Abstract
The prevalence of type 2 diabetes mellitus (T2DM) has reached epidemic proportions globally, posing a significant burden on public health. Dysregulation of lipid metabolism and insulin resistance in T2DM often leads to hepatic complications, making the modulation of microRNAs (miRNAs) associated with these pathways a promising therapeutic target. This study aimed to investigate the protective effects of aerobic training (AT) and vitamin D supplementation on the liver of individuals with T2DM by examining the modulation of miRNAs related to lipid metabolism and insulin resistance. Specifically, the miRNAs examined in this study were miR-33, miR-122, miR-29, and miR-9. Thirty-two male Wistar rats with T2DM were randomly assigned to four groups: Control (C), AT, moderate dose of Vitamin D supplementation (MD; 5,000 IU), and high dose of Vitamin D supplementation (HD; 10,000 IU). The AT group underwent an eight-week program consisting of treadmill running sessions, five days per week, with a gradual increase in intensity and duration. The vitamin D supplementation groups received either 5,000 or 10,000 IU of vitamin D, administered via injection once weekly for 8 weeks. The study used the STZ + HFD rat model and collected liver tissue samples for analysis. Total RNA, including miRNA, was extracted from the liver tissue samples, and the miRNA expression levels were quantified using quantitative real-time PCR (qRT-PCR). Statistical analyses were performed using one-way ANOVA followed by Tukey’s post hoc test. AT led to significantly lower fasting plasma insulin levels (p < 0.05) and a notable improvement in the homeostatic model assessment of insulin resistance (HOMA-IR) index, indicating enhanced insulin sensitivity compared with the control and other groups. It also resulted in significantly lower triglyceride levels (p < 0.01) and a favorable shift in the HDL/LDL ratio, indicative of improved lipid metabolism. Vitamin D supplementation showed a dose-dependent reduction in insulin resistance, with the 10,000 IU group demonstrating a more pronounced improvement compared with the 5,000 IU group. Rats supplemented with vitamin D exhibited a dose-dependent modulation of lipid profile, with the 10,000 IU group demonstrating a more significant decrease in triglycerides and an increase in HDL/LDL ratio. The expression of miR-33, miR-122, miR-29, and miR-9 differed significantly among the experimental groups. The AT group exhibited a significant downregulation of miR-122 and miR-9 while showing a significant upregulation of miR-33 and miR-29 compared to the C and the MD groups. The HD group showed significant downregulation of miR-122 and miR-9 compared to the C and the MD groups. Both AT and high-dose vitamin D supplementation have beneficial effects on insulin levels, insulin resistance, and lipid metabolism in rats with T2DM by modulating miRNA expression, thereby inhibiting insulin resistance and improving T2DM.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is a complex metabolic disorder characterized by dysregulated glucose homeostasis, impaired insulin signaling, and aberrant lipid metabolism1. The liver plays a pivotal role in maintaining metabolic equilibrium, and its dysfunction significantly contributes to the pathogenesis and progression of T2DM-related complications2. MicroRNAs (miRNAs), which are small non-coding RNA molecules, have emerged as critical post-transcriptional regulators involved in modulating lipid metabolism and insulin sensitivity3. Among the miRNAs implicated in these processes, miR-33, miR-122, miR-29, and miR-9 have garnered considerable attention for their roles in hepatic lipid homeostasis and glucose metabolism3.
The selection of specific miRNAs (miR-33, miR-122, miR-29, and miR-9) for this study was guided by their well-documented roles in key metabolic pathways related to lipid metabolism and insulin resistance. MiR-122 is a critical regulator of hepatic lipid metabolism, influencing fatty acid synthesis and oxidation4,5. MiR-9 has been implicated in glucose homeostasis through its effects on gluconeogenesis-related enzymes such as PEPCK and G6Pase6. MiR-33 plays a pivotal role in cholesterol metabolism and fatty acid oxidation by targeting SREBP-1c and FAS, key enzymes in lipogenesis6,7. Additionally, miR-29 is associated with fibrosis regulation and insulin signaling, potentially modulating pathways central to energy metabolism and mitochondrial biogenesis8. These miRNAs were chosen based on their established biological relevance and potential as therapeutic targets in type 2 diabetes mellitus (T2DM). However, further investigation into their regulatory mechanisms and downstream mRNA targets is essential to fully elucidate their biological significance.
The potential of lifestyle interventions, such as aerobic training (AT) and nutritional supplementation, to modulate miRNA expression and improve hepatic dysfunction in T2DM warrants comprehensive investigation9.
AT has been widely recognized for its beneficial effects on insulin sensitivity10, lipid profile11, and hepatic function in individuals with T2DM10. The positive effects of AT are underpinned by a complex interplay of molecular processes; regular AT has been shown to enhance insulin signaling pathways2, leading to improved glucose uptake by skeletal muscles1. In addition, exercise-induced activation of AMP-activated protein kinase (AMPK) facilitates glucose transport and metabolism, contributing to enhanced insulin sensitivity12. Furthermore, AT promotes mitochondrial biogenesis and oxidative capacity, which play pivotal roles in insulin sensitivity and glucose homeostasis13. AT also exerts favorable effects on lipid metabolism by enhancing lipoprotein lipase activity, which facilitates triglyceride clearance from circulation14. Moreover, exercise training promotes the expression of the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), a key regulator of lipid metabolism, leading to an improved lipid profile and reduced cardiovascular risk15. Furthermore, AT modulates the gene expression-promoting pathways involved in lipid oxidation and glucose utilization12,14.
Similarly, vitamin D, a pleiotropic hormone, exhibits regulatory effects on lipid metabolism and insulin action15,16. Vitamin D has been demonstrated to maintain metabolic equilibrium by promoting insulin receptor expression and signaling, modulating the expression and activity of key enzymes involved in hepatic gluconeogenesis, glycogen synthesis, and glucose utilization17. Moreover, vitamin D promotes the activation of peroxisome proliferator-activated receptors (PPARs), which are key regulators of lipid metabolism, leading to enhanced lipid oxidation18. The beneficial effects of AT and vitamin D supplementation on insulin sensitivity, lipid profile, and hepatic function may also be intricately regulated by molecular processes that involve post-transcriptional regulatory pathways, including miRNAs12. miRNAs play a crucial role in modulating gene expression at the post-transcriptional level, thereby influencing key metabolic pathways19. AT may alter the expression of specific miRNAs involved in insulin signaling, lipid metabolism, and hepatic function20. However, the mechanistic underpinnings of how these interventions impact miRNA-mediated pathways in the liver of T2DM individuals remain incompletely understood.
Notably, this study showed that exercise and vitamin D supplementation improved insulin resistance by modulating hepatic miRNA levels. These results provide a new insight into the molecular leveling of the metabolic actions of these interventions. However, to reinforce the results obtained in the study, it is also possible that some methodological details could be highlighted, such as the techniques used to quantify miRNA expression or standardization of collection and processing of samples. Additionally, the associations between miRNA levels and other metabolic parameters, such as insulin resistance, lipid profiles, and glucose homeostasis, should be discussed in more detail. The roles of miRNA-mediated pathways deserve further investigation in terms of their contributions to the metabolic benefits associated with AT and vitamin D supplementation .
This study aims to fill this gap by investigating the impact of eight weeks of an AT program and vitamin D supplementation at two doses (5,000 or 10,000 IU) on the liver tissue expression levels of miR-33 , miR-122, miR-29, and miR-9 in male Wistar rats with T2DM. The results are expected to further our understanding of the complex interrelationships between lifestyle interventions, miRNA expression, and relevant metabolic pathways in hepatic tissue within the context of T2DM, which may have important implications for the development of targeted therapeutic interventions .
.
Methodology
Study design
This study investigated the effects of an eight-week AT program and vitamin D supplementation on the expression of miR-33, miR-122, miR-29, and miR-9 in the liver tissue of male Wistar rats with type 2 diabetes mellitus (T2DM). The study was conducted in compliance with the ethical guidelines and was approved by the Institutional Animal Care and Use of the Ethics in Research Committee of Razi University (no. IR.RAZI.REC.1401.065).
Animal model and housing
Thirty-two male Wistar rats (age: 8–10 weeks; weight: 200–250 g) were used in this study. The rats were housed in a controlled environment with a 12-h light/dark cycle, a temperature of 22 ± 2 °C, and humidity of 50 ± 10%. Standard rodent chow and water were provided ad libitum unless otherwise specified.
Induction of T2DM
The rats were fed a high-fat diet (HFD) for 4 weeks to induce insulin resistance, followed by a single intraperitoneal (IP) injection of streptozotocin (STZ; 35 mg/kg body weight) to induce hyperglycemia. Blood glucose levels were measured 72 h after STZ injection, and rats with fasting blood glucose levels ≥ 250 mg/dL were considered diabetic and included in the study.
Experimental groups
The rats were randomly assigned to four groups (n = 8 per group):
-
1.
Control group (C): No intervention.
-
2.
Aerobic training group (AT): Rats underwent an eight-week AT program.
-
3.
Moderate-dose vitamin D group (MD): Rats received 5,000 IU of vitamin D (cholecalciferol) weekly via intraperitoneal (IP) injection.
-
4.
High-dose vitamin D group (HD): Rats received 10,000 IU of vitamin D (cholecalciferol) weekly via intraperitoneal (IP) injection.
Intervention programs
Vitamin D supplementation
The vitamin D doses were selected based on previous studies demonstrating their safety and efficacy in rodent models15,21. Due to the high concentration of vitamin D, which makes it unsuitable for injection using an insulin syringe, it was dissolved in sesame oil to enable precise and consistent administration. The selected doses of 5,000 and 10,000 IU/week were chosen to ensure a biologically effective concentration of vitamin D that modulates metabolic pathways while remaining within the safe and well-tolerated range established in previous rodent studies. Higher doses are often required in rodents to achieve physiological effects comparable to those in humans due to differences in metabolic rates and vitamin D metabolism. To control for potential stress effects, the control group received an equivalent volume of sesame oil without vitamin D21,22.
The AT protocol
Rats in the AT group underwent an eight-week treadmill running program, conducted five days per week. Before the formal training, all rats completed a 1-week familiarization period, which included daily 5-minute sessions at a speed of 8–10 m/min on a zero-incline treadmill to minimize stress and ensure acclimation. The formal exercise regimen began with 15-minute sessions at a speed of 10 m/min and gradually increased to 30-minute sessions at a speed of 25 m/min by the end of the program. The treadmill was set at a 0-degree slope, and the exercise intensity was maintained at 60–70% of the rats’ maximal oxygen consumption (VO₂ max), as estimated using established rodent protocols )23. Each session included a 10-minute warm-up and cool-down period at 5 m/min to prevent injury and promote recovery. Compliance with the training protocol was closely monitored, and all rats completed the program without signs of excessive fatigue or injury. Detailed progression and monitoring data are provided in Table 1.
Measurement variables
Food intake, body weight, and body mass index
All animals were reweighed on a scale weekly between the hours of 9:00 and 11:30 a.m. The body mass index (BMI) was then computed in nose-to-anus length units. Food intake (FI) was calculated by subtracting the weight of uneaten food from the total 20 g/day given period .
Sample collection
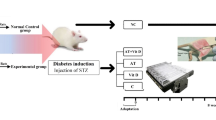
Forty-eight hours after the last training session, the rats were anesthetized using a combination of xylazine (5 mg/kg) and ketamine (40 mg/kg) administered via intraperitoneal injection. Blood samples were collected from the vena cava for the assessment of insulin levels and lipid profile, including triglycerides (TG), total cholesterol (TC), low-density lipoprotein (LDL), and high-density lipoprotein (HDL). Liver tissue was immediately excised, snap-frozen in liquid nitrogen, and stored at − 80 °C until further processing (Fig. 1). Following sample collection, the rats were euthanized using pentobarbital sodium (100 mg/kg body weight) administered via intraperitoneal injection.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA, including miRNA, was extracted from the liver tissue using the Qiagen RNeasy Mini Kit (Qiagen, Germany) following the manufacturer’s instructions. RNA quality and quantity were assessed using a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA). The miRNA fraction was enriched using the Ambion mirVana miRNA Isolation Kit (Thermo Fisher Scientific, USA). Reverse transcription was performed using the Applied Biosystems TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific, USA), and miRNA expression levels (miR-33, miR-122, miR-29, and miR-9) were quantified using TaqMan MicroRNA Assays on a real-time PCR system. U6 small nuclear RNA (RNU6-2) served as the endogenous control. Relative miRNA expression was calculated using the 2−ΔΔCT method.
Statistical analysis
Data were analyzed using GraphPad Prism software (GraphPad Software, USA). Differences between the groups were assessed using one-way ANOVA followed by Tukey’s post hoc test. A p-value < 0.05 was considered statistically significant. miRNA expression patterns were validated using northern blotting to ensure the reliability of the qRT–PCR results.
Results
Body composition, food intake, insulin resistance, and lipid profile
The one-way ANOVA in Tables 2 and 3 revealed a statistically significant difference in body weight, BMI, FI, glucose, insulin, and HOMA-IR among the experimental groups. AT resulted in a significantly lower body weight, BMI, FI, glucose, insulin, and HOMA-IR (p < 0.05) compared to HD, MD, and C. Likewise, vitamin D supplementation led to a dose-dependent reduction in insulin resistance, and both HD and MD caused a significant reduction in body weight, BMI, FI, glucose, insulin, and HOMA-IR (p < 0.05) compared to C. There was also a significant difference in body weight, BMI, FI, glucose, insulin, and HOMA-IR (p < 0.05) between HD and MD (p < 0.05).
Regarding lipid profile parameters, significantly higher HDL/LDL ratio and lower TC and TG levels (p < 0.01) have been observed in AT compared to HD, MD, and C. There were significant differences between AT and HD in the lipid profile parameters. However, HD demonstrated significantly lower TC and TG levels (p < 0.01) and a higher HDL/LDL ratio compared to MD and C. Also, rats in the MD group exhibited a significant modulation of lipid profile, with significantly decreased TC and TG levels (p < 0.01) and increased HDL/LDL ratio compared to C (Table 3).
MiRNA expression
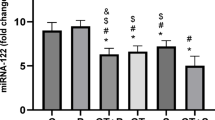
The one-way ANOVA in Fig. 2A–D revealed a statistically significant difference in the expression of miR-33, miR-122, miR-29, and miR-9 among the experimental groups (p < 0.01). The post hoc tests indicated that the AT exhibited a significant downregulation of miR-122 and miR-9 and a significant upregulation of miR-33 and miR-29 compared with the MD and C (p < 0.01). Also, there was a significant difference in the miR-33 and miR-29 expression between AT and HD, with AT exhibiting a significant upregulation. However, there were no such significant differences in miR-122 and miR-9 between AT and HD.
miRNAs fold change compared to the control. (A) miR-122, (B) miR-9, (C) miR-33, and (D) miR-29 expression normalized to U6 small nuclear RNA (Significant changes compared to control represented as *p ≤ 0.05, ** p < 0.01, ***p < 0.001, and ****p < 0.0001). Data expressed are expressed as means ± SEM.
Additionally, the HD showed significant downregulation of miR-122 and miR-9 compared to the C (p = 0.005) and MD (p = 0.009). However, the HD showed no significant alteration in miR-33 and miR-29 compared to the control. MD resulted in the downregulation of miR-122 compared with C; however, there were no significant differences in miR-33, miR-29, and miR-9 between MD and C (p = 0.005).
Discussion
The present study aimed to investigate the effects of 8-week AT and vitamin D supplementation on miRNAs related to lipid metabolism and insulin resistance in the liver tissue of male Wistar rats with T2DM. The results demonstrated significant improvements in insulin sensitivity, lipid profiles, and specific miRNA expression profiles.
The results indicated a statistically significant difference in body weight, BMI, FI, glucose, insulin, and HOMA-IR among the experimental groups. AT notably improved insulin sensitivity, as evidenced by lower fasting plasma insulin levels and a better HOMA-IR. These results are consistent with previous research that has established the efficacy of exercise in enhancing insulin sensitivity and glucose metabolism in diabetic conditions24,25,26. AT improves insulin sensitivity through a complex interplay of cellular and molecular mechanisms that involve the upregulation of the expression and activity27, which facilitates glucose uptake28, reduction of adipokine and inflammatory responses29 by improving the adipokine profile30, enhancement of insulin signal transduction by activating key proteins and enzymes31 such as AMP-activated protein kinase (AMPK)14, and modulation of oxidative stress13,32, thereby protecting the insulin signaling pathway and improving insulin sensitivity33.
Vitamin D supplementation also contributed to a dose-dependent reduction in insulin resistance, with the higher dose (10,000 IU) showing a more pronounced effect. This aligns with the existing literature on the role of vitamin D in modulating insulin resistance and glycemic control34,35. Previous studies have shown a similar reduction following both 8-week AT and vitamin D supplementation9,36,37. The cellular and molecular mechanisms by which vitamin D supplementation reduces insulin resistance involve the phosphorylation of insulin receptor substrate (IRS) proteins35, modulation of the production and activity of various pro-inflammatory cytokines38,39, and support for pancreatic beta cell function40 via enhancing the secretion of insulin from beta cells41 and promoting beta cell survival42. These mechanisms collectively contribute to improved insulin sensitivity and glycemic control.
This study also observed significant improvements in lipid metabolism, with AT leading to lower TC and TG levels and a favorable HDL/LDL ratio. The cellular mechanisms behind the beneficial effects of AT on lipid metabolism include increased lipid utilization leading to TG lipolysis43,44, enhanced lipid oxidation via optimizing beta-oxidation45, improved lipoprotein lipase activity46, and regulation of cholesterol transport47. The observed improvements in metabolic parameters may also be attributed to the modulation of key signaling pathways and gene expression. AT activates pathways such as AMPK and PGC-1α, which play crucial roles in energy metabolism and mitochondrial biogenesis48. Other underlying mechanisms might involve the regulation of gene expressions related to lipid metabolism, the inhibition of the activity of lipogenic enzymes, thus reducing the synthesis of fatty acids and TG in the liver, and the enhancement of insulin sensitivity through the modulation of Akt, PEPCK, and G6Pase expression1.
The results of this study highlight the potential molecular mechanisms underlying the improvements in insulin resistance and lipid profile observed in T2DM following AT and high-dose vitamin D supplementation. Specifically, this study examined the impact of these interventions on the expression levels of key miRNAs known to be involved in metabolic regulation.
The results revealed that AT downregulated miR-122 and miR-9. MiR-122 has been previously implicated in the regulation of hepatic lipid metabolism and insulin resistance. By downregulating miR-122, AT may promote a reduction in hepatic lipid accumulation and improve insulin sensitivity15,21. Similarly, miR-9 was associated with insulin resistance and inflammatory processes. The downregulation of miR-9 through AT suggests a potential role in mitigating insulin resistance and inflammation in T2DM21. Conversely, AT was found to upregulate miR-33 and miR-29. MiR-33 modulates cholesterol metabolism and fatty acid oxidation49. The upregulation of miR-33 following AT suggests a potential mechanism through which lipid metabolism is improved, leading to a favorable lipid profile50. Moreover, the upregulation of miR-29 through AT may contribute to the prevention or reduction of fibrosis-related complications in T2DM.
In addition to AT, high-dose vitamin D supplementation demonstrated significant effects on miRNA expression. Specifically, it downregulated miR-122 and miR-9. This finding suggests that vitamin D supplementation may exert a similar effect as AT on hepatic lipid metabolism and insulin resistance through the regulation of these miRNAs. However, miR-33 and miR-29 expression showed no significant alteration following high-dose vitamin D supplementation. This indicates that the impact of vitamin D on these particular miRNAs may be different from that of AT.
The observed changes in miR-33, miR-122, miR-29, and miR-9 expression are closely linked to their downstream targets and their roles in key metabolic pathways. For instance, miR-33 regulates cholesterol and fatty acid metabolism by targeting SREBP-1c and FAS, which are key enzymes in lipogenesis51. The downregulation of miR-122, a well-established regulator of hepatic lipid metabolism, may reduce lipid accumulation by modulating genes involved in fatty acid synthesis and oxidation52. Similarly, miR-9 has been implicated in glucose homeostasis by targeting enzymes such as PEPCK and G6Pase, which are critical for gluconeogenesis51,52. Additionally, miR-29 plays a role in fibrosis and insulin signaling, potentially influencing pathways like AMPK and PGC-1α, which are central to energy metabolism and mitochondrial biogenesis53. Although this study did not directly measure these target genes, the observed miRNA changes suggest potential mechanisms through which AT and vitamin D supplementation improve metabolic outcomes. Although the differences in miRNA expression between the moderate-dose vitamin D (MD) and control (C) groups were not statistically significant, several glucose-related indicators, such as fasting insulin, HOMA-IR, and blood glucose levels, showed notable improvements in the MD group. This discrepancy suggests that the beneficial effects of vitamin D supplementation on glucose metabolism may not be solely mediated by changes in the expression of the miRNAs examined in this study (miR-33, miR-122, miR-29, and miR-9)49,50. It is possible that other molecular mechanisms, such as the modulation of insulin signaling pathways, inflammatory responses, or oxidative stress, play a more direct role in the observed improvements in glucose homeostasis21,50. Alternatively, the lack of significant changes in miRNA expression in the MD group may indicate that the dose of 5,000 IU was insufficient to induce detectable alterations in these specific miRNAs, despite its positive impact on metabolic parameters21.
While the observed changes in miRNA expression provide valuable insights into the molecular mechanisms underlying the metabolic improvements induced by aerobic training (AT) and vitamin D supplementation, the biological significance of these findings remains incomplete without directly measuring the expression of mRNAs regulated by the selected miRNAs. For instance, miR-122 and miR-9 are known to regulate genes involved in lipid metabolism and gluconeogenesis, respectively, while miR-33 and miR-29 target pathways critical for cholesterol homeostasis and fibrosis prevention51,52. Direct measurement of these mRNA targets would help clarify the functional implications of miRNA modulation and strengthen the interpretation of our results. Additionally, conducting further experiments to explore the interactions between these miRNAs and broader molecular pathways, such as AMPK signaling and mitochondrial biogenesis, would enhance our understanding of the mechanisms driving the observed metabolic benefits. Future studies should aim to address these gaps to provide a more comprehensive view of the therapeutic potential of AT and vitamin D interventions in T2DM.
These results highlight the complexity of the relationship between miRNA expression and metabolic outcomes. While the study focused on the role of miRNAs in mediating the effects of AT and vitamin D supplementation, the improvements in glucose-related indicators in the absence of significant miRNA changes suggest that additional pathways may be involved. Future studies could explore the correlation between miRNA expression levels and specific metabolic parameters, such as blood glucose and insulin concentrations, to better understand the interplay between these factors. Such investigations could provide deeper insights into the mechanisms underlying the metabolic benefits of these interventions and identify potential biomarkers for monitoring therapeutic responses.
The observed changes in miRNA expression patterns in response to both AT and high-dose vitamin D supplementation provide potential molecular mechanisms through which these interventions may improve insulin resistance and lipid profile in T2DM. The downregulation of miR-122 and miR-9, which are associated with insulin resistance and inflammation, suggests a reduction in these harmful processes. Conversely, the upregulation of miR-33 and miR-29, which are involved in lipid metabolism and fibrosis regulation, respectively, supports the beneficial effects on the lipid profile and prevention of fibrosis-related complications. miRNAs are only one piece of the complex regulatory puzzle governing insulin resistance and lipid metabolism.
The results of this study align with the existing literature demonstrating the beneficial effects of AT and vitamin D supplementation on metabolic health. For instance, similar to our results, previous studies have shown that AT enhances mitochondrial biogenesis and AMPK signaling, leading to improved glucose and lipid metabolism54,55. Likewise, vitamin D supplementation reduces insulin resistance and inflammation, supporting our observations of improved metabolic parameters56,57. However, data on the combined effects of AT and vitamin D in rodent models of metabolic disorders remain limited. While some human studies suggest the synergistic benefits of combining exercise and vitamin D supplementation, direct comparisons with rodent studies are scarce. Our study contributes to this emerging field by demonstrating that both interventions, either alone or in combination, modulate miRNA expression and metabolic outcomes in a T2DM rodent model.
Nevertheless, further research is warranted to validate these findings and elucidate the underlying mechanisms in greater detail, particularly through rodent-specific studies. Such investigations will help bridge the gap between preclinical and clinical research, offering a more comprehensive understanding of the therapeutic potential of combined AT and vitamin D interventions. In addition, future studies should explore the precise mechanisms through which these miRNAs interact with other molecular pathways and cellular processes, such as AMPK signaling, mitochondrial biogenesis, and inflammatory responses. Despite these gaps, the results of this study contribute to the growing body of knowledge in this field and identify potential molecular targets for future therapeutic strategies aimed at improving metabolic outcomes in individuals with type 2 diabetes mellitus (T2DM).
Limitations
Although this study provides valuable insights into the effects of AT and vitamin D supplementation on miRNA expression and metabolic outcomes in a T2DM rodent model, several limitations should be acknowledged. First, the absence of baseline vitamin D levels limits our ability to assess the magnitude of supplementation effects and the initial vitamin D status of the animals. Second, although changes in miRNA expression were observed, downstream protein or gene targets (e.g., AMPK, GLUT4, and hepatic enzymes such as PEPCK and G6Pase) were not measured, which restricts our ability to draw causal conclusions about the mechanisms underlying the observed improvements. Third, the relatively small sample size (eight rats per group) may increase susceptibility to inter-individual variability, potentially affecting the robustness of the miRNA expression data. Larger cohorts or repeated measures in future studies could strengthen the reliability of these findings. Finally, while efforts were made to minimize stress during the intervention, the lack of detailed information on compliance and potential stress effects on the rodents may have confounded the interpretation of the results. Addressing these limitations in future research will provide a more comprehensive understanding of the molecular and metabolic effects of AT and vitamin D supplementation in T2DM.
Conclusion
In conclusion, this study demonstrated that 8 weeks of AT and vitamin D supplementation significantly improved insulin sensitivity, lipid profiles, and hepatic miRNA expression in a T2DM rodent model. AT downregulated miR-122 and miR-9, which are associated with insulin resistance and inflammation, while upregulating miR-33 and miR-29, which play roles in lipid metabolism and fibrosis regulation. High-dose vitamin D supplementation similarly downregulated miR-122 and miR-9, suggesting overlapping mechanisms with AT. These findings highlight the potential of AT and vitamin D to modulate miRNA expression and improve metabolic outcomes in T2DM. However, the lack of significant miRNA changes in the moderate-dose vitamin D group, despite improvements in glucose-related indicators, suggests that additional pathways, such as insulin signaling and oxidative stress modulation, may also contribute to the observed benefits. Future studies should explore the precise molecular mechanisms underlying these effects and investigate the translational potential of these interventions in clinical settings. Overall, this study provides valuable insights into the therapeutic potential of lifestyle interventions and identifies miRNA targets for further research in the management of T2DM and its complications.
Data availability
“The datasets generated and analyzed during the current study are not publicly available due to ongoing data analysis but are available from the corresponding author upon reasonable request.”
References
Hoseini, Z., Behpour, N. & Hoseini, R. Vitamin D improves the antidiabetic effectiveness of aerobic training via modulation of Akt, PEPCK, and G6Pase expression. Diabetol. Metab. Syndr. 15 (1), 184 (2023).
Hoseini, Z., Behpour, N. & Hoseini, R. Aerobic training with moderate or high doses of vitamin D improve liver enzymes, LXRα and PGC-1α levels in rats with T2DM. Sci. Rep. 14 (1), 6409 (2024).
Nigi, L. et al. MicroRNAs as regulators of insulin signaling: research updates and potential therapeutic perspectives in type 2 diabetes. Int. J. Mol. Sci. 19 (12), 3705 (2018).
Qiang, J. et al. High fat diet-induced miR-122 regulates lipid metabolism and fat deposition in genetically improved farmed tilapia (GIFT, Oreochromis niloticus) liver. Front. Physiol. 9, 1422 (2018).
Masoudi, F., Sharifi, M. R. & Pourfarzam, M. Investigation of the relationship between miR-33a, miR-122, erythrocyte membrane fatty acids profile, and serum lipids with components of metabolic syndrome in type 2 diabetic patients. Res. Pharm. Sci. 17 (3), 242–251 (2022).
Liu, H. et al. Enhanced alleviation of insulin resistance via the IRS-1/Akt/FOXO1 pathway by combining Quercetin and EGCG and involving miR-27a-3p and miR-96–5p. Free Radic. Biol. Med. 181, 105–117 (2022).
Ortega, R., Liu, B. & Persaud, S. J. Effects of miR-33 deficiency on metabolic and cardiovascular diseases: implications for therapeutic intervention. Int. J. Mol. Sci. 24 (13), 10777 (2023).
Wang, M. et al. The role of MiR-29 in the mechanism of fibrosis. Mini Rev. Med. Chem. 23 (19), 1846–1858 (2023).
Hoseini, R., Rahim, H. A. & Ahmed, J. K. Decreased inflammatory gene expression accompanies the improvement of liver enzyme and lipid profile following aerobic training and vitamin D supplementation in T2DM patients. BMC Endocr. Disorders. 22 (1), 245 (2022).
Hoseini, Z., Behpour, N. & Hoseini, R. Co-treatment with vitamin D supplementation and aerobic training in elderly women with Vit D deficiency and NAFLD: A single-blind controlled trial. Hepat. Mon. 20(2). (2020).
Hoseini, Z., Behpour, N. & Hoseini, R. Vitamin D improves lipid profile and promotes beneficial effects of aerobic training in elderly women with NAFLD. Sci. Sports. 35 (6), 399–401 (2020).
Khaledi, K., Hoseini, R. & Gharzi, A. Effects of aerobic training and vitamin D supplementation on glycemic indices and adipose tissue gene expression in type 2 diabetic rats. Sci. Rep. 13 (1), 10218 (2023).
Hoseini, R., Rahim, H. A. & Ahmed, J. K. Concurrent alteration in inflammatory biomarker gene expression and oxidative stress: how aerobic training and vitamin D improve T2DM. BMC Complement. Med. Ther. 22 (1), 165 (2022).
Khaledi, K., Hoseini, R. & Gharzi, A. The impact of vitamin D on type 2 diabetes management: boosting PTP1B gene expression and physical activity benefits in rats. Genes Nutr. 19 (1), 1–11 (2024).
Hoseini, R., Damirchi, A. & Babaei, P. Vitamin D increases PPARγ expression and promotes beneficial effects of physical activity in metabolic syndrome. Nutrition 36, 54–59 (2017).
Bahmani, E., Hoseini, R. & Amiri, E. The effect of home-based aerobic training and vitamin D supplementation on fatigue and quality of life in patients with multiple sclerosis during COVID-19 outbreak. Sci. Sports. 37 (8), 710–719 (2022).
Szymczak-Pajor, I. & Śliwińska, A. Analysis of association between vitamin D deficiency and insulin resistance. Nutrients 11 (4), 794 (2019).
Marino, M. et al. Vitamin D counteracts lipid accumulation, augments free fatty acid-induced ABCA1 and CPT-1A expression while reducing CD36 and C/EBPβ protein levels in monocyte-derived macrophages. Biomedicines 10 (4), 775 (2022).
Alshahrani, S. H. et al. Metabolic reprogramming by MiRNAs in the tumor microenvironment: focused on immunometabolism. Front. Oncol. 12, 1042196 (2022).
Improta Caria, A. C. et al. Exercise training-induced changes in MicroRNAs: beneficial regulatory effects in hypertension, type 2 diabetes, and obesity. Int. J. Mol. Sci. 19 (11), 3608 (2018).
Mazaheri, F., Hoseini, R. & Gharzi, A. Vitamin D and exercise improve VEGF-B production and IGF-1 levels in diabetic rats: insights the role of miR-1 suppression. Sci. Rep. 15 (1), 1328 (2025).
Khaledi, K., Hoseini, R. & Gharzi, A. The impact of vitamin D on type 2 diabetes management: boosting PTP1B gene expression and physical activity benefits in rats. Genes Nutr. 19 (1), 4 (2024).
Høydal, M. A., Wisløff, U., Kemi, O. J. & Ellingsen, Ø. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur. J. Prev. Cardiol. 14 (6), 753–760 (2007).
Syeda, U. A., Battillo, D., Visaria, A. & Malin, S. K. The importance of exercise for glycemic control in type 2 diabetes. Am. J. Med. Open. 9, 100031 (2023).
Gallardo-Gómez, D. et al. Optimal dose and type of physical activity to improve glycemic control in people diagnosed with type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 47 (2), 295–303 (2024).
Marcotte-Chénard, A. & Little, J. P. Towards optimizing exercise prescription for type 2 diabetes: Modulating exercise parameters to strategically improve glucose control. Transl. Exerc. Biomed. (2024).
Richter, E. A., Sylow, L. & Hargreaves, M. Interactions between insulin and exercise. Biochem. J. 478 (21), 3827–3846 (2021).
Wang, T., Wang, J., Hu, X., Huang, X-J. & Chen, G-X. Current Understanding of glucose transporter 4 expression and functional mechanisms. World J. Biol. Chem. 11 (3), 76 (2020).
Gonzalez-Gil, A. M. & Elizondo-Montemayor, L. The role of exercise in the interplay between myokines, hepatokines, osteokines, adipokines, and modulation of inflammation for energy substrate redistribution and fat mass loss: a review. Nutrients 12 (6), 1899 (2020).
Babaei, P. & Hoseini, R. Exercise training modulates adipokine dysregulations in metabolic syndrome. Sports Med. Health Sci. 4 (1), 18–28 (2022).
Imierska, M., Kurianiuk, A. & Błachnio-Zabielska, A. The influence of physical activity on the bioactive lipids metabolism in obesity-induced muscle insulin resistance. Biomolecules 10 (12), 1665 (2020).
Suzuki, K., Tominaga, T., Ruhee, R. T. & Ma, S. Characterization and modulation of systemic inflammatory response to exhaustive exercise in relation to oxidative stress. Antioxidants 9 (5), 401 (2020).
Bronczek, G. A. et al. Resistance exercise training improves glucose homeostasis by enhancing insulin secretion in C57BL/6 mice. Sci. Rep. 11 (1), 8574 (2021).
Pramono, A., Jocken, J. W., Blaak, E. E. & van Baak, M. A. The effect of vitamin D supplementation on insulin sensitivity: a systematic review and meta-analysis. Diabetes Care. 43 (7), 1659–1669 (2020).
Krisnamurti, D. G. B. et al. Vitamin D supplementation alleviates insulin resistance in prediabetic rats by modifying IRS-1 and PPARγ/NF-κB expressions. Front. Endocrinol. 14, 1089298 (2023).
Nazarabadi, P. N., Etemad, Z., Hoseini, R. & Moradi, F. Anti-Inflammatory effects of a period of aerobic training and vitamin D supplementation in postmenopausal women with metabolic syndrome. Int. J. Prev. Med. 13 (1), 60 (2022).
Najafi Nazar Abadi, P., Etemad, Z. & Hoseini, R. How combined exercise and vitamin D supplementation affect metabolic syndrome risk factors: a clinical trial in menopausal women. J. Fasa Univ. Med. Sci. 10 (3), 2498–2508 (2020).
Krajewska, M., Witkowska-Sędek, E. & Rumińska, M. Vitamin D effects on selected anti-inflammatory and pro-inflammatory markers of obesity-related chronic inflammation. Front. Endocrinol. 13, 920340 (2022).
Zughaier, S. M., Lubberts, E. & Bener, A. Immune-modulatory effects of vitamin D. Front. Immunol. 11, 596611 (2020).
Rasouli, N. et al. Effects of vitamin D supplementation on insulin sensitivity and secretion in prediabetes. J. Clin. Endocrinol. Metabolism. 107 (1), 230–240 (2022).
Wu, M., Lu, L., Guo, K., Lu, J. & Chen, H. Vitamin D protects against high glucose-induced pancreatic β-cell dysfunction via AMPK-NLRP3 inflammasome pathway. Mol. Cell. Endocrinol. 547, 111596 (2022).
Szymczak-Pajor, I., Drzewoski, J. & Śliwińska, A. The molecular mechanisms by which vitamin D prevents insulin resistance and associated disorders. Int. J. Mol. Sci. 21 (18), 6644 (2020).
Muscella, A., Stefàno, E., Lunetti, P., Capobianco, L. & Marsigliante, S. The regulation of fat metabolism during aerobic exercise. Biomolecules 10 (12), 1699 (2020).
Rodrigues, A., Prímola-Gomes, T., Peluzio, M., Hermsdorff, H. & Natali, A. Aerobic exercise and lipolysis: A review of the β-adrenergic signaling pathways in adipose tissue. Sci. Sports. 36 (1), 16–26 (2021).
Langlois, A., Forterre, A., Pinget, M. & Bouzakri, K. Impact of moderate exercise on fatty acid oxidation in pancreatic β-cells and skeletal muscle. J. Endocrinol. Investig. 44, 1815–1825 (2021).
Greiwe, J. S., Holloszy, J. O. & Semenkovich, C. F. Exercise induces lipoprotein lipase and GLUT-4 protein in muscle independent of adrenergic-receptor signaling. J. Appl. Physiol. 89 (1), 176–181 (2000).
Rahmati-Ahmadabad, S., Broom, D. R., Ghanbari-Niaki, A. & Shirvani, H. Effects of exercise on reverse cholesterol transport: A systemized narrative review of animal studies. Life Sci. 224, 139–148 (2019).
Gu, C., Yan, J., Zhao, L., Wu, G. & Wang, Y. Regulation of mitochondrial dynamics by aerobic exercise in cardiovascular diseases. Front. Cardiovasc. Med. 8, 788505 (2022).
Kurtz, C. L. et al. MicroRNA-29 fine-tunes the expression of key FOXA2-activated lipid metabolism genes and is dysregulated in animal models of insulin resistance and diabetes. Diabetes 63 (9), 3141–3148 (2014).
Wang, X-C., Zhan, X-R., Li, X-Y., Yu, J-J. & Liu, X-M. MicroRNA-185 regulates expression of lipid metabolism genes and improves insulin sensitivity in mice with non-alcoholic fatty liver disease. World J. Gastroenterology: WJG. 20 (47), 17914 (2014).
Goedeke, L. et al. Long-term therapeutic Silencing of miR‐33 increases Circulating triglyceride levels and hepatic lipid accumulation in mice. EMBO Mol. Med. 6 (9), 1133–1141 (2014).
López-Pastor, A. R., Infante-Menéndez, J., Escribano, Ó. & Gómez-Hernández, A. MiRNA dysregulation in the development of non-alcoholic fatty liver disease and the related disorders type 2 diabetes mellitus and cardiovascular disease. Front. Med. 7, 527059 (2020).
Caravia, X. M. et al. The microRNA-29/PGC1α regulatory axis is critical for metabolic control of cardiac function. PLoS Biol. 16 (10), e2006247 (2018).
Marcinko, K. & Steinberg, G. R. The role of AMPK in controlling metabolism and mitochondrial biogenesis during exercise. Exp. Physiol. 99 (12), 1581–1585 (2014).
Yan, W. et al. Impaired mitochondrial biogenesis due to dysfunctional adiponectin-AMPK-PGC-1α signaling contributing to increased vulnerability in diabetic heart. Basic Res. Cardiol. 108, 1–15 (2013).
Garbossa, S. G., Folli, F. & Vitamin, D. sub-inflammation and insulin resistance. A window on a potential role for the interaction between bone and glucose metabolism. Reviews Endocr. Metabolic Disorders. 18 (2), 243–258 (2017).
Wenclewska, S., Szymczak-Pajor, I., Drzewoski, J., Bunk, M. & Śliwińska, A. Vitamin D supplementation reduces both oxidative DNA damage and insulin resistance in the elderly with metabolic disorders. Int. J. Mol. Sci. 20 (12), 2891 (2019).
Acknowledgements
The authors are thankful to Dr. Ahmad Gharezi for his technical advice during the study.
Funding
The authors declare that the research did not receive any financial grants.
Author information
Authors and Affiliations
Contributions
“ZH and NB were responsible for the study concept and design, the data curation, project administration, and formal analysis, and wrote the original draft. RH contributed to the investigation and the methodology. All authors wrote, read, and approved the final manuscript draft.”
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All actions performed on the animals followed the guidelines of the Ethics Committee of the Razi University of Kermanshah (IR.RAZI.REC.1401.065 on 2022/11/30) and followed the ARRIVE guidelines.
Consent for publication
All the authors have provided their consent for the publication of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hoseini, Z., Behpour, N. & Hoseini, R. Aerobic training and vitamin D modulate hepatic miRNA expression to improve lipid metabolism and insulin resistance in type 2 diabetes. Sci Rep 15, 16764 (2025). https://doi.org/10.1038/s41598-025-01757-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01757-x