ethics Committee. All research was performed in accordance with relevant guidelines/regulations. Since this is a retrospective study, the Huizhou Central People’s Hospital Ethics Committee approved the waiver of informed consent in accordance with national regulations and institutional requirements. This study only collected clinical information and test data of patients, and no informed consent was required from patients and their families. To protect patient privacy, all patient identity information has been anonymized.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mao, M., Zhou, R., Chen, Y. et al. Construction of a nomogram model for predicting delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage patients. Sci Rep 15, 17739 (2025). https://doi.org/10.1038/s41598-025-01693-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01693-w