Abstract
This study examined the association between gestational weight gain (GWG) patterns and preterm birth (PTB) subtypes using data from a registry system in Wuhan, China. A total of 57,138 women with live singleton births were included. Total GWG in the first and second trimesters was categorized as insufficient, appropriate, or excessive based on Chinese guidelines, and weekly early GWG (< 20 weeks; eGWG) was classified into Class I (< 100 g/week), Class II (100–399 g/week), and Class III (≥ 400 g/week). Multiple logistic regression analyses assessed the relationships between GWG patterns and PTB subtypes, including spontaneous PTB, medically indicated PTB, and preterm premature rupture of membranes (PPROM), and adjusted for covariates such as age, education, pregnancy, parity, and offspring sex. Subgroup analyses were conducted by pre-pregnancy BMI categories (underweight: <18.5 kg/m², normal weight: 18.5–23.9 kg/m², overweight/obesity: ≥24 kg/m²). Results showed that underweight women with excessive GWG or Class III eGWG had increased risks of all PTB subtypes. Normal weight women with excessive or Class III eGWG, as well as insufficient or Class I eGWG, exhibited elevated risks for all PTB types. Overweight/obesity women with insufficient or Class I eGWG were at higher risks for spontaneous PTB and PPROM. These findings underscore the importance of optimal GWG across all BMI categories to mitigate PTB risks, highlighting the need for tailored prenatal weight management strategies.
Similar content being viewed by others
Introduction
Preterm birth (PTB), defined as the delivery of a born infant before 37 completed gestational weeks1, is the leading cause of neonatal mortality and morbidity worldwide2 and has been reported to be strongly associated with long-term health problems such as neurological disabilities and various chronic diseases3,4. In recent decades, the burden of PTB has been substantial and increasing5. Therefore, it is crucial to identify the potential modifiable risk factors to prevent PTB. The etiology of PTB is multifactorial and involves a complex interplay of genetic, environmental, and socioeconomic factors6. While significant progress has been made in understanding these contributors, many aspects remain under investigation.
Several previous studies have indicated that maternal overweight/obesity is one potentially modifiable risk factor for PTB7,8 and thus provided a target for intervention of PTB during pre-conception care. Besides, as weight control is considered more feasible during pregnancy than before conception, there is increasing concern about the association of gestational weight gain (GWG) with PTB. However, conclusions of previous investigations have been inconsistent, as several studies reported an association of lower GWG with an elevated risk of PTB9. In contrast, some studies indicated that the risk of PTB increased with higher GWG10.
Most previous studies only evaluated the total GWG throughout pregnancy, which may lead to biased associations because GWG differs by term and preterm birth11 and is not linear throughout pregnancy12. The early GWG (< 20 weeks gestation, eGWG) was considered to be critical for embryogenesis and fetal growth13. However, few studies have specifically examined eGWG related to PTB. Furthermore, PTB is a heterogeneous condition, but few studies have examined whether associations of GWG with PTB differ according to different subtypes of PTB, such as spontaneous PTB, preterm premature rupture of membrane (PPROM), and medically indicated PTB. Besides, most of the previous studies were conducted in developed countries. However, in developing countries, women of reproductive age generally have a lower body mass index (BMI) than those in developed countries14 and was less investigated. Furthermore, existing GWG guidelines, such as those from the Institute of Medicine (IOM)12 of the USA, may not be fully applicable to Chinese women due to differences in body composition and lifestyle.
Therefore, the relationship between GWG patterns and PTB subtypes remains inadequately explored, particularly in the context of Chinese women and the recommended values outlined in Chinese guidelines. To address this gap, we conducted a retrospective cohort study involving 57,138 women in China, utilizing data from a registration system. This study aimed to investigate the association between total GWG in the first and second trimesters, and weekly eGWG with the risk of PTB subtypes in singleton pregnancies.
Results
General characteristics of the study subjects
Table 1 presents the maternal characteristics of women in the study. Of the 57,138 women, 1999 (3.5%) gave birth to a PTB infant. Among all the PTBs, 577 (28.8%) were spontaneous PTB, 567 (28.3%) were PPROM, and 855 (42.7%) were medically indicated PTB. The total GWG of most pregnant women (61.9%) in the first and second trimesters is excessive, and the average weekly eGWG of most pregnant women (68.3%) ranges from 100 to 399 g.
Association of weight gain patterns with PTB subtypes
Table 2 (crude model) and Table 3 (adjusted model) shows the associations of total GWG in the first and second trimesters, and weekly eGWG with PTB subtypes.
In the adjusted model, compared with women who had a total GWG in the first and second trimesters within the Chinese recommendation, women who were below and above the recommended value had an increased risk of PTB (below, OR: 1.88, 95% CI: 1.59–2.22, P < 0.001; above, OR: 1.43, 95% CI: 1.28–1.60, P < 0.001). When the outcomes were stratified according to different PTB subtypes, the aforementioned associations remained statistically significant, with the highest risk observed in medically indicated PTB.
In the adjusted model, compared with women with weekly eGWG of 100–399 g (Class II), pregnant women with a weekly eGWG of < 100 g (Class I) and ≥ 400 g (Class III) had a higher risk of PTB (Class I, OR: 1.70, 95% CI: 1.50–1.92, P < 0.001; Class III, OR: 2.21, 95% CI: 1.99–2.46, P < 0.001). When analyzing different subtypes of PTB as the outcome, the associations described above retained their statistical significance, with the highest risk observed in PPROM.
Association of weight gain patterns with PTB subtypes in pregnant women with different pre-pregnancy BMI
Further, we examined the associations of total GWG in the first and second trimesters, and weekly eGWG with PTB subtypes stratified by pre-pregnancy BMI in both crude (Table 4) and adjusted (Table 5) models.
In normal (pre-pregnancy BMI: 18.5–23.9 kg/m2) pregnant women, except for the association between total GWG and PPROM, which did not reach statistical significance (OR: 1.24, 95% CI: 0.99–1.54, P = 0.059), both insufficient and excessive weight gain—whether measured as total GWG in the first and second trimesters or as weekly eGWG—were statistically associated with an increased risk of all three PTB subtypes.
Among underweight (pre-pregnancy BMI < 18.5 kg/m2) pregnant women, excessive weight gain (both total GWG in the first and second trimesters and weekly eGWG) was positively associated with an increased risk of all subtypes of PTB, while insufficient weight gain showed no significant association. However, among overweight/obesity (pre-pregnancy BMI ≥ 24 kg/m2) pregnant women, only insufficient total GWG during the first and second trimesters was associated with an increased risk of spontaneous PTB (OR: 3.75, 95% CI: 1.23–11.43, P = 0.020) and PPROM (OR: 3.80, 95% CI: 1.25–11.49, P = 0.018).
Discussion
In this cohort study conducted among Chinese women based on a registry system, we found that total GWG in the first and second trimesters and weekly eGWG were associated with an increased risk of PTB as well as its subtypes. When stratified by pre-pregnancy BMI, excessive weight gain in underweight women, insufficient or excessive weight gain in normal-weight women, and insufficient weight gain in overweight/obese women were identified as risk factors for PTB and its subtypes.
GWG is a critical modifiable factor influencing perinatal maternal and neonatal outcomes and appropriate GWG has been implicated as a causal factor in various adverse pregnancy outcomes, including PTB15,16. According to published studies, the association between insufficient GWG and an increased risk of PTB is well-established17,18,19,20,21,9, while the relationship between excessive GWG and PTB remains controversial. The study by Pigatti Silva et al.18 identified excessive GWG as a risk factor for PTB, which is consistent with our findings. However, other studies19,20,21,22 have reported that excessive GWG may act as a protective factor against PTB. For instance, a study conducted in China20 demonstrated an inverse association between GWG and PTB (OR: 0.81, 95% CI: 0.72–0.91). Similarly, a study from Puerto Rico21 indicated that excessive GWG reduced the risk of both moderate PTB (OR: 0.77, 95% CI: 0.67–0.88) and late PTB (OR: 0.93, 95% CI: 0.88–0.98) among overweight/obese pregnant women. The conflicting findings may be attributed to the fact that these studies did not account for the differences in the duration of GWG between preterm and term delivery groups12. Specifically, PTB inherently shortens the time available for weight gain, potentially leading to lower total GWG among women delivering preterm, which could confound the observed association between GWG and PTB.
Additionally, pre-pregnancy BMI plays a significant role in the relationship between GWG and PTB. Both Chinese23 and IOM12 guidelines specify distinct recommended GWG ranges based on pre-pregnancy BMI categories. Previous studies18,20 have shown that the impact of GWG on PTB risk differs according to pre-pregnancy BMI. In line with this, our study found that underweight women with excessive GWG and overweight/obese women with insufficient GWG had a higher risk of PTB. It is worth noting that, unlike these two studies18,20 conducted in East Asian populations which applied the IOM recommendations12—a standard more suitable for White populations—our study utilized the Chinese guidelines23 to classify both pre-pregnancy BMI categories and GWG ranges.
Moreover, eGWG was critical for embryogenesis and fetal growth12. However, few studies have specifically examined eGWG related to PTB. Therefore, in this study, we also investigated the association of weekly eGWG with PTB, and our results indicated that low or high weekly eGWG was significantly associated with a higher risk of all types of PTB. Specifically, high weekly eGWG was most strongly linked to PTB in underweight women, but not significantly associated in overweight/obese women. These findings highlight the clinical importance of managing early gestational weight gain, particularly in underweight and normal-weight women, to reduce the risk of PTB.
While GWG in the first half of pregnancy is mainly the result of maternal tissue deposition and placental growth, gains from that point on until the end of pregnancy might be influenced by the accumulation of amniotic fluid. Inappropriate GWG is associated with inflammatory up-regulation through increased production of adipokines by adipose tissue and augmented systemic secretion of pro-inflammatory cytokines, which may contribute to the biological pathway of PTB24. Another potential biological pathway is that inappropriate GWG may disrupt maternal endocrine homeostasis. Excessive weight gain during early pregnancy can disrupt metabolic homeostasis, leading to insulin resistance, hyperglycemia, and dyslipidemia, which may impair placental function and increase the risk of PTB6.
Our findings suggest that public health recommendations should emphasize personalized GWG targets based on pre-pregnancy BMI. For underweight women, avoiding excessive GWG during early pregnancy may reduce PTB risk, while overweight/obese women should be encouraged to achieve adequate GWG to prevent adverse outcomes. Prenatal care programs and community-based interventions could play a key role in supporting women in achieving these goals.
This study has several notable strengths. First, it mitigates potential confounding due to differences in the duration of GWG between preterm and term birth populations. Second, it employs GWG recommendations specifically tailored to the Chinese population, ensuring more accurate classification of GWG categories. We also hope that this study can provide valuable insights for future research in low- and middle-income countries. Third, the large sample size allows for in-depth stratified analyses based on PTB subtypes and pre-pregnancy BMI categories.
However, this study also has certain limitations. A potential limitation of the study is that our data relies on a self-reported pre-pregnancy weight, which could be underestimated, and the potential misclassification bias may exist. However, previous studies suggest that the resulting BMI category from self-reported data rarely alters, and the self-reported weight may be considered an acceptable substitute for actual measurements25,26. Another limitation is that even if we adjusted for several potential confounders, residual confounding from unmeasured factors (e.g., diet or physical activity) cannot be ruled out. Future studies with more comprehensive data are needed to further evaluate the potential impact of residual confounding on these associations.
In conclusion, we conducted a population-based cohort study in China to explore the association of GWG patterns with the risk of PTB and its subtypes. Excessive weight gain in underweight women, insufficient or excessive weight gain in women with normal pre-pregnancy BMI, and inadequate weight gain in overweight/obese women were all significantly associated with a higher risk of PTB. These findings underscore the importance of optimal GWG across all BMI categories to mitigate PTB risks, highlighting the need for tailored prenatal weight management strategies. Future research should validate these findings in prospective cohorts and diverse populations to confirm their generalizability. Such studies will also provide an opportunity to explore the mechanisms underlying the observed associations.
Methods
Study population and design
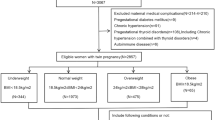
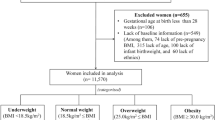
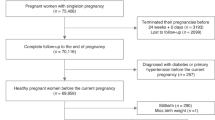
This retrospective cohort study was conducted in Wuhan, China, using electronic medical record data from a registry system, the Maternal and Children Healthcare Information Tracking System of Wuhan, which is an extensive integrated healthcare system including information on maternal demographic characteristics, medical history, antenatal examinations and delivery information from all the 93 hospitals and 121 community health centers in Wuhan. Eligible participants in this study were women who delivered a live singleton newborn with no congenital disabilities within 28–41 weeks’ gestational age between March 1, 2011, to May 31, 2013 and lived in the urban area of Wuhan during pregnancy (n = 106682). Individuals with gestational age < 28 weeks were not included due to the small sample size (n = 35) and to ensure a consistent observation period for GWG between preterm and term births. Women younger than 16 years or older than 50 at delivery were excluded (n = 6). Also, participants with unknown height (n = 547), pre-pregnancy weight (n = 23033), or weight at 22–28 weeks of gestation (n = 25958) were excluded. A total of 57,138 women met the eligibility criteria and were included in the study. Among them, 55,414 had their weight measured at least once during the early pregnancy (8–20 weeks).
The Medical Ethics Committee of Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital) approved the study and analysis plan (Approve Number: 2016003). All methods were carried out in accordance with relevant guidelines and regulations. Since this is a retrospective study based on registry system, the written informed consent was not applicable. The Medical Ethics Committee of Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital) agreed to waive the need for written informed consent from all study participants.
Assessment of study variables
Gestational age was calculated from the delivery date and the date of the last menstrual period. Preterm delivery was defined as a delivery between 28 weeks, 0 days and 36 weeks, 6 days of gestation. Very preterm birth (< 28 weeks gestation) was not included in our study as there were few in this cohort (35 cases in the study time).
We additionally categorized the PTB subtype as either spontaneous PTB, PPROM, or medically indicated PTB based on the records of clinical diagnosis reported by the obstetrician at birth. Medically indicated PTB was defined by either induction or cesarean section without uterine contractions or rupture of membranes prior to delivery. PPROM was defined as the PTB with premature rupture of membranes (PROM), and spontaneous PTB was identified as early onset of delivery and no identifiable medical indication without a PROM diagnosis.
The gender of the infants was obtained from birth records. Pre-pregnancy weight and height were self-reported at the first antenatal care visit (usually during the first trimester). Pre-pregnancy BMI was calculated as weight (kg)/height (m2 and then categorized into four groups based on recommendations by the Working Group On Obesity in China of the Chinese Ministry of Health (Underweight: <18.5 kg/m2, normal weight: 18.5–23.9 kg/m2, overweight: 24–27.9 kg/m2, and obesity: ≥28 kg/m2)27.
The total GWG in the first and second trimesters was calculated by subtracting maternal pre-pregnancy weight from the weight before 28 weeks of gestation (if multiple measurements were available, the one closest to 28 weeks was selected). Subsequently, based on the Chinese guidelines (Standard of Recommendation for Weight Gain during Pregnancy Period)23, total GWG in the first and second trimesters was classified as insufficient, adequate, or excessive according to the recommended ranges for the specific gestational week at which the actual weight measurement was taken. To provide a clearer understanding of the classification criteria used in this study, Table 6 shows the specific ranges for recommended GWG according to Chinese guideline23. A weight measurement taken between 8 and 20 weeks of gestation (if multiple measurements were available, the one closest to 20 weeks was selected) was used to evaluate eGWG that reported as a rate/week in grams. It was calculated by subtracting self-reported pre-pregnancy weight from measured weight during early gestation (8–20 weeks) and dividing by gestational week at measurement. It was categorized as class I (< 100 g/week), class II (100–399 g/week), and class III (≥ 400 g/week)28.
Statistical analysis
Categorical variables were expressed as frequencies and percentages. Unconditional logistic regression was conducted to calculate odds ratios (ORs) and 95% confidence intervals (CIs) to evaluate the associations of total GWG in the first and second trimesters, and weekly eGWG with PTB subtypes. Models were adjusted for potential confounders, including maternal age at delivery, education level, gravidity, parity, and offspring gender. To assess effect modification, we included an interaction term between pre-pregnancy BMI and total GWG/eGWG in the logistic regression model. A significant (p value < 0.10) interaction term indicated heterogeneity across pre-pregnancy BMI categories. We further stratified the analysis by pre-pregnancy BMI and created separate logistic regression models for each stratum to evaluate the association within subgroups. All the statistical analyses were conducted with SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina), and p value < 0.05 was considered statistically significant.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- eGWG:
-
Early gestational weight gain
- GWG:
-
Gestational weight gain
- OR:
-
Odds ratio
- PTB:
-
Preterm birth
- PPROM:
-
Preterm premature rupture of membrane
References
Goldenberg, R. L., Culhane, J. F., Iams, J. D. & Romero, R. Epidemiology and causes of preterm birth. Lancet 371, 75–84 (2008).
Katz, J. et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet 382, 417–425 (2013).
Mwaniki, M. K., Atieno, M., Lawn, J. E. & Newton, C. R. J. C. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet 379, 445–452 (2012).
Robbins, C. L., Hutchings, Y., Dietz, P. M., Kuklina, E. V. & Callaghan, W. M. History of preterm birth and subsequent cardiovascular disease: a systematic review. Am. J. Obstet. Gynecol. 210, 285–297 (2014).
Blencowe, H. et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379, 2162–2172 (2012).
Hunter, P. J. et al. Biological and pathological mechanisms leading to the birth of a small vulnerable newborn. Lancet 401, 1720–1732 (2023).
Cnattingius, S. et al. Maternal obesity and risk of preterm delivery. JAMA 309, 2362–2370 (2013).
Shaw GM,et al. Maternal prepregnancy body mass index and risk of spontaneous preterm birth. Paediatr Perinat Epidemiol. 28, 302–11 (2014).https://pubmed.ncbi.nlm.nih.gov/24810721/
Mamun, A. A. et al. Associations of maternal pre-pregnancy obesity and excess pregnancy weight gains with adverse pregnancy outcomes and length of hospital stay. BMC Preg. Childbirth. 11, 62 (2011).
Faucher, M. A., Hastings-Tolsma, M., Song, J. J., Willoughby, D. S. & Bader, S. G. Gestational weight gain and preterm birth in obese women: a systematic review and meta-analysis. BJOG 123, 199–206 (2016).
Sharma, A. J. et al. Associations of gestational weight gain with preterm birth among underweight and normal weight women. Matern. Child. Health J. 19, 2066–2073 (2015).
Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain during Pregnancy: Reexamining the Guidelines (National Academies Press (US), 2009).
Laitinen, J. et al. Maternal weight gain during the first half of pregnancy and offspring obesity at 16 years: a prospective cohort study. BJOG 119, 716–723 (2012).
Yazdani, S., Yosofniyapasha, Y., Nasab, B. H., Mojaveri, M. H. & Bouzari, Z. Effect of maternal body mass index on pregnancy outcome and newborn weight. BMC Res. Notes. 5, 34 (2012).
LifeCycle Project-Maternal Obesity and Childhood Outcomes Study Group. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA 321, 1702–1715 (2019).
Louise, J., Deussen, A. R. & Dodd, J. M. Gestational weight Gain-Re-Examining the current paradigm. Nutrients 12, 2314 (2020).
Kominiarek, M. A. et al. Association between gestational weight gain and perinatal outcomes. Obstet. Gynecol. 132, 875–881 (2018).
Pigatti Silva, F. et al. Role of body mass index and gestational weight gain on preterm birth and adverse perinatal outcomes. Sci. Rep. 9, 13093 (2019).
Enomoto, K. et al. Pregnancy outcomes based on Pre-Pregnancy body mass index in Japanese women. PLoS One. 11, e0157081 (2016).
Hu, Y. et al. Association between maternal gestational weight gain and preterm birth according to body mass index and maternal age in Quzhou, China. Sci. Rep. 10, 15863 (2020).
Eick, S. M. et al. Associations between gestational weight gain and preterm birth in Puerto Rico. BMC Preg. Childbirth. 20, 599 (2020).
McDonald SD, et al. High gestational weight gain and the risk of preterm birth and low birth weight: a systematic review and meta-analysis. J Obstet Gynaecol Can. 33, 1223–1233 (2011). https://pubmed.ncbi.nlm.nih.gov/22166276/
Standard of Recommendation for Weight. Gain during pregnancy period. Biomed. Environ. Sci. 35, 875–877 (2022).
Lee, K. K. et al. Maternal obesity during pregnancy associates with premature mortality and major cardiovascular events in later life. Hypertension 66, 938–944 (2015).
Vlachadis, N. Pregnancy outcomes with weight gain above or below the 2009 Institute of medicine guidelines. Obstet. Gynecol. 122, 696–697 (2013).
Brunner Huber, L. R. Validity of self-reported height and weight in women of reproductive age. Matern. Child. Health J. 11, 137–144 (2007).
Zhou, B. F. & Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed. Environ. Sci. 15, 83–96 (2002).
Nohr, E. A. et al. Cause-Specific stillbirth and neonatal death according to prepregnancy obesity and early gestational weight gain: A study in the Danish National birth cohort. Nutrients 13, 1676 (2021).
Acknowledgements
We are extremely grateful to Wuhan Health Bureau, and all the hospitals and community health centers involved in this study.
Funding
This work was supported by the Knowledge Innovation Specialized Project of Wuhan Science and Technology Bureau (2023020201010198), National Natural Science Foundation of China (81903331) and Open project of Key Laboratory of Environment and Health, Ministry of Education (2022GWKFJJ05).
Author information
Authors and Affiliations
Contributions
Yiyang Guo, Kai Chen, and Jin’e Zhang contributed equally to this work. Guo Yiyang and Kai Chen made substantial contributions to the conception and design of the study, data analysis, drafting of the manuscript. Jin’e Zhang made substantial contributions to the data analysis and revision of the manuscript. Chao Xiong and Aifen Zhou made substantial contributions to study design, intellectual direction, and revision of the manuscript. Yiming Zhang, Zhiguo Xia, Yuji Wang, Xiaoxuan Fan, Xiaofeng Mu, Luli Xu, and Menglan Guo made contributions to data collection and revision of the drafting of the manuscript. All authors were involved in drafting of the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The Medical Ethics Committee of Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital) approved the study and analysis plan (Approve Number: 2016003). Since this is a retrospective study based on registry system, the written informed consent was not applicable. The Medical Ethics Committee of Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital) agreed to waive the need for written informed consent from all study participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, Y., Chen, K., Zhang, J. et al. Association of gestational weight gain patterns with preterm birth subtypes in a population based cohort study from China. Sci Rep 15, 23324 (2025). https://doi.org/10.1038/s41598-025-03995-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03995-5