Abstract
Spatial neglect is commonly attributed to lesions of a predominantly right-hemispheric cortical network. Although spatial neglect was also repeatedly observed after lesions to the basal ganglia and the thalamus, many anatomical network models omit these structures. We investigated if disruption of functional or structural connectivity can explain spatial neglect in subcortical stroke. We retrospectively investigated data of first-ever, acute stroke patients with right-sided lesions of the basal ganglia (n = 27) or the thalamus (n = 16). Based on lesion location, we estimated (i) functional connectivity via lesion-network mapping with normative resting state fMRI data, (ii) structural white matter disconnection using a white matter atlas and (iii) tract-wise disconnection of association fibres based on normative tractography data to investigate the association of spatial neglect and disconnection measures. Apart from very small clusters of functional disconnection observed in inferior/middle frontal regions in lesion-network symptom mapping for basal ganglia lesions, our analyses found no evidence of functional or structural subcortico-cortical disconnection. Instead, the multivariate consideration of lesion load to several association fibres predicted the occurrence of spatial neglect (p = 0.0048; AUC = 0.76), which were the superior longitudinal fasciculus, inferior occipitofrontal fasciculus, superior occipitofrontal fasciculus, and the uncinate fasciculus. Disconnection of long (cortico-cortical) association fibres can explain spatial neglect in subcortical stroke. Like the competing theory of remote cortical hypoperfusion, our finding does not support a genuine role for subcortical grey matter structures in spatial neglect.
Similar content being viewed by others
Introduction
Spatial attention deficits are a typical and debilitating consequence of right hemispheric stroke. The syndrome of spatial neglect, with an egocentric core deficit of an attentional deviation towards the ipsilesional side and neglect of contralesional objects1,2, impacts stroke outcome and activities of daily living3,4. Several theories have been proposed to explain the cognitive processes behind spatial neglect (see, e.g1,5,6,7)., and extensive research has been dedicated to uncovering its neural foundations. The last two decades saw a shift from theories that focussed on single cortical loci in the right hemisphere, for example within the inferior parietal lobe8,9, the superior or middle temporal lobes10,11, and the inferior frontal lobe12,13, to cortical network theories that unified these previous findings to various degrees1,2,14. This development was stimulated by advances in the accessibility of the human brain connectome and new insights into the impact of white matter disconnection on spatial neglect15,16,17,18,19,20,21.
Infarcts in the thalamus and the basal ganglia were also repeatedly implicated in spatial neglect22,23,24. A few theories incorporated such structures based on subcortico-cortical connections with cortical key regions of spatial neglect10,25,26,27 or multimodal connectivity data28, but subcortical grey matter structures only play a niche role in current network theories of spatial neglect. The functional role of subcortical grey matter structures is also challenged by an alternative explanation of spatial neglect after subcortical stroke that emphasizes the role of cortical hypoperfusion. In acute stroke with large vessel occlusion, hypoperfusion typically goes beyond the core lesion area visible in diffusion-weighted MRI and includes areas where perfusion is sufficient to sustain tissue integrity but insufficient to sustain neural activity29,30. In basal ganglia stroke, hypoperfusion and functional underactivation in cortical areas such as the superior temporal gyrus, the inferior parietal lobule, and the inferior frontal gyrus31 can explain spatial neglect31,32.
Yet another alternative account likewise dismisses the functional role of subcortical grey matter structures and explains spatial neglect after subcortical stroke with the disconnection of long association fibre tracts18,33,34, i.e. intrahemispheric cortico-cortical long-range white matter connections. Indeed, the disconnection of several major fibre bundles has been implicated in spatial neglect15,16,18,19,35. These fibre bundles are located in the subcortical white matter directly adjacent to the thalamus and basal ganglia. Hence, even when a lesion of subcortical grey matter structures falls largely into the grey matter, it may still include damage to white matter fibre bundles which might suffice to cause spatial neglect through cortico-cortical disconnection.
In the current study, we aimed to re-evaluate the possible causes of spatial neglect in acute subcortical stroke using state-of-the-art analyses of indirect connectivity estimation. We studied patients whose lesions focussed on subcortical grey matter structures, without damage to cortical structures, and, at most, minor white matter damage. First, we used functional lesion-network symptom mapping, an approach to assess functional brain networks affected by focal structural lesions to gain insight into the possible role of subcortico-cortical connectivity. Second, we evaluated structural brain connectivity, including subcortico-cortical disconnection estimated based on normative connectome data. Third, we estimated the cortico-cortical disconnectome of each patient on the level of major fibre bundles to evaluate a possible role of cortico-cortical white matter disconnection.
General methods
Patient recruitment and behavioural assessment
We re-analysed data of stroke patients admitted to the Centre of Neurology at the University of Tübingen. Patients were retrospectively identified in datasets collected in previous studies11,23,36,37,38. All patients had a first-ever unilateral cerebral stroke to the right hemisphere confirmed by CT or MRI imaging. Patients with a medical history of a neurological or psychiatric disease that could interfere with the neuropsychological assessment as well as patients with diffuse lesions or tumours were excluded. Selection criteria were: (1) stroke affecting the basal ganglia or the thalamus, (2) no visible damage to cortical areas, and (3) no or only minor damage to white matter (average white matter lesion volume was 1.9 ± 2.25 cm³; range = 0.0–10.8 cm³). These criteria were first liberally checked by an automated search across available datasets by reference to the AICHA brain atlas39. A second, visual evaluation of the neuroimaging of potentially suitable datasets resulted in a sample of 43 stroke patients. Demographic and clinical data are shown in Table 1; lesion topographies in Fig. 1. The original studies that collected clinical data as well as the re-analysis of the data were approved by the local ethics board and have been performed in accordance with the revised Declaration of Helsinki. Patients or their relatives gave their informed consent for participation in the study.
Spatial neglect was assessed with pencil and paper cancellation tests presented on sheets of paper measuring 21 cm x 29.7 cm. In the letter cancellation task40 60 target letters ‘A’ are embedded among distractor letters; in the bells cancellation task41 35 bell-shaped black symbols are shown among other similarly depicted distractor objects. The sheets were placed horizontally and their centre was aligned with the patient’s sagittal midline. Patients were instructed to cancel all target items with the pencil. No time limit was set and the assessment was completed after the patient had confirmed twice that they were satisfied with the performance. To quantify performance with a continuous measure, we computed the Centre of Cancellation (CoC)42 using freely available software (https://github.com/neurolabusc/Cancel). The CoC predicts the typical neurological signs of spatial neglect and ranges from − 1 (maximum neglect of right stimuli) to 0 (symmetrical test performance) up to 1 (maximum neglect of left stimuli). Briefly, this measure refers to the mean horizontal position of all successfully cancelled targets relative to the midline of the page, which is defined to have a horizontal coordinate of 0. The average CoC score of both the letter and bells cancellation tests was used as the measure of spatial neglect severity. For analyses with binary data, patients were classified as suffering from spatial neglect if the CoC in at least one of the two cancellation tasks was above the empirically established cut-offs42. For 7 patients, only the measurement in one of the two cancellation tests was available, and this was used as the sole measure.
Imaging and lesion segmentation
Structural brain imaging was acquired by clinical CT (n = 30) or MRI (n = 13) on average 2.3 ± 4.1 days after stroke onset. In patients with an available MRI, diffusion-weighted imaging was used in the first 48 h after stroke onset and T2 fluid-attenuated inversion recovery imaging afterwards. Part of the data included in the present study reached back to times before digitalization of imaging data. For 25 of such older cases, lesions were manually drawn on axial slices of the ch2 MNI T1 template in MRIcron11, and interpolated in the z-direction as described previously37. For the remaining 18 cases, lesions were delineated semi-automatically on axial slices of the clinical scan using the Clusterize Toolbox43. If possible, images were co-registered with high-resolution T1 MRI. Scans were warped to 1 × 1 × 1 mm³ MNI coordinates using age-specific templates in the Clinical Toolbox44, situation-dependently either using cost-function masking or enantiomorphic normalisation. The normalisation parameters were then applied to the lesion masks.
Lesion-network symptom mapping based on normative rs-fMRI data
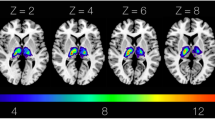
We mapped the whole-brain functional connectivity of each lesion with ‘lesion-network mapping’45. In this approach, only the patients’ lesion masks are needed, and the brain-wide disturbance caused by a lesion is determined by reference to normative resting state functional MRI (rs-fMRI) of a healthy control group (Fig. 2). In short, the binary lesion mask of each patient is used as a seed in a rs-fMRI analysis. For each patient, the correlation of spontaneous resting brain activity within voxels inside the seed area is computed for all healthy control subjects for each voxel in the brain. This results in a single, full-brain lesion-network topography per patient, indicating both positive and negative functional connectivity with the lesioned area, which can be used within common statistical topographic approaches to map the brain-wide disruption of the functional connectome. For our study, this method has significant advantages over the direct measurement of functional connectivity: firstly, it allows statements to be made about the connectivity of the damaged tissue − which is no longer possible with direct methods − and secondly, it is widely applicable, as it only relies on data that is already recorded as part of standard clinical protocols. The procedures followed previous studies46 and utilised public rs-fMRI data of 100 healthy young subjects. The detailed procedures are described in the supplementary.
We utilised patient-wise maps of averaged Fisher-transformed Pearson correlations of rs-fMRI as lesion-network maps. For statistical analyses, we have masked the full-brain lesion-network maps to only include cerebral cortical grey matter areas with the AICHA brain atlas39, henceforth termed cortical lesion-network maps. The masking step removed white matter areas and subcortical brain structures, i.e. the thalamus and basal ganglia, effectively removing areas where autocorrelation of resting state activity inside the lesion seed maps would inflate lesion-network connectivity.
Estimated structural disconnectivity profiles
We indirectly estimated the lesion-induced disconnection of white matter fibres by reference to a healthy reference streamline connectome. Our method closely resembled the estimation of white matter disconnection in a popular toolkit47, but extended this approach to the level of individual streamlines. We loaded all lesion masks as regions of interest to DSI studio (https://dsi-studio.labsolver.org/) and tracked streamlines based on the HCP84248 intersecting the lesion mask. The resulting set of streamlines included both streamlines that terminated in the lesion mask, i.e. disrupted connections of the subcortical structures themselves, and streamlines that passed the lesion mask, which included cortico-cortical streamlines.
Tract-wise lesion load
We assessed the tract-wise lesion load of several long association (i.e. cortico-cortical) fibre tracts that were previously found to be implicated in spatial neglect15,19,21,36,49 and which, due to proximity to subcortical structures, may be disrupted in subcortical stroke. These included the superior longitudinal fasciculus (SLF), the inferior occipitofrontal fasciculus (IOF), the superior occipitofrontal fasciculus (SOF), and the uncinate fasciculus (UF). We defined the location of these fibre tracts with a histology-based probabilistic cytoarchitectonic fibre tract atlas50. We created a binary region of interest for each tract by binarisation of the probabilistic map at p ≥ 0.3 (the cut-off was derived from previous literature19. The lesion load of a fibre tract was defined as the proportional overlap of the lesion map with this binarised map. While such overlap maps do not necessarily represent the proportion of disconnected fibres, they were found (for motor deficits after damage to the corticospinal tract) not only to predict motor outcome but also to have higher predictive value than lesion volume51. In a control condition, we accounted for the full probabilistic information of the fibre tract atlas. We computed the weighted lesion load with the entire (non-thresholded) probabilistic fibre tract and the lesion map, whereas the lesion load of each voxel was weighted by its probability to be part of the fibre tract and summed up.
Multivariate prediction analysis on tract-wise lesion load
We used a random forest classifier to evaluate if multivariate tract-wise lesion load can predict spatial neglect (methodologically similar to52). We trained random forests with the scikit-learn package in Python to predict the binary spatial neglect diagnosis based on the four tract-wise lesion load measures. Each random forest contained 100 trees and, to prevent over-fitting, we limited the maximum depth to 3 and the minimum number of samples required to split an internal node to 5. We assessed the out-of-sample classifier performance in a looped cross-validation with 100 stratified splits of the total sample into 80% training and 20% test data. Classification performance was assessed in the test data by the area under the curve (AUC) of the receiver operator characteristic. We tested the classification performance against chance level by permutation testing. For 2500 times, we randomly shuffled the target variable and re-computed a random forest with the same procedures as for the original data. With this procedure, we assessed the distribution of the AUC under the null hypothesis and estimated the statistical significance of the original model. We also planned to repeat the multivariate random forest classification analysis with the indirectly estimated streamline-wise disconnectivity profiles aggregated for the major cortico-cortical fibre bundles based on the HCP84248. To do so, we derived patients’ tract disconnection measures using the lesion quantification toolkit47.
Experiment 1: lesion network symptom mapping
Experimental design and statistical analysis
First, we used frequentist statistical parametric mapping to identify subcortico-cortical lesion-network connectivity related to spatial neglect after stroke to either the basal ganglia or the thalamus. We computed voxel-wise general linear models using NiiStat (https://github.com/neurolabusc/NiiStat) to test the association between spatial neglect severity as measured by average CoC scores and indirectly estimated connectivity in cortical lesion-network maps. Family-wise correction for multiple comparisons was implemented by maximum statistic permutation thresholding at p < 0.05 (two-tailed) with 10,000 permutations. We interpreted the results with the rs-fMRI-based AICHA atlas39.
Second, we replicated the analysis with a Bayesian approach with Bayes factor mapping to gain insights into evidence for the null hypothesis h0 and statistical power. We used the Bayesian Lesion-Deficit Inference toolbox53 with a design equivalent to the frequentist analysis. Bayesian general linear models tested if an association between cortical lesion-network connectivity and spatial neglect exists in two-sided tests against the null hypothesis that no such association exists. For voxels without evidence for an association between spatial neglect and lesion networks, it can differentiate between the absence of evidence, i.e. a lack of statistical power, and evidence for the null hypothesis. We classified the degree of evidence provided by the mapped Bayes factors according to established conventions54.
Results
The frequentist analyses failed to find cortical lesion-network correlates of spatial neglect after correction for multiple comparisons both for basal ganglia and thalamic stroke.
For basal ganglia stroke, the Bayesian analysis (Fig. 3A) was, as expected53, more liberal and found scattered clusters of evidence for lesion network-neglect associations (i.e. Bayes Factors [BF] > 3, meaning that h1 is at least 3 times more likely than h0). However, the level of evidence was mostly only moderate and clusters were very small, creating largely non-interpretable results. We found a single small, potentially interpretable cluster in the right inferior and middle frontal gyrus (in regions 20 and 30 in the AICHA atlas), albeit maximum BFs did not exceed 12. On the other hand, due to the small sample size, the Bayesian analysis was unable to provide evidence in favour of the null hypothesis (BF < 1/3). Hence, we sub-differentiated BFs that suggested no evidence for any hypothesis (BFs > 1/3 and < 3) into anecdotal evidence for h1 (BF > 1 and < 3) and anecdotal evidence for h0 (BF < 1 and > 1/3; see54). Anecdotal evidence for h0 was found in the majority of tested voxels (74.1%) and comprised large parts of the right cerebral cortex, including most of the temporal and parietal lobes.
Results of the Bayesian lesion-network mapping analysis. (A) Statistical results of the Bayesian voxel-wise lesion-network analysis with normative rsfMRI data for basal ganglia stroke and (B) thalamic stroke. The analyses resulted in Bayes factors that weigh the evidence for the alternative hypothesis h1 (i.e. an association between the voxel-wise lesion-network connectivity and spatial neglect exists) and the null hypothesis h0 (i.e. no such association exists). For visualization, we binned these Bayes factors according to common conventions54 into evidence in favour of h1 (red; moderate BF > 3, strong BF > 10, very strong BF > 30), and inconclusive, anecdotal evidence for h1 (yellow; BF > 1) anecdotal evidence for h0 (turquoise; BF < 1). Due to the small sample sizes, the smallest Bayes factor was 0.35 (i.e. > 1/3), hence no moderate or stronger evidence for h0 was found. See supplementary Fig. 2 for results not restricted to the cortical grey matter, i.e. also covering white matter and subcortical areas.
For thalamic stroke, the Bayesian analysis found anecdotal evidence for h0 across the majority of tested voxels (97.9%; Fig. 3B) and no interpretable clusters of voxels with evidence for h1.
Experiment 2: structural disconnectivity profile analysis
Experimental design and statistical analysis
We only tested streamlines disconnected in at least 5 patients, resulting in 88781 for basal ganglia lesions and 25873 streamlines for thalamic lesions (for a visualisation, see Supplementary Fig. 3). We tested the association of streamline disconnection with the mean CoC score with general linear models corrected by a permutation-based family-wise error correction with 10000 permutations at p < 0.05. The tests were one-tailed because we expected that neural damage would be associated with more severe, not less severe, deficits. To assign significant streamlines to major fibre tracts we used the recognize and cluster function in DSI Studio.
Results
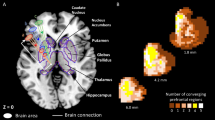
In basal ganglia stroke, we found a small set of 12 streamlines in the corticostriatal tract (Fig. 4A) where disconnection was significantly associated with spatial neglect. The identified streamlines appear to connect the putamen (AICHA region 366 in39) and a portion of the superior frontal gyrus (AICHA region 4 and 14) in the right hemisphere. In thalamic stroke (Fig. 4B), disconnection of 141 streamlines in the right corticospinal tract, as well as a single streamline each from the right anterior thalamic radiation, right fornix, and the right dentato-rubro-thalamic tract were observed.
Streamline disconnections associated with neglect severity. (A) Significant disconnections of streamlines found in basal ganglia lesions. All fibres found were classified as part of the corticostriatal tract and are depicted in orange. (B) Significant disconnections in thalamic lesions. These included the corticospinal tract (yellow), anterior thalamic radiation (red), fornix (green) and dentatorubrothalamic tract (blue).
Experiment 3: white matter tract-wise analysis
Experimental design and statistical analysis
With the third experiment, we investigated if spatial neglect can be explained by damage to white matter fibre tracts that pass in close proximity to the basal ganglia and thalamus. First, we assessed the association between tract-wise lesion load and spatial neglect. As the analysis focussed on cortico-cortical disconnection – independent of the subcortical lesion site in either the basal ganglia or thalamus – we performed analyses for all 43 patients combined. However, to improve comparability with analyses 1 and 2, we also report results for basal ganglia and thalamic stroke patients separately. Second, we used the random forest classifier to evaluate to what degree tract-wise lesion load can explain spatial neglect. In contrast to the simple tract-wise lesion load analysis, the random forest classification could not be repeated for the patient groups separately, since the overall sample size would be too small.
Results
We found a lesion load in at least one of the four fibre tracts in 28 out of 43 patients. This was the case for 12 out of 15 patients with spatial neglect and 12 out of 29 patients without spatial neglect. This ratio was significantly larger in patients with spatial neglect (χ2(1, N = 43) = 3.88; p = 0.049). For all four fibre tracts, at least some patients with a lesion load were identified ranging from 13 to 23 patients (Fig. 5). The rank correlation between the mean CoC score and the lesion load in each fibre tract ranged from τ = 0.20 to 0.27. However, while the correlation coefficients were significant for all fibres except the SLF, no results remained significant after Bonferroni correction. The correlation between the total lesion load across the four fibre tracts and the mean CoC was highly significant (τ = 0.31; p = 0.005; Fig. 5), the correlation remained significant when only patients with damage to the basal ganglia were included (τ = 0.32; p = 0.023), but not for the thalamic stroke patient sample (τ = 0.24; p = 0.27). In a control analysis, we looked at the weighted lesion load that incorporated the full probabilistic information of the fibre tract atlas. This analysis closely replicated the correlation between lesion load and mean CoC (τ = 0.32; p = 0.003). The out-of-sample prediction accuracy of the random forest classifier was highly significant above chance (p = 0.0048; AUC = 0.76), meaning that the multivariate consideration of tract-wise disconnection can predict spatial neglect in subcortical stroke.
We aimed to repeat the multivariate classification with the estimated streamline-wise disconnectivity across major cortico-cortical fibre bundles based on the HCP84248. However, not only the known neglect-related association fibre bundles (see above) were rarely or never disconnected, but association fibres in general. Disconnection of the SLF was found in only 2 patients. Only the IOF and the UF were more often found to be disconnected. Hence, we deemed the data to be unfit for multivariate model training.
Discussion
Our study investigated whether indirectly estimated functional or structural disconnection can explain spatial neglect in acute subcortical stroke to either the basal ganglia or the thalamus. Neither a functional analysis – i.e., lesion network mapping using normative resting-state fMRI – nor a structural analysis – i.e., the estimation of white matter disconnection using a normative streamline atlas – found convincing evidence for subcortico-cortical connectivity that could explain spatial neglect. However, we identified disconnection of cortico-cortical fibres that pass the subcortical structures in close proximity as a possible cause of spatial neglect.
The first analysis used lesion network mapping to evaluate possible disruption of functional subcortico-cortial connectivity underlying spatial neglect after subcortical stroke. Voxel-wise univariate mapping failed to identify significant contributions of lesion network connectivity to spatial neglect. Moreover, Bayesian analyses partially provided anecdotal evidence for the absence of any association between lesion-network connectivity and spatial neglect in several known cortical key areas of spatial neglect. If anything, our analyses indicate functional connectivity of the basal ganglia with inferior/middle frontal due to very small clusters observed in these regions. However, due to the small sample sizes, we could not find at least moderate evidence in favour of the null hypothesis.
The second analysis estimated disconnection of white matter fibre streamlines to evaluate possible disruption of structural subcortico-cortial connectivity. In basal ganglia stroke, cortical projections of fibres more often affected in neglect patients were located in the right superior frontal gyrus, i.e. more superiorly than those frontal regions commonly associated with spatial neglect (namely, the right ventrolateral frontal cortex35). In thalamic stroke, the results were almost exclusively limited to the corticospinal tract. We consider this to be a coincidental finding unrelated to spatial neglect but linked to the high rate of additional hemiparesis (Table 1). In summary, neither of the first analyses provided convincing evidence of disconnectivity between subcortical and cortical regions to underlie spatial neglect.
The third analysis estimated the lesion load of cortico-cortical white matter tracts using a probabilistic atlas of white matter bundles. The multivariate consideration of lesion load to several association fibres by the random forest classifier predicted the occurrence of spatial neglect in cross-validation. This suggests that disconnection of cortico-cortical long association fibres situated in proximity of the basal ganglia and thalamus, namely the SLF, IOF, SOF, and the UF, could be a cause of spatial neglect in subcortical stroke. However, the same analysis of disconnectivity profiles created with a non-probabilistic streamline atlas was not possible, as this method rarely or never captured disconnection of most of the potentially relevant fibres. At first glance, it may seem surprising that this simple analysis has yielded a positive finding. But there are good reasons that can explain this finding. Firstly, the spatial definition of white matter fibres varies markedly across atlases, especially between atlases derived from histological versus from diffusion-tensor imaging data55. Secondly, the histological atlas, which was used in the analysis with positive findings, is a probabilistic atlas, i.e. it represents inter-individual variation in the location of fibre bundles. Disconnection of cortico-cortical fibre bundles may occur in subcortical strokes depending on individual anatomy, in the sense that the fibre bundles are located inter-individually differently close to the basal ganglia and the thalamus and their disconnection is differently likely when the subcortical structures are damaged. It should be noted that the lesion load approach also has limitations. Relevant for the disconnection of a fibre tract is less the total lesion load than the disconnection of fibre bundles. More complex lesion load approaches attempt to take this into account56 and could yield better predictors, although our approach for the current study yielded positive findings and was therefore sufficient.
Disconnection of long association fibres is a well-known cause of spatial neglect, as suggested by studies in stroke15,19,21,33,36,49, tumour resection57, and using electrical stimulation16. Several studies suggested disconnection of the SLF and the IOF as a cause of spatial neglect and found disconnection of these fibres to be linked to different attentional sub-deficits21,49. Intrasurgical electrical stimulation of the SOF was found to induce neglect-like deviations in a line bisection task16. Lesions to the UF were found to be linked to spatial neglect in chronic stroke patients36. The assumed relevance of these fibre bundles is consistent with the known correlates of spatial neglect in the grey matter as described in the introduction. The association fibre bundles connect the frontal, temporal, and parietal correlates of spatial neglect, which culminated in anatomical theories of spatial neglect that assume disturbances of a fronto-temporo-parietal network to cause spatial neglect1,2,14,35.
Since aphasia and spatial neglect in humans are caused by damage to somewhat homologue networks in opposing hemispheres, the state of research on aphasia following subcortical grey matter lesions might help to put our current findings into context. Aphasia after basal ganglia damage in the left hemisphere also remains controversial with heterogeneous findings and a lack of consensus on the involvement of specific structures58. In line with the assumption of parallel mechanisms when considering our current results, white matter damage was found to be a potential explanation for the occurrence of aphasia in subcortical stroke59,60. Other studies suggested, for example, left hemispheric thalamocortical functional disconnection as a possible mechanism61. In our study, we did not find any results that would suggest similar mechanisms for spatial neglect after right-hemispheric thalamic stroke. However, we must note that our sample of right thalamic stroke cases (as opposed to right basal ganglia cases) was too small to draw or reject parallels with the explanation by Stockert and colleagues61.
Some anatomical network models have attributed a direct anatomical role to the basal ganglia, the thalamus, or both in the evocation of spatial neglect. These models were based on findings from lesion studies in subcortical stroke in the context of existing knowledge about brain connections (e.g10,26).,. Other network models solely focused on cortico-cortical, intra- or interhemispheric networks and did not include any subcortical structures1,17. In line with these theories, disconnection of long association fibres subserving a right fronto-temporo-parietal network has been found to underlie spatial neglect15,16,18,19,35. According to our results, these theories about the general anatomy of spatial neglect may – at least in some cases – also explain spatial neglect after subcortical stroke. The possibility of such a mechanism underlying spatial neglect in subcortical stroke adds − in addition to remotely induced hypoperfusion in intact cortical regions following lesions of subcortical grey matter structures31 − yet another explanation that does not assume a direct role of subcortical structures in the spatial neglect network.
Given contradicting findings (e.g28). and the possibility of multiple, parallel causal mechanisms, further studies are needed to understand the neural correlates of spatial neglect after subcortical stroke. This is of particular importance as null results in our study are of limited significance due to the relatively small sample size, as shown by the results of the Bayesian lesion network analysis. Bayesian statistics may be well suited for the analysis of small samples, as they transparently highlight any lack of evidence due to small sample or effect sizes. However, even though they are capable of indicating evidence in favour of the null hypothesis, Bayesian statistics typically require larger samples to gather evidence for h0 than for h162. Furthermore, even if a null effect was likely, a possible role of subcortical structures in spatial neglect could not be excluded. A possible multitude of causes for spatial neglect in subcortical stroke – cortical hypoperfusion31,32, cortico-cortical white matter disconnection, and a direct impact of damaged or disconnected subcortical structures – could statistically dilute an effect.
In summary, our study suggests that disconnection of cortico-cortical fibre bundles can explain spatial neglect after subcortical stroke. This explanation does not contradict the assumption that remote cortical hypoperfusion caused by subcortical damage causes spatial neglect31,32. Both mechanisms seem plausible and, depending on the case, could underlie the occurrence of spatial neglect either separately or in combination. Apart from very small clusters of functional disconnection observed in inferior/middle frontal regions in lesion-network symptom mapping for basal ganglia lesions, our study found no evidence for the involvement of subcortico-cortical disconnection in the known network of spatial neglect. However, due to the small sample size in our study, we cannot exclude this possibility, and future studies with larger samples are needed to draw more precise conclusions on this question.
Data availability
The data of the current study are not publicly available due to the data protection agreement approved by the local ethics committee and signed by the participants. Reasonable requests concerning patient dats are to be addressed to HOK. Analysis scripts can be obtained from CS.
Abbreviations
- CoC :
-
Centre of Cancellation
- IOF:
-
inferior occipitofrontal fasciculus
- rs-fMRI:
-
Resting state functional magnetic resonance imaging
- SLF:
-
superior longitudinal fasciculus
- SOF:
-
superior occipitofrontal fasciculus
- UF:
-
uncinate fasciculus
References
Corbetta, M. & Shulman, G. L. Spatial neglect and attention networks. Annu. Rev. Neurosci. 34, 569–599 (2011).
Karnath, H. O. & Rorden, C. The anatomy of Spatial neglect. Neuropsychologia 50, 1010–1017 (2012).
Nijboer, T., van de Port, I., Schepers, V., Post, M. & Visser-Meily, A. Predicting functional outcome after stroke: the influence of neglect on basic activities in daily living. Front. Hum. Neurosci. 7, 182 (2013).
Moore, M. J., Vancleef, K., Riddoch, M. J., Gillebert, C. R. & Demeyere, N. Recovery of visuospatial neglect subtypes and relationship to functional outcome six months after stroke. Neurorehabil Neural Repair. 35, 823–835 (2021).
Karnath, H. O. Spatial attention systems in Spatial neglect. Neuropsychologia 75, 61–73 (2015).
Ptak, R. & Bourgeois, A. Disengagement of attention with Spatial neglect: A systematic review of behavioral and anatomical findings. Neurosci. Biobehav Rev. 160, 105622 (2024).
Zebhauser, P. T., Vernet, M., Unterburger, E. & Brem, A. K. Visuospatial Neglect - a Theory-Informed overview of current and emerging strategies and a systematic review on the therapeutic use of Non-invasive brain stimulation. Neuropsychol. Rev. 29, 397–420 (2019).
Mort, D. J. et al. The anatomy of visual neglect. Brain 126, 1986–1997 (2003).
Chechlacz, M. et al. Separating neural correlates of allocentric and egocentric neglect: distinct cortical sites and common white matter disconnections. Cogn. Neuropsychol. 27, 277–303 (2010).
Karnath, H. O., Ferber, S. & Himmelbach, M. Spatial awareness is a function of the Temporal not the posterior parietal lobe. Nature 411, 950–953 (2001).
Karnath, H. O., Fruhmann Berger, M., Küker, W. & Rorden, C. The anatomy of Spatial neglect based on Voxelwise statistical analysis: a study of 140 patients. Cereb. Cortex. 14, 1164–1172 (2004).
Husain, M. & Kennard, C. Visual neglect associated with frontal lobe infarction. J. Neurol. 243, 652–657 (1996).
Committeri, G. et al. Neural bases of personal and extrapersonal neglect in humans. Brain 130, 431–441 (2007).
Lunven, M. & Bartolomeo, P. Attention and Spatial cognition: neural and anatomical substrates of visual neglect. Ann. Phys. Rehabil Med. 60, 124–129 (2017).
De Thiebaut, M. et al. Damage to white matter pathways in subacute and chronic Spatial neglect: A group study and 2 single-case studies with complete virtual ‘in vivo’ tractography dissection. Cereb. Cortex. 24, 691–706 (2014).
de Thiebaut, M. et al. Direct evidence for a parietal-frontal pathway subserving Spatial awareness in humans. Science 309, 2226–2228 (2005).
Bartolomeo, P., Thiebaut de Schotten, M. & Doricchi, F. Left unilateral neglect as a Disconnection syndrome. Cereb. Cortex. 17, 2479–2490 (2007).
Doricchi, F., Thiebaut de Schotten, M., Tomaiuolo, F. & Bartolomeo, P. White matter (dis)connections and Gray matter (dys)functions in visual neglect: gaining insights into the brain networks of Spatial awareness. Cortex 44, 983–995 (2008).
Karnath, H. O., Rorden, C. & Ticini, L. F. Damage to white matter fiber tracts in acute Spatial neglect. Cereb. Cortex. 19, 2331–2337 (2009).
Umarova, R. M. et al. Attention-network specific alterations of structural connectivity in the undamaged white matter in acute neglect. Hum. Brain Mapp. 35, 4678–4692 (2014).
Toba, M. N. et al. Common brain networks for distinct deficits in visual neglect. A combined structural and tractography MRI approach. Neuropsychologia 115, 167–178 (2018).
Graff-Radford, N. R., Damasio, H., Yamada, T., Eslinger, P. J. & Damasio, A. R. Nonhaemorrhagic thalamic infarction. Clinical, neuropsychological and electrophysiological findings in four anatomical groups defined by computerized tomography. Brain 108, 485–516 (1985).
Karnath, H. O., Himmelbach, M. & Rorden, C. The subcortical anatomy of human Spatial neglect: putamen, caudate nucleus and pulvinar. Brain 125, 350–360 (2002).
Ten Brink, A. F., Biesbroek, J. M., Oort, Q., Visser-Meily, J. M. A. & Nijboer, T. C. W. Peripersonal and extrapersonal visuospatial neglect in different frames of reference: A brain lesion-symptom mapping study. Behav. Brain Res. 356, 504–515 (2019).
Watson, R. T., Valenstein, E. & Heilman, K. M. Thalamic neglect. Possible role of the medial thalamus and nucleus reticularis in behavior. Arch. Neurol. 38, 501–506 (1981).
Parr, T. & Friston, K. J. The computational anatomy of visual neglect. Cereb. Cortex. 28, 777–790 (2018).
Saxena, S. et al. Disruptions of the human connectome associated with hemispatial neglect. Neurology 98, e107–e114 (2022).
Alves, P. N., Forkel, S. J. & Corbetta, M. & Thiebaut de Schotten, M. The subcortical and neurochemical organization of the ventral and dorsal attention networks. Commun. Biol. 5, (2022).
Warach, S., Dashe, J. F. & Edelman, R. R. Clinical outcome in ischemic stroke predicted by early diffusion-weighted and perfusion magnetic resonance imaging: A preliminary analysis. J. Cereb. Blood Flow. Metab. 16, 53–59 (1996).
Saur, D., Buchert, R., Knab, R., Weiller, C. & Röther, J. Iomazenil-Single-Photon emission computed tomography reveals selective neuronal loss in magnetic Resonance-Defined mismatch areas. Stroke 37, 2713–2719 (2006).
Karnath, H. O. et al. Normalized perfusion MRI to identify common areas of dysfunction: patients with basal ganglia neglect. Brain 128, 2462–2469 (2005).
Hillis, A. E., Tuffiash, E., Wityk, R. J. & Barker, P. B. Regions of neural dysfunction associated with impaired naming of actions and objects in acute stroke. Cogn. Neuropsychol. 19, 523–534 (2002).
Doricchi, F. & Tomaiuolo, F. The anatomy of neglect without hemianopia: A key role for parietal-frontal disconnection? Neuroreport 14, 26–36 (2003).
Cha, S. et al. White matter tracts involved in subcortical unilateral Spatial neglect in subacute stroke. Front. Neurol. 13, (2022).
Karnath, H. O. A right Perisylvian neural network for human Spatial orienting in The Cognitive Neurosciences IV (ed Gazzaniga, M. S.) 259–268 (MIT Press, (2009).
Karnath, H. O., Rennig, J., Johannsen, L. & Rorden, C. The anatomy underlying acute versus chronic Spatial neglect: a longitudinal study. Brain 134, 903–912 (2011).
Sperber, C. & Karnath, H. O. Topography of acute stroke in a sample of 439 right brain damaged patients. NeuroImage Clin. 10, (2016).
Wiesen, D., Sperber, C., Yourganov, G., Rorden, C. & Karnath, H. O. Using machine learning-based lesion behavior mapping to identify anatomical networks of cognitive dysfunction: Spatial neglect and attention. Neuroimage 201, 116000 (2019).
Joliot, M. et al. An atlas of intrinsic connectivity of homotopic areas. J. Neurosci. Methods 254. AICHA, 46–59 (2015).
Weintraub, S. & Mesulam, M. M. Mental State Assessment of Young and Elderly Adults in Behavioral Neurology in Principles of Behavioral Neurology, (ed. Mesulam, M.M.) 71–123 (F.A. Davis Company,1985).
Gauthier, L., Dehaut, F. & Joanette, Y. The bells test: A quantitative and qualitative test for visual neglect. Int. J. Clin. Neuropsychol. 11, 49–54 (1989).
Rorden, C. & Karnath, H. O. A simple measure of neglect severity. Neuropsychologia 48, 2758–2763 (2010).
De Haan, B., Clas, P., Juenger, H., Wilke, M. & Karnath, H. O. Fast semi-automated lesion demarcation in stroke. NeuroImage Clin. 9, 69–74 (2015).
Rorden, C., Bonilha, L., Fridriksson, J., Bender, B. & Karnath, H. O. Age-specific CT and MRI templates for Spatial normalization. Neuroimage 61, 957–965 (2012).
Boes, A. D. et al. Network localization of neurological symptoms from focal brain lesions. Brain 138, 3061–3075 (2015).
Wawrzyniak, M., Klingbeil, J., Zeller, D., Saur, D. & Classen, J. The neuronal network involved in self-attribution of an artificial hand: A lesion network-symptom-mapping study. Neuroimage 166, 317–324 (2018).
Griffis, J. C., Metcalf, N. V., Corbetta, M. & Shulman, G. L. Lesion quantification toolkit: A MATLAB software tool for estimating grey matter damage and white matter disconnections in patients with focal brain lesions. NeuroImage Clin. 30, 102639 (2021).
Yeh, F. C. et al. Population-averaged atlas of the macroscale human structural connectome and its network topology. Neuroimage 178, 57–68 (2018).
Vaessen, M. J., Saj, A., Lovblad, K. O., Gschwind, M. & Vuilleumier, P. Structural white-matter connections mediating distinct behavioral components of Spatial neglect in right brain-damaged patients. Cortex 77, 54–68 (2016).
Bürgel, U. et al. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage 29, 1092–1105 (2006).
Rickers, E. et al. Corticospinal tract lesion quantification: distinct approaches and their association with motor impairment after stroke. Clin. Neurophysiol. 159, e24–e25 (2024).
Mrah, S. et al. Network-level prediction of set-shifting deterioration after lower-grade glioma resection. J. Neurosurg. 1–9. https://doi.org/10.3171/2022.1.jns212257 (2022).
Sperber, C., Gallucci, L., Smaczny, S. & Umarova, R. Bayesian lesion-deficit inference with Bayes factor mapping: key advantages, limitations, and a toolbox. Neuroimage 271, 120008 (2023).
Wagenmakers, E. J. et al. Bayesian inference for psychology. Part II: example applications with JASP. Psychon Bull. Rev. 25, 58–76 (2018).
de Haan, B. & Karnath, H. O. Whose atlas I use, his song I sing?’ – The impact of anatomical atlases on fiber tract contributions to cognitive deficits after stroke. Neuroimage 163, 301–309 (2017).
Riley, J. D. et al. Anatomy of stroke injury predicts gains from therapy. Stroke 42, 421–426 (2011).
Herbet, G. & Duffau, H. Contribution of the medial eye field network to the voluntary deployment of visuospatial attention. Nat. Commun. 13, 328 (2022).
Radanovic, M. & Almeida, V. N. Subcortical aphasia. Curr. Neurol. Neurosci. Rep. 21, 73 (2021).
Damasio, A. R., Damasio, H., Rizzo, M., Varney, N. & Gersh, F. Aphasia with nonhemorrhagic lesions in the basal ganglia and internal capsule. Arch. Neurol. 39, 15–20 (1982).
Alexander, M. P., Naeser, M. A. & Palumbo, C. L. Correlations of subcorticalct lesion sites and aphasia profiles. Brain 110, 961–988 (1987).
Stockert, A. et al. Involvement of thalamocortical networks in patients with poststroke thalamic aphasia. Neurology 100, e485–e496 (2023).
van Ravenzwaaij, D. & Wagenmakers, E. J. Advantages Masquerading as Issues in Bayesian Hypothesis Testing: A Commentary on Tendeiro and Kiers Psychol. Methods 27, 451–465 (2022). (2019).
Funding
Open Access funding enabled and organized by Projekt DEAL.
This work was supported by the Deutsche Forschungsgemeinschaft (KA 1258/23 − 1; SA 1723/5 − 1). Max Wawrzyniak was supported by the Clinician Scientist Program of the Medical Faculty of Leipzig University.
Author information
Authors and Affiliations
Contributions
CS: Conceptualization, Methodology, Data Curation, Formal Analysis, Writing - Original Draft; HR: Methodology, Formal Analysis, Writing - Original Draft; MW: Methodology, Formal Analysis, Writing - Review and Editing; JK: Methodology, Formal Analysis, Writing - Review and Editing; DS: Supervision, Writing - Review and Editing; HOK: Conceptualization, Resources, Investigation, Data Curation, Supervision, Writing - Review and Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sperber, C., Rosenzopf, H., Wawrzyniak, M. et al. Spatial neglect after subcortical stroke may reflect cortico-cortical disconnection. Sci Rep 15, 18544 (2025). https://doi.org/10.1038/s41598-025-01703-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01703-x