Abstract
The purpose of this study was to determine the recurrence rate, factors, incidence of polypoidal lesions, and risk factors for developing polypoidal lesions after a reduced dose photodynamic therapy (PDT) for pachychoroid neovasculopathy (PNV) without polypoidal lesions. This study is an observational case-control study. The study included 105 patients (77 men, 28 women; mean age, 61.6 years) with PNV in 105 eyes treated with reduced PDT. Twenty-eight eyes were treated with half-dose PDT and 77 eyes with two-thirds dose PDT, with an average follow-up of 588 days. Logistic regression analysis was used to examine factors of recurrence and polypoidal lesions. Recurrence was observed in six patients (21%) receiving half-dose PDT and 24 patients (31%) receiving two-thirds dose PDT. Polypoidal lesions developed in seven patients (7%). Recurrence factors were duration of follow-up (P < 0.0001), macular neovascularization (MNV) thickness before PDT (P = 0.010), and age (P = 0.002). MNV area before PDT was an independent risk factor for the development of polypoidal lesions (P = 0.001). Long-term follow-up after reduced PDT for PNV is necessary because recurrence factors include follow-up duration, age, and MNV thickness. A risk factor for polypoidal lesions after PNV treatment was the MNV area before PDT.
Similar content being viewed by others
Introduction
Pachychoroid neovasculopathy (PNV) is a concept proposed by Pang et al. in 20151. PNV is a type of pachychoroid disease characterized by thickening of the choroid, dilation of large choroidal vessels, increased choroidal vascular hyperpermeability on indocyanine green angiography (ICGA), and the presence of macular neovascularization (MNV)1,2. Pachychoroid spectrum diseases include pachychoroid pigment epitheliopathy, central serous chorioretinopathy (CSC), focal choroidal excavation, peripapillary syndrome, and polypoidal choroidal vasculopathy (PCV)3.
Although progression from CSC to PNV is common4, reports of PNV transitioning to PCV are very rare. Siedlecki et al. reported that 13% of patients treated with intravitreal anti-vascular endothelial growth factor (VEGF) injections for PNV developed PCV after approximately three years of follow-up5, with risk factors including young age and a thick choroid.
Many treatments for PNV without PCV have been reported. The three main treatments are intravitreal injection alone6, intravitreal injection combined with photodynamic therapy (PDT)7,8, and PDT alone9,10. PDT was originally used to treat nAMD, where verteporfin binds to LDL in the blood and is taken up by LDL receptors in the MNV. The MNV is then occluded by laser irradiation, which damages the endothelial cells. Full-dose PDT can cause macular atrophy by occluding the choriocapillaris and massive SRH by occluding the MNV11,12. Therefore, reducing the dose by half can prevent this risk. We previously performed two-thirds dose PDT for PNV and reported good results at one year10. Although all treatments have shown positive outcomes, it is still uncertain which is superior. Flat, irregular elevations of the retinal pigment epithelium on optical coherence tomography (OCT) are known to indicate MNV, with a greater thickness being associated with a higher risk of recurrence7. Additionally, PNV is more likely to recur with a larger MNV area13.
In this study, we report the results of a retrospective analysis examining factors associated with recurrence rate, the duration of recurrence, and the appearance of polypoidal lesions in patients treated with half-dose PDT and two-thirds dose PDT as monotherapy for PNV.
Materials and methods
Patients
This study is an observational case-control study. The study investigated 105 eyes from 105 patients (78 men, 27 women; mean age, 61.6 years) diagnosed with PNV who presented at Nihon University Hospital from 2015 to 2022 and underwent reduced PDT monotherapy for the first time. PNV was defined as the presence of flat, irregular pigment epithelium detachment (PED) on OCT, macular neovascularization (MNV on OCT angiography (OCTA), dilation of the choroidal large vessels, and serous retinal detachment (SRD) on OCT. Patients who had previously undergone half-dose PDT for CSC were also included, but in such cases, OCTA was assessed at the time of half-dose PDT, and only those cases in which the absence of MNV was confirmed were included. Cases with PCV, suspected secondary MNV due to inflammatory disease, a history of vitreous surgery, or myopia greater than − 6.0 diopters were excluded.
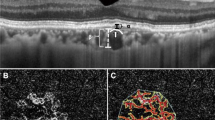
Central macular thickness, central choroidal thickness, and the double layer of shallow PED were measured using enhanced depth imaging OCT with the SPECTRALIS HRA-OCT (Heidelberg Engineering Inc., Franklin, MA). The thickness of the shallow PED was defined as MNV thickness and measured at the fovea. OCTA was performed using the RTVue XR Avanti with AngioVue (Optovue Inc., Fremont, CA). Macular cubes (3 × 3 mm) were acquired. The area of MNV was measured by manually selecting the largest slab on OCTA and using the OCTA built-in measurement function.
Recurrence was defined as the need for re-treatment with aflibercept after PDT. Aflibercept was not injected if the SRD decreased after PDT, but it was injected if the SRD remained unchanged or increased, including in patients who did not achieve dryness from the initial treatment. The presence of a polypoidal lesion was confirmed by OCT and ICGA. A polypoidal lesion was defined as a steep, high PED on OCT and as a lesion that becomes hyperfluorescent in the late phase on ICGA at a location consistent with the OCT findings. Visiting intervals ranged from one to four months, though these were not consistent due to the retrospective nature of the study.
This study adhered to the tenets of the Declaration of Helsinki. This was a retrospective, single-center study, and the procedures used were approved by the ethics committee of Nihon University School of Medicine on 11 November 2021 (No. 20211105). Informed consent was obtained from all individual participants in this study.
Statistical analysis
Data are presented as mean ± standard deviation. Paired t-tests were used to assess changes in visual acuity, foveal retinal thickness, and choroidal thickness before and after treatment. Logistic regression was performed to analyze factors in patients requiring additional treatment. Recurrence and polypoidal lesion development in the half-dose PDT and two-thirds dose PDT groups, respectively, were examined as events using the Kaplan-Meier method. Logistic regression analysis was also used to examine factors associated with recurrence and polypoidal lesion development. SPSS version 26 was used for these analyses. A P-value < 0.05 was considered to indicate a significant difference.
Results
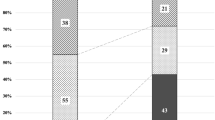
The clinical features of the participants are shown in Table 1. Values are mean ± standard deviation, unless indicated otherwise. The mean follow-up period was 454 days in the half-dose PDT group, 636 days in the two-thirds dose PDT group, and 588 days overall (range, 27–1866 days). When comparing the two groups, more patients in the half-dose PDT group were treatment-naïve, while more patients in the two-thirds dose PDT group had a history of PDT and a larger MNV area. The treatment results for each group are shown in Table 2. At one month, 93% of patients had a reduction in SRD or dryness after half-dose PDT compared to 95% of patients for two-thirds dose PDT. After three months of PDT, 76% of patients had dryness after half-dose PDT and 88% of patients after two-thirds dose PDT. During the follow-up period, recurrence occurred in 6 patients (21%) in the half-dose PDT group and 24 patients (31%) in the two-thirds dose PDT group. Final visual acuity significantly improved in both groups compared to pre-PDT visual acuity (P < 0.0001). Polypoidal lesions developed in one patient (3%) receiving half-dose PDT and in six patients (8%) receiving two-thirds dose PDT during the follow-up period. One of these patients developed a subretinal hemorrhage measuring 5 disc diameters, but vision improved after treatment with gas injection and intravitreal aflibercept. (Fig. 1)
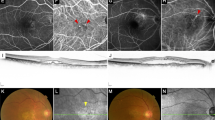
56 year-old female patient who underwent two-third PDT. (a) baseline color fundus photograph, b: baseline OCT; yellow arrow showed “ double layer sign ” (c) baseline OCT angiography showed MNV in choriocapillaris slab (yellow circle) (d) color fundus photograph; one year after two-third PDT. (E) OCT; one year after PDT, (F) OCT angiography; one year after PDT; yellow circle showed increased MNV, no additional treatment was needed. (g, h) 19 months after two- third PDT. Massive subretinal hemorrhage appeared. Orange-red elevated lesion and OCT shows dome-like elevations of RPE.
Risk factors for recurrence in the combined half-dose and two-thirds dose groups are shown in Table 3. Older age, greater pre-treatment MNV thickness, and a longer follow-up period were also significant risk factors for recurrence in the multivariate analysis.
Risk factors for the development of polypoidal lesions are shown in Table 4. In the multivariate analysis, a large pre-treatment MNV area was identified as a significant risk factor. Figure 2 shows the recurrence duration according to the Kaplan-Meier method. The median recurrence duration was 1021 days in the half-dose PDT group and similar in the two-thirds dose PDT group at 1127 days (P = 0.680). In Fig. 3, the duration of polypoidal lesion development was estimated using the Kaplan-Meier method and did not differ between the two groups (P = 0.736).
Discussion
There is some controversy regarding whether PCV should be included in PNV. However, the treatment for PCV has been reported in several studies and was excluded in this report. Treatment for PNV can be broadly categorized into anti-VEGF intravitreal injection, combined anti-VEGF intravitreal injection and PDT, and PDT monotherapy, although most reports have covered periods of less than one year. In the present study, we report on a relatively large cohort of 105 patients treated with PDT alone, who were followed for an average of more than one year. We treated 28 patients with half-dose PDT and 77 patients with two-thirds dose PDT. After one month of treatment, dryness or reduction in SRD was observed in more than 90% of patients, and after three months, 76% of patients in the half-dose group and 88% of patients in the two-thirds dose group were dry. The dry rate after three intravitreal injections of anti-VEGF drugs for age-related macular degeneration (AMD) is reported to be 67% after one injection and 91% after three injections14, which is comparable to the results of reduced-dose PDT. The recurrence rate was 21% in the half-dose group and 31% in the two-thirds dose group. This may be because 82% of patients in the half-dose group were treatment-naïve at baseline, while only 51% of patients in the two-thirds dose group were treatment-naïve. Another reason is that many patients in the two-thirds-dose PDT group had a long follow-up from the initial visit, and there may be cases in which MNV developed from CSC or were misdiagnosed as CSC. Additionally, as this study was retrospective, there may have been selection bias where half-dose PDT was chosen for patients more likely to respond to treatment. Factors contributing to recurrence include older age, longer follow-up duration, and greater baseline MNV thickness. While ‘treat and extend’ is commonly used for nAMD, pro re nata’ is considered sufficient for cases with low recurrence rates like PNV. Nonetheless, due to the low number of recurrences, regular follow-up remains important.
A report of intravitreal aflibercept combined with half-dose PDT treatment for PNV in 21 eyes had described a higher likelihood of requiring additional treatment when the choroidal neovascularization thickness is greater7. Additionally, a thicker double layer in intermediate AMD is correlated with a higher propensity to develop neovascular AMD13. These findings are consistent with our report that those with a thicker MNV are more likely to develop recurrence. In addition, a study of 30 patients receiving half-dose PDT with intravitreal aflibercept for PNV showed that a larger preoperative MNV area is associated with a greater likelihood of recurrence15. In our results, patients with a larger MNV area were also more likely to develop recurrence. However, patients with chronic CSC with MNV experienced a significant increase in MNV area after three years of follow-up16, and MNV is known to increase over time. Given these considerations, it should be noted that patients with a large MNV area at baseline are more likely to relapse.
Siedlecki et al. previously treated PNV with intravitreal injections of anti-VEGF drugs and reported that 13% developed PCV after approximately three years of follow-up5. In our study, polypoidal lesions developed in 7% of patients during a mean follow-up of 588 days. Although the treatments differed between anti-VEGF drugs and PDT, the incidence of polypoidal lesions was generally comparable. There was no significant difference in the incidence of polypoidal lesions between the half-dose and two-thirds dose PDT groups. Given the small number of patients with polypoidal lesions, it is not possible to determine which dose is more likely to result in the development of polypoidal lesions. However, the Kaplan-Meier analysis did not show a significant difference, and there is no apparent difference based on the dose. We believe this report is valuable, as there have been no previous reports on the development of polypoidal lesions following PDT monotherapy for PNV. The results indicate that the baseline MNV area is a relevant risk factor for the development of polypoidal lesions. PCV with polypoidal lesions is characterized by the presence of a branching vascular network. Whether PCV originates from choroidal neovascularization or from abnormal vessels remains a controversial issue17,18. In some cases, polypoidal lesions arise from the branching vascular network, while in others, they may appear independently. We previously reported two types of PCV: type 1 PCV, where polypoidal lesions develop beyond a branching vascular network; and type 2 PCV, with few abnormal vascular networks and no feeding vessels19. The development of polypoidal lesions from PNV is considered to be similar to type 1 PCV, but further investigation using ICGA and other methods will be needed to verify this issue.
Polypoidal lesions recur in PCV when the branching vascular network increases20. Given that the branching vascular network is the PNV, this is consistent with our findings that polypoidal lesions are more likely to develop from PNV when the MNV area is large. Massive submacular hemorrhage (SMH) can be a concern when polypoidal lesions develop. Cho et al. reported that the rate of massive SMH within one year was 2.45%, and that 30% of PCV patients experienced massive SMH within 10 years21. Unfortunately, one of our study patients experienced a massive SMH. Fortunately, her vision recovered after treatment, but PCV carries its risks. This study found that polypoidal lesions are more likely to develop when the MNV area is large. Therefore, when the MNV area is large on OCTA or ICGA, careful follow-up is necessary.
In the present study, we have, for the first time, reported the outcomes and recurrence rates, risk factors for recurrence, and the incidence of polypoidal lesions and their risk factors in 105 patients treated with half-dose PDT or two-thirds dose PDT for PNV. We were able to show that monotherapy of reduced PDT for PNV has good long-term results even without anti-VEGF drugs. It is a treatment with both physical and economic benefits for patients. However, it should be noted that a certain percentage of patients will develop PCV.
The limitations of this study include the presence of cases with a short follow-up period, the retrospective nature of the study, and the small number of cases that developed polypoidal lesions. In the future, it will be necessary to increase the number of cases and conduct further research on the causes of polypoidal lesion development.
An additional limitation is the lack of evaluation of macular atrophy after PDT. We would like to discuss it in future studies.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Pang, C. E. & Freund, K. B. Pachychoroid neovasculopathy. Retina 35, 1–9. https://doi.org/10.1097/iae.0000000000000331 (2015).
WarrowDJ, HoangQV & FreundKB Pachychoroid pigment epitheliopathy. Retina 33, 1659–1672. https://doi.org/10.1097/iae.0b013e3182953df4 (2013).
Cheung, C. M. G. et al. Pachychoroid disease. Eye (Lond). 33, 14–33. https://doi.org/10.1038/s41433-018-0158-4 (2019).
Fung, A. T., Yannuzzi, L. A. & Freund, K. B. Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina 32, 1829–1837. https://doi.org/10.1097/iae.0b013e3182680a66 (2012).
Siedlecki, J. et al. Progression of pachychoroid neovasculopathy into aneurysmal type 1 choroidal neovascularization or polypoidal choroidal vasculopathy. Ophthalmol. Retina. 6, 807–813. https://doi.org/10.1016/j.oret.2022.04.004 (2022).
Jung, B. J. et al. Intravitreal Aflibercept and Ranibizumab for pachychoroid neovasculopathy. Sci. Rep. 9, 2055. https://doi.org/10.1038/s41598-019-38504-y (2019).
Matsumoto, H., Mukai, R., Kikuchi, Y., Morimoto, M. & Akiyama, H. One-year outcomes of half-fluence photodynamic therapy combined with intravitreal injection of Aflibercept for pachychoroid neovasculopathy without polypoidal lesions. Jpn J. Ophthalmol. 64, 203–209. https://doi.org/10.1007/s10384-020-00722-7 (2020).
Kitajima, Y. et al. One-year outcome of combination therapy with intravitreal anti-vascular endothelial growth factor and photodynamic therapy in patients with pachychoroid neovasculopathy. Graefes Arch. Clin. Exp. Ophthalmol. 258, 1279–1285. https://doi.org/10.1007/s00417-020-04661-4 (2020).
Hikichi, T., Kubo, N. & Yamauchi, M. One-year comparison of anti-vascular endothelial growth factor and half dose photodynamic therapies for pachychoroid neovasculopathy. Eye (Lond). 35, 3367–3375. https://doi.org/10.1038/s41433-021-01418-z (2021).
Tanaka, K. et al. Two-thirds dose photodynamic therapy for pachychoroid neovasculopathy. J Clin Med 2021;10:2168. (2021). https://doi.org/10.3390/jcm10102168
Kawai, K. et al. Macular atrophy at 5 years after photodynamic therapy for polypoidal choroidal vasculopathy. Eye (Lond). 37, 1067–1072. https://doi.org/10.1038/s41433-022-02067-6 (2023).
Hirami, Y. et al. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina 27, 335–341. https://doi.org/10.1097/01.iae.0000233647.78726.46 (2007).
Takeuchi, J. et al. Predictive factors for outcomes of half dose photodynamic therapy combined with Aflibercept for pachychoroid neovasculopathy. Graefes Arch. Clin. Exp. Ophthalmol. 261, 2235–2243. https://doi.org/10.1007/s00417-023-06030-3 (2023).
Tanaka, K. et al. Short-term results for Brolucizumab in treatment-naïve neovascular age-related macular degeneration: a Japanese multicenter study. Jpn J. Ophthalmol. 66, 379–385. https://doi.org/10.1007/s10384-022-00922-3 (2022).
Wakatsuki, Y. et al. Optical coherence tomography biomarkers for conversion to exudative neovascular age-related macular degeneration. Am. J. Ophthalmol. 247, 137–144. https://doi.org/10.1016/j.ajo.2022.09.018 (2023).
Chen, Y. C. & Chen, S. N. Three-year follow-up of choroidal neovascularization in eyes of chronic central serous chorioretinopathy. Br. J. Ophthalmol. 104, 1561–1566. https://doi.org/10.1136/bjophthalmol-2019-315302 (2020).
Yuzawa, M., Mori, R. & Kawamura, A. The origins of polypoidal choroidal vasculopathy. Br. J. Ophthalmol. 89, 602–607. https://doi.org/10.1136/bjo.2004.049296 (2005).
Cheung, C. M. G. Macular neovascularization and polypoidal choroidal vasculopathy: phenotypic variations, pathogenic mechanisms and implications in management. Eye (Lond). 38, 659–667. https://doi.org/10.1038/s41433-023-02764-w (2024).
Kawamura, A. et al. Indocyanine green angiographic and optical coherence tomographic findings support classification of polypoidal choroidal vasculopathy into two types. Acta Ophthalmol. 91, e474–e481. https://doi.org/10.1111/aos.12110 (2013).
Bo, Q. et al. Progression of polypoidal lesions associated with exudative recurrence in polypoidal choroidal vasculopathy. Ophthalmology 2023;130:167 – 78. (2023). https://doi.org/10.1016/j.ophtha.2022.09.013
Cho, J. H. et al. Incidence rate of massive submacular hemorrhage and its risk factors in polypoidal choroidal vasculopathy. Am. J. Ophthalmol. 169, 79–88. https://doi.org/10.1016/j.ajo.2016.06.014 (2016).
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
KT designed the research and wrote the main manuscript. RM, YW, HO, KT, and MK collected the data and prepared the figures. HN and AK reviewed the study design and the results. KT contributed to the manuscript as the first author and as the corresponding author. All authors read and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tanaka, K., Onoe, H., Takeshima, K. et al. Incidence of recurrence and development of polypoidal lesions following half-dose and two-thirds dose photodynamic therapy for pachychoroid neovasculopathy. Sci Rep 15, 18975 (2025). https://doi.org/10.1038/s41598-025-03782-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03782-2
Keywords
This article is cited by
-
Subtype-specific shifts in age, axial length, and clinical profile of neovascular age-related macular degeneration: a five-year study in Japan
Japanese Journal of Ophthalmology (2025)