Abstract
Background and objectives Vestibular schwannomas are known to demonstrate tumor expansion, commonly referred to as pseudoprogression, after SRS. It is critical to differentiate between true tumor progression and pseudoprogression as this may entail performing an unnecessary intervention, such as surgery or repeat radiosurgery. This study aims to identify the fate of tumor enlargement that may occur after SRS for vestibular schwannomas and to propose a management algorithm for vestibular schwannoma enlargement after SRS. Methods In this retrospective study, we included 171 patients with sporadic vestibular schwannomas who showed tumor enlargement after SRS. The mean dose was 11.9 Gy (10–12 Gy). The mean tumor volume was 4.1 cc (0.1–19.7 cc). More than half of the tumors were Koos grade 4 (Koos 1: 8 (5%), 2: 20 (12%), 3: 43 (25%), 4: 100 (58%)). Volumetric changes and clinical outcomes were recorded. Different progression patterns were recorded according to the tumor volume changes (TVC) and the timings of TVC. Results The pseudoprogression rate among the patients who showed tumor enlargement after SRS was 83% (142/171). The mean follow-up duration was 64 months (12–241 months). The actuarial progression-free survival at 5-,7- and 10-years was 95%, 92%, and 90%, respectively. The mean follow-up duration was 64 months (12–241 months). The mean TVC at progression (TVCp) was 72% (11–439%). The mean time to tumor progression was 13 months (2–160 months) and the mean duration of TVC was 15 months (2–164 months). Late pseudoprogression (after 3 years) occurred in 30 patients (21%). In early PP, there was a shorter duration of volume change, and a presence of CLC. In true progression, there was a bigger TVCp, a bigger TVCf and a bigger tumor volume at the final follow-up (TVf). Clinical decline was observed with tumor enlargement in 36% of the patients, but in most of them, improvement occurred without the need for tumor intervention. Conclusion GKR for VS is associated with radiation-induced tumor enlargement in a group of patients. Pseudoprogression may occur beyond 5 years after treatment. A more conservative approach may be adopted in most vestibular schwannomas that exhibit tumor enlargement after SRS, as in most cases, they will eventually be controlled.
Similar content being viewed by others
Introduction
Vestibular schwannomas are estimated to comprise 8% of intracranial tumors1. More than 90% are of the sporadic type2. Depending on the radiological and clinical context, management may be observation, surgery or SRS. Tumor control after SRS ranges from 90 to 99% depending on but not limited to the tumor size, follow-up duration, and dose3. Vestibular schwannomas are known to demonstrate transient expansion after SRS, known as pseudoprogression4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20. Pseudoprogression is defined as a temporary increase in tumor size followed by tumor shrinkage or stability. It is critical to differentiate between true tumor progression and pseudoprogression, as this may entail performing an unnecessary intervention, such as surgery or repeat radiosurgery.
In this study, we aim to demonstrate the fate of tumor enlargement after SRS for sporadic vestibular schwannomas and associated clinical manifestations and suggest a management algorithm.
Methods
This was a retrospective study. Between June 2001 and June 2022, 1667 patients with sporadic VS were treated by single-session GK. From these, 1411 patients were available for follow-up. Tumor progression was defined as a volume increase of > 10% from the initial tumor volume. Inclusion criteria were patients with sporadic vestibular schwannomas, showing more than 10% increase in tumor volume after SRS and with more than one year follow-up after recorded tumor progression. Exclusion criteria were patients with NF2 and less than one year follow-up. One hundred and seventy-one patients fulfilled the inclusion criteria in this study. The patient demographics and treatment parameters are summarized in Tables 1 and 2, respectively.
True progression, synonymous with treatment failure, was considered if there was a progressive increase in tumor size and/or progressive neurological deterioration, requiring some form of tumor intervention. Pseudoprogression was defined as tumor progression after treatment followed by tumor stability or shrinkage without the need for tumor intervention. All methods were carried out in accordance with appropriate guidelines. Approval was obtained from the relevant IRB (Ethical Committee of the Specialized Medical Centers (SMC)). Informed consent was obtained from all subjects and/or their legal guardian(s). This study was conducted and reported in accordance with the STROBE guidelines for observational studies. The STROBE checklist was used to ensure comprehensive and transparent reporting of study design, data collection, and analysis.
Follow-up volumetric analysis
Volumetric assessment was performed. A thin slice contrasted MRI was done then imported and co-registered in Leksell GammaPlan software (version 11.3.2) with MRI done on the day of treatment. The tumor was then drawn on all slices and the volume were determined from dose-volume histogram, the same way as the treatment planning day. From the follow-up images, the tumor volumes (TV), tumor volume changes from the original volume (TVC) and the timings of TVC were recorded. Other image changes were reported, including edema (T2 hyperintense signal) and central loss of contrast (CLC).
Statistical analysis
Tumor volume at the time of treatment (TVt), tumor volume (peak volume) at progression (TVp), tumor volume change at progression (TVCp), tumor volume at the final follow-up (TVf), and tumor volume change at the final follow-up (TVCf) were reported. The time to progression was also reported. Serial volume measurements were made after treatment. Mean values were tested using independent t-test and ANOVA. Categorical variables were tested for by X2 or Fisher exact test. Secondary outcomes such as edema, hydrocephalus, cyst formation, and clinical outcome were also tested for. Case matching for tumor volume was done. Statistical analysis was done using SPSS version 26 (SPSS Inc, Chicago, Illinois, USA).
Results
The mean follow-up duration was 64 months (12–241 months). The mean TVCp was 72% (11–439%). The mean time to tumor progression was 13 months (2–160 months) and the mean duration of TVC was 15 months (2–164 months).
Progression patterns
Progression patterns were identified according to tumor behavior: Type 1 in which tumor enlargement was followed by eventual shrinkage (95/171;56%), Type 2 in which tumor enlargement was followed by stability (47/171;27%) and Type 3 in which there was tumor enlargement with a progressive increase in tumor size on serial images (29/171;17%). In a univariate analysis of the progression pattern type we found the following significant factors: in Type 1: CLC with progression 85/95 (90%) (p < 0.0001), smaller TVCp (45.7% vs. 69% vs. 161%) (p < 0.0001), earlier progression (17 months vs. 28 months vs. 56 months) (p < 0.0001), shorter duration of volume change (19 months vs. 39 months) (p < 0.0001). In Type 3: Cyst formation 4/7 (57%) (p 0.01) and a shorter follow-up (p < 0.0001). In a multivariate analysis only, a shorter follow-up was significant (p 0.02) for Type 3.
There was also a biphasic progression pattern in which the tumor showed two progression peaks with a period of tumor stability or regression in between then eventual regression or stability. This pattern was identified in 6 patients. The first peak occurred at a mean of 12 months (7–34 months) and the second peak at 47 months (24–76 months). The mean TVt was 5.5 cc (0.7–14.3 cc). In univariate analysis, follow-up duration (141 months vs. 79 months) (p 0.04) was significantly associated with this phenomenon. The tumor eventually shrank in 4 patients and remained stable in 2 patients.
Tumor enlargement
Pseudoprogression was identified in 142 patients (83%). A temporal classification of tumor pseudoprogression was devised categorizing them into 3 groups:
Early: tumor enlargement occurred during the first year after treatment (Supplementary Fig. 1). It was observed in 85 patients (60%) starting at a mean of 7 months (2–12 months).
Intermediate: tumor enlargement occurred after the first year and within 3 years of treatment (Supplementary Fig. 2). It was observed in 27 patients (19%) starting at a mean of 22 months (13–36 months).
Late: tumor enlargement occurred after 3 years of treatment (Fig. 1). It was found in 30 patients (21%) starting at a mean of 59 months (37–162 months).
True progression was found in 29 patients (17%) at a median of 56 months (19–152 months). In 23 patients, serial radiological tumor progression was established. In 6 patients progressive neurological deterioration, without further radiological progression, necessitated surgical intervention for the tumor.
A univariate analysis of the progression type was done. In early PP, there was a shorter duration of volume change, type 1 pattern and a presence of CLC. In true progression, there was a bigger TVCp, a bigger TVt and a bigger TVCf (Supplementary Tables 1 and 2).
Hydrocephalus
Hydrocephalus occurred after GK in 18 patients (11%) predominantly in intermediate pseudoprogression (p 0.04). Fourteen patients were found to have pseudoprogression and 4 were found to be true progression. In 14 patients VP shunt was enough to correct the patient’s clinical deterioration with no need for further intervention. Four patients required additional tumor debulking or excision.
Cyst formation
Cyst formation was observed in 7 patients (4%). One patient underwent surgical tumor intervention because of clinical deterioration and radiological tumor and cyst progression, followed by GK treatment for the residual tumor. In two patients, spontaneous cyst regression occurred, while one patient underwent cyst aspiration, and no further intervention was required as the tumor itself eventually shrank. In three patients, GK re-treatment was performed. Cyst formation was predominantly associated with late PP (p 0.02).
Tumor control
The pseudoprogression rate among the patients who showed tumor enlargement after SRS was 83% (142/171). The actuarial progression-free survival at 5-,7- and 10-years was 95%, 92%, and 90%, respectively. Most cases of treatment failure were recorded within 5 years from GK (Fig. 2). The mean time to true progression was 56 months (Supplementary Table 1). In a univariate analysis, factors favoring tumor control were CLC with progression 112/142 (79%) (p < 0.0001), shorter follow up (72 vs. 126 months) (< 0.0001), lesser TVCp (53% vs. 161%) (< 0.0001), lesser TVf 4.4 cc vs. 8.5 cc (p < 0.0001), TVCf − 3.2% vs. 165.9% (p < 0.0001), and earlier progression 21 months vs. 56 months (p < 0.0001). In a multivariate analysis, CLC with progression (p 0.02), follow-up duration (p 0.03) and, TVCf (p < 0.0001) were significant (Table 3). The highest tumor volume reduction was found in patients with early PP (p < 0.0001) while the most volume increase was seen in patients with true tumor progression (p < 0.0001) (Fig. 3).
ROC analyses were done to determine the cut-off values for true tumor progression and clinical progression/decline. The cut-off for TVCf for true progression was 51% (< 0.0001), and the AUCwas 0.96. Fifty patients (29%) showed more than 50% volume increase at the final follow-up, but only 8 patients had large tumors with a volume of more than 10 cc (p 0.8). The cut-off for TVCf associated with clinical decline was 40% (< 0.0001), and the AUC was 0.88. The cut-off for TVCp associated with clinical decline was 61% (p 0.66) but was not significant. (Supplementary Fig. 3).
Clinical status
New or worsened clinical status at the time of tumor enlargement was observed in 61 patients (36%) (Table 4). Neurological deterioration was due to hydrocephalus in 18 patients (30%), due to cyst formation in 3 patients (5%), and in the remaining patients due to tumor compression. In 41/61 patients (67%), neurological improvement occurred, including 14 patients after VP shunt, two patients with cysts that experienced spontaneous regression, and one patient who underwent cyst evacuation. The remaining patients improved spontaneously or with the aid of steroid therapy. In a univariate analysis, the factors determining the final clinical decline were cyst formation (p 0.03), greater TVCp (p < 0.0001), greater TVf (p 0.01), greater TVCf (p < 0.0001), radiological progression (p < 0.0001), and longer time to progression (p < 0.0001). In a multivariate analysis, TVf (p < 0.0001) and radiological progression (p < 0.0001) were significant (Tables 5).
Salvage treatment after GK failure
Twenty-seven patients underwent salvage treatment. Nineteen patients (71%) underwent GK re-treatment alone, six patients (22%) underwent surgery alone, and two patients (7%) had surgery then GK re-treatment. The median follow-up duration after salvage treatment was 68 months (4–160 months). The tumor volume according to salvage treatment was; mean 5.4 cc (0.5–15.8 cc) for GK re-treatment alone (Fig. 4), mean 14.9 cc (5–30 cc) for surgery alone, and mean 12.6 cc (12–13.2 cc) for surgery followed by GK re-treatment. The five patients who underwent surgery alone were lost to follow-up. Tumor control was achieved in 19/21 patients (90%). The actuarial tumor control was 94% at 5 years. The tumors shrank in 15 patients (71%), remained stable in 4 patients (19%), and progressed in 2 patients (10%). The two patients with tumor progression are still being followed up.
GK salvage treatment A At the time of treatment; 3.3 cc tumor was treated with 12 Gy to the 50% isodose with 97% cover, B 6 months after treatment 4.8 cc tumor volume (44% TVCp), C 61 months after treatment; 7.2 cc tumor volume (118% TVCf). GK re-treatment was performed to a 7.2 cc tumor that was treated with 12 Gy to the 50% isodose with 97% cover, D 70 months after 2nd GK treatment; 4.6 cc tumor volume (−36% TVC).
Discussion
Tumor control
The definition of tumor control after SRS was traditionally regarded as either stability or shrinkage of tumor size on follow-up images. However, this does not apply to all tumor types, as some tumors may transiently swell after SRS. This is in addition to the lack of universally accepted criteria for tumor control after SRS; most depend on an absolute or relative change in tumor size. To avert this, it has been proposed that tumor control be defined as the absence of the need for a second tumor intervention3,21,22. Considering the cohort of patients with sporadic VS treated by GK, from which the cases of this study were taken, the tumor control would be 98% (1382/1411), including the patients that turned out to be pseudoprogression. The tumor control of VS treated by SRS is commonly reported to exceed 90%3,21. We observed that tumors showing CLC were less likely to exhibit true progression. Earlier studies reported similar findings5. Tumors that exhibited earlier tumor enlargement, usually within the first two years, were more likely to be controlled, as we found earlier progression was significantly associated with tumor control. True progression was significantly associated with greater volume changes in this study, specially more than 100%. Matsuo et al. found that a greater than two-fold increase in tumor size was associated with continued tumor growth.
Most cases of treatment failure in the current study were recorded within 5 years from GK. However, most were earlier patients in our practice, in whom treatment failure may have been determined prematurely. The current study found that the mean time to treatment failure was determined much later, around 5 years from treatment. Later on we advocated more careful assessment of the clinical status of the patients and weighing conservative management against surgical intervention for the tumor, in addition to resorting to non-tumor-based interventions for other complications such as cysts or hydrocephalus. Most reports determined that treatment failure occurs at around 5 years6,13,23,24,25,26.
Pseudoprogression
Pseudoprogression is defined as tumor expansion following SRS followed by either tumor stability or regression14. The reported incidence of vestibular schwannoma enlargement after SRS is 3–80%5,6,7,9,10,11,12,13,14,15,16,17,18,19,20,27 (Table 6). In the current series, VS enlargement was present in 12% of treated cases. The high variability may be because of the criteria and the method of measurement used to assess tumor enlargement. Some might rely on computer-generated volume measurements or manually obtained through two-dimensional measurements on MRI images and then using a formula. They then report a percentage change from the original size, which can vary from 10 to 25%. Others would report an increase in size of a few millimeters in one or more dimensions using only two-dimensional measurements3,28. Volume measurements appear to be more accurate, as reported by several studies13,15,29, which we used in the current study. The current study used the 10% volume increase as a threshold indicating tumor enlargement. Snell et al., using computational geometrical methods, indicated that volume changes of less than 10% are within the error levels with volumetric measurements30. Further analysis of our cohort showed that 91% (155 patients) had a volume increase of more than 20%, and only 9% (16 patients) had a volume increase of less than 20%. This shows little difference between the 10% and 20% volume change thresholds. The rationale for using a lower threshold was being more meticulous, which is in the patient’s interest, so although almost 90% of the tumors surpassed the 20% threshold, it also means that 10% would not have been detected as growth, giving the patients a false sense of security. It also means that, with the lower threshold, if the patient continues to follow up and turns out to show continuous tumor progression, then the true progression can be detected earlier than with a higher threshold, and intervention will be done in a timely manner.
Conventional tumor response criteria, such as Macdonald and RECIST, were originally developed for oncological purposes, mainly for malignant and systemic tumors. However, these criteria have limited value in the response assessment of benign tumors, as these are slow-responding tumors. Consequently, volume change occurs after extended periods and in a portion of patients only. More recently, modified RANO criteria were developed for meningiomas31 and vestibular schwannomas32. They have proposed that the cut-off for progressive disease be a volume increase of more than 40%. This value aligns with our findings, which suggest a cut-off for true progression and clinical progression/decline of 51% and 40%, respectively. It should be noted that pseudoprogression is less common in meningiomas, reportedly in 5–11%33, so the suggested cut-off values for vestibular schwannomas should be more generous. Our results also show that most tumors with these volume changes were less than 10 cc volume, probably because larger tumors may have required intervention.
Several hypotheses have been proposed for the development of pseudoprogression. It has been suggested that radiation-induced inflammation results in increased vascular permeability34. There is the associated release of cytokines, VEGF, and other mediators, as well as tumor infiltration by macrophages35,36. Other explanations include the induction of an immune response by radiation resulting in tumor infiltration by immune cells and disruption of the blood-brain barrier37.
Previously it was thought pseudoprogression occurred as early as 2 months and as late as 36 months after SRS14,17,38. Early pseudoprogression is commonly reported8,19,39. 50% of the patients in the current study had early pseudoprogression, and the mean time for pseudoprogression was 13 months. Wage et al. reported that there were two types of pseudoprogression, one as early as 6 months after treatment and one later at around 3 years from treatment14. Among the identified progression patterns, Type 1 had a shorter duration of pseudoprogression, and Type 2 lasted longer. In the current study, we identified a third type of pseudoprogression (late), which occurred later than 3 years, at around 5 years from treatment. The early type had a significantly shorter duration of swelling (15 months) compared to the other two types (39 and 44 months, respectively). Recently, Balossier et al.40 reported on radiological outcome using clustering to determine the different response patterns to gamma knife treatment of vestibular schwannomas. They attempted to predict the response trajectories in the five patterns they identified. In their study, pattern 2 showed either stability or continuous progression and was the most likely to show treatment failure. The remaining patterns were more likely destined for tumor control. Central loss of contrast is a common postradiosurgical finding in VS, which usually occurs in the first year and is followed by a return of central contrast enhancement41,42,43. We found that most tumors showed CLC in the early type PP and progression pattern type 1. The early type of PP had lesser volume change compared to other types at progression and final follow-up. In addition, the early type was all without any clinical consequences. Type2 progression was mentioned in two previous studies, occurring in 19% and 29% of patients with pseudoprogression13,44. In the current study, it was found in 27% of patients. A more complex progression pattern, referred to in this study as biphasic, in which the tumor showed two instances of progression along the timeline of their follow-up, separated by periods of tumor stability or even shrinkage. Similarly, Matsuo et al. mentioned 3 patients with a bimodal peak at one and three years15in their study. This phenomenon suggests that if the tumor progresses even after initial tumor regression, one should not rush to consider this treatment failure.
Complications
Post-SRS hydrocephalus has been reported in 1–19% of cases45,46,47,48,49,50. In the current study, it was observed in 11% of the patients. However, this cohort is a subgroup from the total number of treated patients, meaning the incidence is likely much lower. We found hydrocephalus was associated with larger tumors. Lee et al. found that post-SRS hydrocephalus occurs within 3–4 years at around the time of tumor expansion51. We found hydrocephalus occurred predominantly in late PP at around 2 years after treatment.
The incidence of cyst formation after SRS may be as low as 2%. Pikis et al. reported cyst formation in 6% of patients17. We observed this phenomenon in 4% of patients. Two patients did not undergo any intervention and regressed spontaneously, and one patient only required cyst evacuation.
Salvage treatment
Repeat SRS for VS after treatment failure has been reported with a tumor control rate of 85–95%. Better control rates were observed after the second radiosurgical treatment52,53,54,55,56. This study found a similar control rate to the first treatment. It may be related to the longer follow-up duration compared to previous studies. As with primary radiosurgery treatments, treatment failures tend to occur after many years. Slightly higher rates of facial and trigeminal neuropathy were reported.
Surgery after radiosurgical treatment has been a matter of controversy and debate for years. Unfortunately, we could not record the difficulty or ease of the surgeries after treatment failure because they were not performed at our center, as we are a stand-alone gamma knife center. It was suggested that better results may be associated with subtotal or near-total as opposed to gross total resection13.
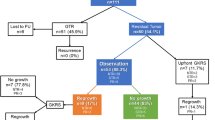
Management algorithm
We observed that two-thirds of the patients in whom clinical deterioration occurred at the time of tumor progression were effectively managed expectantly or with a VP shunt. It is also possible that a more patient wait-and-see strategy may have been adopted in some of these patients, especially for the smaller-sized tumors and asymptomatic patients (Fig. 5). More recent studies have adopted more stringent criteria for treatment failure and a more patient approach to tumor enlargement after SRS (Table 6). In the current study, we have demonstrated that pseudoprogression can occur beyond 5 years and as late as 10 years after treatment. We suggest that guided by the patient’s clinical condition, we proceed with only serial imaging if the symptoms are mild or absent. In the case of gross clinical deterioration, the least invasive strategy is adopted, as in the case of shunt insertion for hydrocephalus or cyst evacuation. In the presence of radiological progression on serial MRIs with minimal or no symptoms, depending on the size of the tumor we may adopt a wait-and-see strategy for smaller tumors or proceed for tumor intervention for larger tumors. The suggested cut-off values for tumor volume change should not be taken at face value as more tolerance should be practiced in smaller, asymptomatic, or minimally symptomatic tumors. Finally, the timing of PP may be an important guide in management. It was noted that early PP should not be of concern as most cases will regress, while intermediate and late PP will either follow a regressive or stable path. Tumors that showed true progression exhibited continuous serial radiological progression on follow-up, unlike those with PP, which remained stable over a period of at least one year.
Limitations
The retrospective nature of the study, related to selection bias and lost follow-up, may affect its strength. A reasonably long follow-up duration, as well as a record of the patients that experienced treatment failure and subsequent salvage treatment, with also an acceptable follow-up, may make up for this.
It may be argued that some patients that remained stable after initial enlargement can grow in the future. However, the results suggest they are more likely to shrink in the long run, specially in intermediate and late PP.
Conclusion
GKR for VS is associated with radiation-induced tumor enlargement in a group of patients. A more conservative approach may be adopted in most vestibular schwannomas that exhibit tumor enlargement after SRS, as they will eventually be controlled in most cases. Pseudoprogression may occur after 5 years.
Data availability
Data will be made available upon reasonable request. An Excel spreadsheet with all deidentified data and software code on which the conclusions of the paper rely will be available for inspection and verification during the peer-review process. Further inquiries can be directed to the corresponding author.
Abbreviations
- GK:
-
Gamma knife
- CLC:
-
Central loss of contrast
- PP:
-
Pseudoprogression
- TTE:
-
Transient tumor expansion
- GI:
-
Gradient index
- RANO:
-
Response Assessment in Neuro-Oncology
- RECIST:
-
Response Evaluation Criteria for Solid Tumors
- ROC:
-
Receiver Operating Characteristic
- SI:
-
Selectivity index
- SRS:
-
Stereotactic radiosurgery
- TV:
-
Tumor volume
- TVC:
-
Tumor volume change
- TVCp:
-
Tumor volume change at progression
- TVCf:
-
Tumor volume change at the final follow-up
- TVf:
-
Tumor volume at the final follow-up
- TVp:
-
Tumor volume at progression
- TVt:
-
Tumor volume at the time of treatment
- VEGF:
-
Vascular endothelial growth factor
References
Rykaczewski, B. & Zabek, M. A meta-analysis of treatment of vestibular Schwannoma using gamma knife radiosurgery. Contemp. Oncol. (Poznan Poland). 18, 60–66 (2014).
Murphy, E. S. & Suh, J. H. Radiotherapy for vestibular schwannomas: a critical review. Int. J. Radiat. Oncol. Biol. Phys. 79, 985–997 (2011).
Balossier, A. et al. Long-Term hearing outcome after radiosurgery for vestibular Schwannoma: A systematic review and Meta-Analysis. Neurosurgery 92, 1130–1141 (2023).
Delsanti, C., Roche, P. H., Thomassin, J. M. & Régis, J. Morphological changes of vestibular schwannomas after radiosurgical treatment: pitfalls and diagnosis of failure. Prog Neurol. Surg. 21, 93–97 (2008).
Kondziolka, D., Lunsford, L. D., McLaughlin, M. R. & Flickinger, J. C. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl. J. Med. 339, 1426–1433 (1998).
Van De Langenberg, R. et al. Volume changes after stereotactic LINAC radiotherapy in vestibular Schwannoma: control rate and growth patterns. Int. J. Radiat. Oncol. Biol. Phys. 84, 343–349 (2012).
Yu, C. P., Cheung, J. Y. C., Leung, S. & Ho, R. Sequential volume mapping for confirmation of negative growth in vestibular schwannomas treated by gamma knife radiosurgery. J. Neurosurg. 93 (Suppl 3), 82–89 (2000).
Nagano, O. et al. Transient expansion of vestibular Schwannoma following stereotactic radiosurgery: clinical Article. J. Neurosurg. 109, 811–816 (2008).
Meijer, O. W. M. et al. Tumor-volume changes after radiosurgery for vestibular Schwannoma: implications for follow-up MR imaging protocol. AJNR Am. J. Neuroradiol. 29, 906–910 (2008).
Mindermann, T. & Schlegel, I. How to distinguish tumor growth from transient expansion of vestibular schwannomas following gamma knife radiosurgery. Acta Neurochir. (Wien). 156, 1121–1123 (2014).
Kobayashi, T., Tanaka, T. & Kida, Y. The early effects of gamma knife on 40 cases of acoustic neurinoma. Acta Neurochir. Suppl. 62, 93–97 (1994).
Hayhurst, C. & Zadeh, G. Tumor pseudoprogression following radiosurgery for vestibular Schwannoma. Neuro Oncol. 14, 87 (2012).
Pollock, B. E. Management of vestibular schwannomas that enlarge after stereotactic radiosurgery: treatment recommendations based on a 15 year experience. Neurosurgery 58, 241–246 (2006).
Wage, J., Mignano, J. & Wu, J. Tufts medical center experience with long-term follow-up of vestibular schwannoma treated with gamma knife stereotactic radiosurgery: novel finding of delayed pseudoprogression. Adv Radiat. Oncol, 6, 4, 100687, 1-10 (2021).
Matsuo, T. et al. Long-term follow-up results of linear accelerator-based radiosurgery for vestibular Schwannoma using serial three-dimensional spoiled gradient-echo MRI. J. Clin. Neurosci. 22, 320–325 (2015).
Hasegawa, T., Kida, Y., Yoshimoto, M., Koike, J. & Goto, K. Evaluation of tumor expansion after stereotactic radiosurgery in patients harboring vestibular schwannomas. Neurosurgery 58, 1119–1126 (2006).
Pikis, S. et al. Stereotactic radiosurgery for Koos grade IV vestibular Schwannoma: a multi-institutional study. J. Neurosurg. 138, 405–412 (2022).
Aoyama, H. et al. Symptomatic outcomes in relation to tumor expansion after fractionated stereotactic radiation therapy for vestibular schwannomas: Single-institutional long-term experience. Int. J. Radiat. Oncol. Biol. Phys. 85, 329–334 (2013).
Breshears, J. D. et al. Temporal dynamics of pseudoprogression after gamma knife radiosurgery for vestibular schwannomas - A retrospective volumetric study. Clin. Neurosurg. 84, 123–131 (2019).
Wowra, B. et al. Outpatient gamma knife surgery for vestibular Schwannoma: definition of the therapeutic profile based on a 10-year experience. J. Neurosurg. 102, 114–118 (2005).
Soltys, S. G. et al. Stereotactic radiosurgery for vestibular schwannomas: tumor control probability analyses and recommended reporting standards. Int. J. Radiat. Oncol. Biol. Phys. 110, 100–111 (2021).
Persson, O. et al. Stereotactic radiosurgery vs. fractionated radiotherapy for tumor control in vestibular Schwannoma patients: a systematic review. Acta Neurochir. (Wien). 159, 1013–1021 (2017).
Hasegawa, T. et al. Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: evaluation of 440 patients more than 10 years after treatment with gamma knife surgery. J. Neurosurg. 118, 557–565 (2013).
Hasegawa, T. et al. Stereotactic radiosurgery for vestibular schwannomas: analysis of 317 patients followed more than 5 years. Neurosurgery 57, 257–263 (2005).
Carlson, M. L. et al. Long-term hearing outcomes following stereotactic radiosurgery for vestibular Schwannoma: patterns of hearing loss and variables influencing audiometric decline. J. Neurosurg. 118, 579–587 (2013).
Régis, J. & Balossier, A. From the perspective of pseudo-progression rather than treatment failure, how long should we wait before considering treatment failure if large cystic enlargement occurs after gamma knife radiosurgery for vestibular Schwannoma?? Insight into pseudo-progression based on two case reports. Acta Neurochir. (Wien). 165, 2101–2103 (2023).
Nagano, O. et al. Transient expansion of vestibular Schwannoma following stereotactic radiosurgery. J. Neurosurg. 109, 811–816 (2008).
Kania, R. et al. EAONO position statement on vestibular Schwannoma: imaging assessment question: how should growth of vestibular Schwannoma be defined? J. Int. Adv. Otol. 14, 90 (2018).
Van De Langenberg, R., De Bondt, B. J., Nelemans, P. J., Baumert, B. G. & Stokroos, R. J. Follow-up assessment of vestibular schwannomas: volume quantification versus two-dimensional measurements. Neuroradiology 51, 517–524 (2009).
Snell, J. W., Sheehan, J., Stroila, M. & Steiner, L. Assessment of imaging studies used with radiosurgery: a volumetric algorithm and an Estimation of its error. Technical note. J. Neurosurg. 104, 157–162 (2006).
Huang, R. Y. et al. Proposed response assessment and endpoints for meningioma clinical trials: report from the response assessment in Neuro-Oncology working group. Neuro Oncol. 21, 26–36 (2019).
Rueß, D. et al. Pseudoprogression of vestibular schwannoma after stereotactic radiosurgery with cyberknife®: proposal for new response criteria. Cancers (Basel) 15, 1496, 1-13 (2023).
Jung, I. H. et al. Pseudoprogression and peritumoral edema due to intratumoral necrosis after gamma knife radiosurgery for meningioma. Sci Rep 12(1), 13663, 1-10 (2022).
De Vries, M. et al. Tumor-associated macrophages are related to volumetric growth of vestibular schwannomas. Otol Neurotol. 34, 347–352 (2013).
Iwai, Y., Yamanaka, K., Yamagata, K. & Yasui, T. Surgery after radiosurgery for acoustic neuromas: surgical strategy and histological findings. Neurosurgery 60(2 Suppl 1):ONS75-82 (2007).
Lewis, D. et al. Inflammation and vascular permeability correlate with growth in sporadic vestibular Schwannoma. Neuro Oncol. 21, 314 (2019).
Dougherty, M. C., Shibata, S. B. & Hansen, M. R. The biological underpinnings of radiation therapy for vestibular schwannomas: review of the literature. Laryngoscope Investig Otolaryngol. 6, 458–468 (2021).
Yang, H. C. et al. Prediction of pseudoprogression and long-term outcome of vestibular Schwannoma after gamma knife radiosurgery based on preradiosurgical MR radiomics. Radiother Oncol. 155, 123–130 (2021).
Nakamura, H. et al. Serial Follow-up MR imaging after gamma knife radiosurgery for vestibular Schwannoma. AJNR Am. J. Neuroradiol. 21, 1540 (2000).
Balossier, A. et al. Dynamics of tumor evolution after gamma knife radiosurgery for sporadic vestibular Schwannoma: defining volumetric patterns characterizing individual trajectory. Neuro Oncol. 27, 545–556 (2025).
Lunsford, L. D., Kondziolka, D., Maitz, A. & Flickinger, J. C. Black holes, white dwarfs and supernovas: imaging after radiosurgery. Stereotact. Funct. Neurosurg. 70 (Suppl 1), 2–10 (1998).
Fukuoka, S. et al. Apoptosis following gamma knife radiosurgery in a case of acoustic Schwannoma. Stereotact. Funct. Neurosurg. 70 (Suppl 1), 88–94 (1998).
Chung, W. Y. et al. Gamma knife surgery for vestibular Schwannoma: 10-year experience of 195 cases. J. Neurosurg. 102, 87–97 (2005).
Okunaga, T. et al. Linear accelerator radiosurgery for vestibular Schwannoma: measuring tumor volume changes on serial three-dimensional spoiled gradient-echo magnetic resonance images. J. Neurosurg. 103, 53–58 (2005).
Yang, H. C. et al. Gamma knife radiosurgery for larger-volume vestibular schwannomas. Clinical Article. J. Neurosurg. 114, 801–807 (2011).
Van De Langenberg, R. et al. Management of large vestibular Schwannoma. Part II. Primary gamma knife surgery: radiological and clinical aspects. J. Neurosurg. 115, 885–893 (2011).
Bailo, M. et al. Gamma knife radiosurgery as primary treatment for large vestibular schwannomas: clinical results at Long-Term Follow-Up in a series of 59 patients. World Neurosurg. 95, 487–501 (2016).
Lefranc, M. et al. Place of gamma knife stereotactic radiosurgery in grade 4 vestibular Schwannoma based on case series of 86 patients with Long-Term Follow-Up. World Neurosurg. 114, e1192–e1198 (2018).
Hasegawa, T. et al. Predictors of long-term tumor control after stereotactic radiosurgery for Koos grade 4 vestibular schwannomas. J. Neurooncol. 151, 145–156 (2021).
Ogino, A. et al. Stereotactic radiosurgery as the primary management for patients with Koos grade IV vestibular schwannomas. J. Neurosurg. 135, 1058–1066 (2021).
Lee, S. et al. Analysis of risk factors to predict communicating hydrocephalus following gamma knife radiosurgery for intracranial Schwannoma. Cancer Med. 5, 3615–3621 (2016).
Lonneville, S., Delbrouck, C., Renier, C. & Devriendt, D. & Massager, N. Repeat gamma knife surgery for vestibular schwannomas. Surg Neurol. Int 6, 153 (2015).
Yomo, S. et al. Repeat gamma knife surgery for regrowth of vestibular schwannomas. Neurosurgery 64, 48–54 (2009).
Dewan, S. & Norén, G. Retreatment of vestibular schwannomas with gamma knife surgery. J. Neurosurg. 109 Suppl, 144–148 (2008).
Iorio-Morin, C. et al. Repeat stereotactic radiosurgery for progressive or recurrent vestibular schwannomas. Neurosurgery 85, 535–542 (2019).
Fu, V. X. et al. Retreatment of vestibular Schwannoma with gamma knife radiosurgery: clinical outcome, tumor control, and review of literature. J. Neurosurg. 129, 137–145 (2018).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
No funding was received for this research.
Author information
Authors and Affiliations
Contributions
Conception and design: Amr M. N. El-Shehaby, Wael Abdel Halim Reda, Ahmed M. Nabeel. Acquisition of data: Amr M. N. El-Shehaby, Khaled M. Abdel Karim, Ahmed M. Nabeel, Reem M. Emad Eldin, Sameh R. Tawadros, Ahmed H. Elazzazi. Analysis and interpretation of data: Amr M. N. El-Shehaby. Drafting the article: Amr M. N. El-Shehaby. Critically revising the article: Wael Abdel Halim Reda, Khaled M. Abdel Karim, Ahmed M. Nabeel, Reem M. Emad Eldin, Sameh R. Tawadros. Reviewed submitted version of manuscript: Wael Abdel Halim Reda, Nabeel, Reem M. Emad Eldin. Approved the final version of the manuscript on behalf of all authors: Amr M. N. El-Shehaby. Statistical analysis: Amr M. N. El-Shehaby. Administrative/technical/material support: Wael Abdel Halim Reda. Study supervision: Amr M. N. El-Shehaby, Wael Abdel Halim Reda.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Ethical approval
Human Ethics and Consent to Participate declarations: not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Shehaby, A.M.N., Reda, W.A., Abdel Karim, K.M. et al. A retrospective study demonstrating the growth patterns and the pseudoprogression temporal classification after stereotactic radiosurgery for sporadic vestibular schwannomas. Sci Rep 15, 18187 (2025). https://doi.org/10.1038/s41598-025-03095-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03095-4