Abstract
This study aims to examine the determinants of contralateral central lymph node metastasis in cases of unilateral papillary thyroid carcinoma, utilizing clinical pathological parameters and ultrasound radiomics. The goal is to develop an optimal predictive model that can inform clinical decisions regarding contralateral central lymph node dissection in patients with unilateral papillary thyroid cancer. A cohort of 329 patients diagnosed with unilateral papillary thyroid carcinoma, all of whom underwent bilateral thyroidectomy and bilateral central lymph node resection, were analyzed. Clinical data were systematically collected, and a logistic regression analysis model was constructed. The patient cohort was randomly divided into a training set and a validation set in an 8:2 ratio. Radiomic features were extracted from ultrasound images using the open-source software pyradiomic, leading to the establishment of three models: a clinical model, a radiomics model, and a combined clinical and radiomics model. The area under the curve (AUC) for the logistic regression model was found to be 0.843. In the training set, the AUCs for the clinical model, the radiomics feature model, and the combined clinical and radiomics feature model developed using random forest were 0.7645, 0.9633, and 0.9726, respectively. In the validation set, the AUCs for the clinical model, the radiomics feature model, and the combined clinical and radiomics feature model developed using random forest were 0.7358, 0.9558, and 0.9694, respectively. The combined model incorporated four clinical features and seven radiomic features, specifically: age, tumor size, presence of microcalcification, presence of ipsilateral central lymph node metastasis, elongation, perimeter, sphericity, maximum probability, large dependence emphasis, first-order range, and cluster shade. The combined clinical and radiomics model developed in this research demonstrates strong predictive diagnostic efficacy. The establishment of this innovative model is anticipated to provide a robust theoretical framework for clinicians considering preventive bilateral central lymph node dissection in patients with unilateral papillary thyroid carcinoma.
Similar content being viewed by others
Introduction
Thyroid carcinoma has been identified as one of the most rapidly escalating malignancies worldwide1. Among them, differentiated thyroid cancer is the most common type of thyroid cancer, characterized by cancer cells that retain some of the function of normal thyroid cells, so the prognosis is usually better. Among its various forms, papillary thyroid carcinoma (PTC) is the predominant subtype, representing approximately 80–85% of cases2. The incidence of early cervical lymph node metastasis in PTC patients can reach as high as 20–50%3. Despite its relatively low malignancy and slow progression, most individuals diagnosed with PTC exhibit favorable prognoses following standard clinical interventions. Nonetheless, some studies have indicated that cervical lymph node metastasis serves as a significant risk factor for postoperative recurrence and diminished survival rates in patients with differentiated thyroid carcinoma (DTC)4.
Recent multicenter cohort studies have established cervical lymph node metastasis as a vital prognostic factor, revealing its substantial correlation with decreased 10-year disease-specific survival and heightened locoregional recurrence risk in multivariate analyses5,6. The revised guidelines from the American Thyroid Association (ATA)7 suggest that patients classified as high-risk for PTC may be candidates for prophylactic central lymph node dissection (PCLND). However, there remains clinical debate regarding the appropriateness of PCLND in patients with central lymph node metastasis (CNM) who do not exhibit clear lymph node involvement through imaging or palpation8,9. It is posited that CNM can manifest early in the progression of PTC, leading experts to recommend ipsilateral central lymph node (Ipsi-CLN) dissection for PTC patients, provided that the parathyroid blood supply is preserved and the recurrent laryngeal nerve is not compromised. However, due to the presence of numerous communicating branches among bilateral central lymph nodes, cancer cells may potentially metastasize to the contralateral central region, suggesting that patients with unilateral papillary thyroid carcinoma (uPTC) may also harbor metastases from contralateral central lymph nodes10. Current guidelines11 do not explicitly address whether such patients should undergo preventive bilateral lymph node dissection. The accurate preoperative identification of Cont-CLNM presents a significant challenge in the management of uPTC. The 2015 ATA guidelines cautiously endorse PCLND in high-risk scenarios while acknowledging the absence of effective preoperative predictors11. Consequently, there is an urgent need for the development of an advanced predictive model to enhance clinical decision-making in this context.
Radiomics is defined as the extraction of high-throughput image data from a substantial volume of medical imaging, followed by focal segmentation, feature extraction, and model development. This concept was initially introduced by Dutch researcher Lambin in 201212. The resulting models have the potential to assist clinicians in predicting and elucidating disease prognosis, thereby enhancing diagnostic accuracy in medical practice13. Radiomics has found extensive application in the diagnosis and treatment of various conditions, including thyroid cancer, breast cancer, lung cancer, liver cancer, and chronic kidney disease, contributing to advancements in medical standards and efficiency14,15,16,17,18.
This study is designed to address practical clinical challenges by developing an optimal model that integrates clinical and imaging features. The objective is to identify factors influencing contralateral central lymph node metastasis (Cont-CLNM) in patients with unifocal papillary thyroid carcinoma (uPTC), thereby informing decisions regarding the necessity of preventive contralateral central lymph node dissection.
Data and methods
All methods were performed in accordance with the relevant guidelines and regulations.
Patients
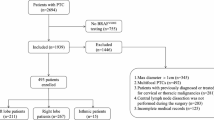
A retrospective analysis was conducted on patients who underwent total thyroidectomy combined with bilateral central lymph node dissection at our institution between July 1, 2017, and February 2, 2019. All participants had successful pathological sampling, comprehensive clinical data, preoperative ultrasonography, and pathological results, with diagnoses confirmed by experienced pathologists serving as the gold standard for routine postoperative evaluations. A total of 329 patients were included in the study, of whom 89 exhibited Cont-CLNM while 240 did not.
The ethics batch number of this research protocol is AF-SOP-07-1.2-01. The waiver was granted through IRB approval and the written informed consent was waived.
Inclusion criteria for the study were as follows: (1) Complete medical history, preoperative examinations, admission diagnoses, treatment processes, and postoperative pathological data for all patients; (2) Confirmation of PTC through postoperative histopathological findings in patients undergoing their first thyroid surgery; (3) Patients who underwent bilateral thyroidectomy and bilateral central lymph node resection.
Exclusion criteria included: (1) Benign thyroid lesions; (2) Pathological findings indicative of other types of thyroid malignancies; (3) Bilateral papillary thyroid carcinoma or isthmic papillary thyroid carcinoma; (4) Patients undergoing a second or subsequent surgery for thyroid cancer; (5) A history of neck radiation exposure or treatment; (6) A history of other cervical malignancies; (7) Incomplete preservation of clinical data; (8) Cases where the tumor size precluded the delineation of the area of interest.
Instruments and methods
Ultrasound images and clinical information
Ultrasound examinations were performed using a high-frequency linear array probe from Philips EPIQ 7, with a frequency range set at 10 MHz. All patients underwent routine preoperative ultrasound assessments. The preoperative data for the included cases were retrospectively analyzed, encompassing variables such as patient gender, age, tumor size, location, margins, echogenicity, structural characteristics, solid component echotexture, microcalcifications, posterior echogenicity, orientation, halo sign presence, and the existence of Hashimoto’s thyroiditis, benign nodules, external thyroid invasion, and ipsilateral central lymph node metastasis. Two sonographers collaboratively interpreted and analyzed the ultrasound images, meticulously documenting the ultrasonographic characteristics of the disease site. In instances of diagnostic discrepancies between the two sonographers, a third experienced expert was consulted to achieve a consensus on the final results.
Ultrasonographic image segmentation
Standard ultrasound images of 2 to 4 lesions were obtained from each patient, with the tumor boundaries manually delineated by an ultrasound resident, thereby defining the area of interest. The accuracy of these delineations was subsequently validated by experts with over a decade of experience in thyroid ultrasound diagnostics. To evaluate the interobserver repeatability of the region of interest (ROI) delineation, a random selection of 30 cases was made, and two independent radiologists, each possessing more than five years of experience in thyroid ultrasound, delineated the ROIs independently. The intraclass correlation coefficient (ICC) was computed to quantify the level of agreement between the two observers. The ICC values were interpreted according to the following criteria: ICC < 0.50 indicated poor agreement, 0.50 ≤ ICC < 0.75 indicated moderate agreement, 0.75 ≤ ICC < 0.90 indicated good agreement, and ICC ≥ 0.90 indicated excellent agreement. Features with ICC values below 0.75 were excluded to enhance the robustness of the radiomic features. Subsequently, radiomic features were extracted from these regions of interest for further analysis.
Radiomics feature extraction and selection
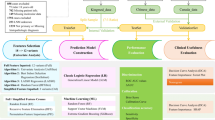
Initially, the original images were converted to grayscale (Fig. 1A), followed by amplification through contrast enhancement (1.5 × and 2x) (Fig. 1B,C), inversion (Fig. 1D), and rotations (45° and 90°) (Fig. 1E,F) to mitigate the effects of insufficient and unbalanced datasets on the predictive model. Various filters were applied to the amplified image data, including Laplacian of Gaussian (LoG, σ = 1, 3), Wavelet (binWidth = 10, 20), Square, Square Root, Exponential, and Gradient filters, to extract the most pertinent features across different scales. Using open source software pyradiomic (v3.0.1, https://github.com/Radiomics/pyradiomics.) in ultrasound images has been drawing ROI extraction of image characteristics of radiomics.
(A): The original images were converted to grayscale. (B) Amplification through contrast enhancement (1.5x). (C) Amplification through contrast enhancement (2.0x). (D) Amplification through inversion. (E) Amplification through inversion rotations (45°). (F) Amplification through inversion rotations (90°).
T-tests were conducted on all features, encompassing both clinical and radiomic features, to ensure correlation and independence among the features. Features exhibiting a Pearson correlation coefficient greater than 0.9 were selected for inclusion in the feature set. Max–min normalization of the radiomic features was performed to eliminate the influence of differing dimensions on data analysis, ensuring that the data were on a comparable scale, which is conducive to comprehensive comparative evaluation. This normalization process also mitigated the potential for excessive influence on model training due to the high values of certain features, thereby enhancing the stability and convergence speed of the model. Finally, recursive feature elimination (RFE) was employed for feature screening, resulting in the identification of the most relevant features from various feature sets through fivefold cross-validation.
Constructing and verifying the radiomics model
Following the pretreatment phase, a diverse array of classifiers was employed to develop clinical models, radiomics models, and integrated clinical-radiomics models utilizing various feature sets. A global grid parameter search was conducted for all models. The training set comprised 263 patients, of whom 193 did not exhibit Cont-CLNM and 70 did. Additionally, the verification set included 66 patients, with 47 lacking Cont-CLNM and 19 presenting with it. During the model construction phase, the training set was randomly partitioned into five folds for cross-validation, and the ultimate performance of the predictive model was assessed using the test set. For testing, the same feature set utilized in the training phase was selected and input into the model.
To facilitate a thorough evaluation, we employed five distinct machine learning algorithms: Random Forest (RF), Extreme Gradient Boosting (XGBoost), Categorical Boosting (CatBoost), Gradient Boosting Decision Trees (GBDT), and Light Gradient Boosting Machine (LGBM). These classifiers were chosen for their robustness, scalability, and capacity to manage high-dimensional data, which are essential for the objectives of our study. RF is an ensemble learning technique that constructs multiple decision trees during the training phase and outputs either the mode of the classes or the mean prediction from the individual trees, thereby enhancing its resilience to overfitting and making it suitable for high-dimensional datasets. XGBoost represents an optimized gradient boosting algorithm that employs a gradient descent framework to minimize loss functions, recognized for its efficiency, scalability, and adeptness at handling sparse data. CatBoost is a gradient boosting algorithm specifically tailored to effectively manage categorical features, utilizing ordered boosting and innovative methodologies for processing such data. GBDT is an ensemble approach that sequentially builds decision trees, with each subsequent tree addressing the errors of its predecessor, thereby optimizing a differentiable loss function to achieve robust predictive performance. LGBM is a gradient boosting framework that employs tree-based learning algorithms, designed for distributed and efficient training, making it particularly suitable for large datasets characterized by high dimensionality. All models were implemented using their respective Python libraries (e.g., scikit-learn for RF and GBDT, xgboost for XGBoost, catboost for CatBoost, and lightgbm for LGBM). Hyperparameter optimization was conducted through grid search or random search in conjunction with cross-validation to enhance model performance. All machine learning experiments were conducted using Python 3.9. The main libraries used include scikit-learn (version 1.2.2), which was used to implement Random Forest and Gradient Boosting Decision Trees (GBDT), XGBoost (version 1.7.5), LightGBM (version 3.3.5), CatBoost (version 1.2), NumPy (version 1.24.2), and Pandas (version 1.5.3).
The evaluation of the model’s performance on both training and validation datasets was conducted using standard clinical metrics, including the area under the curve (AUC), accuracy, specificity, sensitivity, F1 index, and mean square error. The diagnostic performance of model sets was compared to identify those that exhibited superior performance on the test datasets.
Statistical methods
Data analysis was performed using SPSS version 26.0. For categorical data, the Pearson Chi-square test or Fisher’s exact test was applied, while the independent sample t-test was utilized for univariate analysis of continuous data (with P < 0.05 considered statistically significant). In the multifactorial analysis, logistic regression methods were employed to construct regression equations, and the diagnostic efficacy was evaluated through Receiver Operating Characteristic (ROC) curves. An AUC value ranging from 0.7 to 0.8 indicates an average model, 0.8 to 0.9 signifies a good model, and values exceeding 0.9 denote an excellent model.
Results
Patients data
Based on the established inclusion and exclusion criteria, a total of 329 cases were selected following screening, of which 89 cases exhibited contralateral central lymph node metastasis, while 240 cases did not. Among the 329 patients, 16.4% (54/329) were male and 83.6% (275/329) were female. The age of patients ranged from 10 to 85 years, with a median age of 44 years. Tumor sizes varied from 0.32 to 7.51 cm, with a median size of 1.22 cm.
Results of the logistic regression model
Univariate analysis results (refer to Table 1) indicated that the mean age of patients in the Cont-CLNM group was significantly younger than that of the non-Cont-CLNM group (38.1 years vs. 46.6 years, P < 0.001). Additionally, the Cont-CLNM group presented with larger lesions (2.03 cm vs. 1.37 cm, P < 0.001), a higher proportion of irregular or blurred edges (98.9% vs. 90.8%, P = 0.011), a greater incidence of microcalcification (89.9% vs. 55.0%, P < 0.001), and a higher rate of metastasis in ipsilateral central lymph nodes (89.9% vs. 42.9%, P < 0.001).
A total of five independent variables were incorporated into the logistic regression model, specifically the patient’s age, tumor size, tumor margin, microcalcification, and ipsilateral central lymph node metastasis. The analysis of the logistic regression model (refer to Table 2) indicated that a regression value of P ≥ 0.05 was associated with the prediction of Cont-CLNM in patients with unifocal papillary thyroid carcinoma (uPTC), while a P value of < 0.05 indicated the absence of Cont-CLNM in this patient population. The predictive accuracy of the model was determined to be 80.2%. A receiver operating characteristic (ROC) curve was generated based on the logistic regression model, illustrating the predicted probability of metastasis for diagnostic uPTC Cont-CLNM (see Figs. 2 and 3). Within the logistic regression framework, the area under the ROC curve (AUC) for age and tumor size were found to be 0.702 and 0.663, respectively, while the AUC for the model’s predictive probability was 0.843. The standard error was calculated to be 0.034, with a significance level of P < 0.001, and the 95% confidence interval ranged from 0.798 to 0.888.
Receiver operating characteristic (ROC) curves comparing the diagnostic performance of age and tumor size in the logistic regression model for predicting of contralateral central lymph node metastasis in unilateral papillary thyroid carcinoma. Analysis based on a retrospective cohort of 329 patients, with area under the curve (AUC) values of 0.663 (95% CI 0.948–0.996) for age and 0.702 (95% CI 1.032–1.708) for tumor size.
Results of radiomics features
The open-source software PyRadiomics was utilized to extract 1,366 features from images at various scales, encompassing shape, texture, and gray-level features. During the feature selection process, five-fold cross-validation was implemented, resulting in the selection of different features across the folds. Following a series of processing and filtering steps, the final features identified included 13 individual clinical and pathological factors: age, presence of benign nodules, presence of Hashimoto’s thyroiditis, presence of external thyroid invasion, tumor size, location, margin, echogenicity, structure, presence of microcalcification, presence of a halo sign, and presence of ipsilateral central lymph node metastasis. Additionally, five radiomic features were selected: elongation, perimeter, sphericity, variability, and texture complexity. Furthermore, 11 clinical-radiomic joint factors were identified, which comprised seven radiomic features (elongation, circumference, sphericity, maximum probability, measure of large dependent distribution, range of gray values in the region of interest, and clustering shadow) alongside four clinical and pathological factors (age, tumor size, presence or absence of microcalcification, and presence or absence of ipsilateral central lymph node metastasis).
Comparison of diagnostic efficiency of radiomics models
We developed predictive models utilizing clinical and pathological features, radiomic features, and a combination of clinical-radiomic features.
Upon comparing the predictive outcomes of the classifiers and integrating various evaluation metrics, we selected the RF classifier as the final predictive model due to its superior performance. The receiver operating characteristic (ROC) curve and the detection error tradeoff (DET) curve for the integrated learning model of each classifier are presented in Figs. 4A–C and 5A–C, respectively, to illustrate the performance of each classifier. The models’ performance was further assessed using metrics such as the area under the ROC curve (AUC), sensitivity, specificity, Brier Score, Hosmer–Lemeshow (HL) Statistic, F1 Score, and Youden Index, as detailed in Table 3. The AUC values for the clinical, radiomic, and combined models in the training cohort were 0.7645, 0.9633, and 0.9726, respectively. In the validation cohort, the AUC values were 0.7358, 0.9558, and 0.9694, respectively. These findings indicate that the combined model significantly enhanced diagnostic performance compared to the individual clinical or radiomic models, with the combined model yielding a 13% increase in AUC relative to the Logistic Regression model, alongside corresponding improvements in sensitivity and specificity. In the validation cohort, the accuracy rate of the combined model was as high as 0.9245.
The ROC curve was used to compare the discriminative performance of the clinical, radiomics and combined models constructed by the five classifiers respectively in predicting contralateral central lymph node metastasis of uPTC. The analysis included a retrospective cohort of 329 patients, with the combined model (clinical and radiomics features) achieving the highest AUC. (A) Clinical models (B) Radiomics models (C) Combined models.
The DET curve was used to compare the discriminative performance of the clinical, radiomics and combined models constructed by the five classifiers respectively in predicting contralateral central lymph node metastasis of uPTC. The analysis included a retrospective cohort of 329 patients, with the combined model (clinical and radiomics features) achieving the highest AUC. (A) Clinical models (B) Radiomics models (C) Combined models.
We conducted an analysis of the performance metrics for each classifier within the integrated clinical-radiomics model, as illustrated in Fig. 6A,B. The Random Forest (RF) model demonstrated superior performance in terms of accuracy, specificity, and stability, thereby reinforcing its selection as the final model. The Brier score and maximum Youden index for the combined clinical-radiomics model developed using the RF classifier were recorded at 0.0704 and 0.8642, respectively. The threshold proposed in this study (0.8642) was designed to balance the risk of false positives and false negatives. The decision to select this threshold was made by weighing the risk of over dissection (e.g. recurrent laryngeal nerve injury, hypoparathyroidism) against the risk of missing metastasis (e.g. local recurrence). The radiomic features incorporated into the clinical-radiomics model are detailed in Table 4. These features were chosen based on their significant contribution to the model’s predictive efficacy and their relevance in a clinical context.
Analysis of performance indicators of each classifier in the clinical-radiomics comprehensive model. For instance, the RF model demonstrates superior performance in terms of accuracy, specificity and stability. The Brier score and the maximum Youden index of the clinical-radiomics combined model established using the RF classifier were 0.0704 and 0.8642, respectively. (A) Precision-Recall curves assessing classifier performance in the clinical-radiomics combined model for predicting contralateral central lymph node metastasis of uPTC. (B) Calibration curves assessing classifier performance in the clinical-radiomics combined model for predicting contralateral central lymph node metastasis of uPTC.
Discussion
In recent years, there has been a notable increase in the incidence of PTC. While the overall prognosis for PTC is generally favorable, a subset of patients experiences early-stage lymph node metastasis, which is recognized as a significant risk factor for distant metastasis, local recurrence, and reduced survival rates associated with PTC19. Ultrasound imaging is a critical tool in the diagnosis of thyroid cancer; however, the intricate anatomical structure of the central neck region limits the accuracy of ultrasound in detecting central lymph node metastasis. Consequently, accurately assessing the central lymph nodes in patients with unilateral PTC presents a considerable research challenge20. Radiomics, a novel non-invasive analytical approach, has been increasingly utilized in the diagnosis and prognostic evaluation of thyroid cancer21. To date, there have been no reported studies focusing on the radiomic prediction of unilateral central lymph node metastasis in papillary thyroid carcinoma. Therefore, this study aims to provide evidence to support the clinical dissection of contralateral central lymph nodes in cases of uPTC. This investigation analyzed the clinical, ultrasonographic, and radiomic characteristics of thyroid tumors in patients with unilateral PTC, examined their correlation with Cont-CLNM developed various predictive models, and identified the optimal model, which is a combined clinical and radiomic model based on comprehensive performance metrics.
In this study, the mean age of the uPTC group with Cont-CLNM was 38.1 years, while the mean age of the group without Cont-CLNM was 46.6 years. A meta-analysis conducted by Qu et al.22 indicated that patients over the age of 45 with papillary thyroid cancer exhibit a significantly lower risk of central lymph node metastasis. Other studies23 have suggested that patients in the metastatic cohort tend to be younger than those in the non-metastatic cohort. However, some scholars, such as Londero et al.24, argue that patients with papillary thyroid cancer over the age of 45 may experience poorer prognoses and higher recurrence rates, potentially due to age-related declines in immune function. This study posits that a median age of 44 years serves as a threshold, with younger patients being more susceptible to Cont-CLNM. Epidemiological data further suggest that younger thyroid cancer patients exhibit a greater propensity for metastasis, which may be attributable to an age-related metabolically active microenvironment25.
The prevalence of PTC lesions is positively correlated with the likelihood of lymph node metastasis in the central neck region, a phenomenon that is widely recognized in clinical practice. Lesion size serves as a critical parameter in formulating treatment strategies for patients with PTC, as well as determining the extent of central cervical lymph node dissection. Consequently, tumor size is regarded as a significant risk factor for Cont-CLNM in PTC patients26,27. In the present study, the regression coefficient for lesion size in the multivariate analysis was found to be 0.283, with an odds ratio (OR) of 1.328, indicating that for every 1 cm increase in lesion size, the probability of Cont-CLNM escalates by a factor of 1.328. Prior research28 has indicated that when the maximum diameter of the lesion exceeds 10 mm, the likelihood of CNM increases. Furthermore, findings by Ji et al.29 corroborate that an increase in tumor diameter correlates with an elevated risk of CNM in PTC. The results of this investigation align with previously reported data, underscoring the close association between tumor size and the incidence of Cont-CLNM in uPTC.
According to the 2015 Guidelines of the American Thyroid Association11, ultrasonographic indicators of microcalcification are recognized as highly specific markers for suspected malignant thyroid nodules and constitute a significant ultrasonographic characteristic in the diagnosis of PTC. Microcalcifications are typically defined as strong echoes measuring less than 1 mm in diameter, resulting from the aggregation and fusion of calcific particles. Research has demonstrated that PTC patients exhibiting ultrasound features of microcalcification face a heightened risk of CNM30. The findings of this study further validate that the risk factors associated with Cont-CLNM are applicable to uPTC patients. Specifically, uPTC cases accompanied by microcalcification exhibited a Cont-CLNM probability of 37.7% (80 out of 212). In the analysis aimed at predicting Cont-CLNM, ultrasonographic signs of microcalcification were identified as independent risk factors.
Additionally, the research conducted by Chen et al.31 suggests that Ipsi-CLN metastasis constitutes an independent risk factor for CNM. In this study, approximately 43.7% (80 out of 183) of patients with Ipsi-CLN metastasis also presented with Cont-CLNM, a metastasis rate that surpasses the 25.5% reported by Sadowski et al.32 regarding Cont-CLNM. The results of the multifactorial analysis further support the notion that Ipsi-CLN metastasis is an independent risk factor for Cont-CLNM in uPTC patients. This phenomenon may be attributed to the extensive network of lymphatic pathways connecting the central lymph nodes bilaterally, coupled with the unclear and somewhat random mechanisms governing tumor cell dissemination, which can facilitate the metastasis of tumor cells to the contralateral central lymph node region.
In comparison to predictions based solely on individual clinical features, the accuracy of radiomics features extracted from imaging has significantly improved. The characteristics of lesions identified in ultrasonographic images are derived from mathematical computations based on pixel gray values. Our analysis indicates that the majority of features selected during the final screening process are associated with alterations in shape and gray value.
Specifically, elongation, circumference, and sphericity are categorized as shape characteristics of the original ultrasonographic image, each reflecting the morphological attributes of the region of interest (ROI). Elongation and sphericity, to a certain degree, are indicative of the lesion’s tendency towards a circular shape, as evidenced by their characteristic values. A higher elongation value, approaching 1, correlates with a sphericity value also nearing 1, suggesting that the lesion’s shape is more circular, which in turn implies a reduced likelihood of contralateral metastasis. This phenomenon is somewhat analogous to the aspect ratio utilized in ultrasonographic diagnostics. The circumference metric assesses the smoothness of the lesion’s boundary; a larger circumference value indicates a more convoluted boundary, which is associated with an increased propensity for contralateral metastasis.
The gray value range within the ROI reflects the overall pixel gray value distribution within the lesion area. A significant variation in gray values is more likely to be associated with transfer phenomena, which corresponds to the occurrence of micro-calcifications in ultrasonographic images.
The maximum probability, the measure of large dependence distribution, and the clustering shadow metrics all characterize local gray value variations. Fluctuations in these three metrics are indicative of the uniformity of the lesion’s texture in the ultrasound image. Typically, patients with metastasis exhibit low values for maximum probability and the measure of large dependence distribution, suggesting a lack of uniformity in the lesion’s gray value and a significant contrast, which indicates considerable regional texture variation. Concurrently, elevated clustering shadow values suggest asymmetry in pixel changes, thereby confirming that uneven texture changes within the lesion area are associated with a tendency for metastasis.
While the clinical factors of aspect ratio, boundary, and internal echo uniformity do not demonstrate clear significance in traditional clinical feature analysis, the radiomics features associated with these factors are meaningfully incorporated in radiomics feature analysis. This discrepancy may arise from the limitations of sonographers in detecting subtle lesion features through visual inspection and clinical experience alone. In this study, we employed specialized software to analyze these subtle features that are often imperceptible to the human eye, thereby addressing the limitations faced by sonographers. Consequently, the clinical implications represented by these significant radiomics features warrant careful consideration.
This study presents several limitations. Firstly, it is a single-center retrospective analysis with an insufficient sample size. Secondly, the data utilized were not adjusted for propensity scores, which may introduce bias into the findings. Lastly, the study lacks external validation due to resource constraints and challenges in data acquisition. Future research endeavors should focus on conducting multi-center studies with larger sample sizes to enhance the understanding of risk factors associated with contralateral central lymph node metastasis in patients diagnosed with unilateral papillary thyroid carcinoma.
Conclusion
The integrated clinical and radiomic model developed in this research demonstrates commendable predictive diagnostic efficacy. Key risk factors identified include patient age, tumor size, presence of microcalcification, ipsilateral central lymph node metastasis, elongation, circumference, globularity, maximum probability, measures of large dependence distribution, gray value range within the region of interest (ROI), and clustering shadow. Based on the optimal threshold established in this study, contralateral central lymph node dissection is recommended when the Youden index of the combined clinical-radiomic model, as determined by the random forest classifier, exceeds 0.8642. The establishment of this model is anticipated to provide a more robust theoretical framework for clinicians considering preventive bilateral central lymph node dissection in patients with unilateral papillary thyroid carcinoma.
Data availability
Data is provided within the supplementary information files.
Change history
20 September 2025
The original online version of this Article was revised: In the original version of this Article the author, Minghui Zhang was incorrectly affiliated with “Department of Ultrasound, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.” The original article has been corrected.
Abbreviations
- AUC:
-
Area under ROC curve
- CNM:
-
Central lymph node metastasis
- Cont-CLNM:
-
Contralateral central lymph node metastasis
- DTC:
-
Differentiated thyroid carcinoma
- Ipsi-CLNs:
-
Ipsilateral central lymph nodes
- PCLND:
-
Prophylactic central lymph node dissection
- PTC:
-
Papillary thyroid carcinoma
- RF:
-
Random forest
- RFE:
-
Recursive feature elimination
- ROC:
-
Receiver operating characteristic curves
- uPTC:
-
Unilateral papillary thyroid carcinoma
References
Chen, D. W. et al. Thyroid cancer. Lancet 401(10387), 1531–1544 (2023).
Prete, A. et al. Update on fundamental mechanisms of thyroid cancer. Front. Endocrinol. 11, 102 (2020).
Grimm, D. Recent advances in thyroid cancer research. Int. J. Mol. Sci. 23(9), 4631 (2022).
Seok, J. et al. Factors affecting central node metastasis and metastatic lymph node ratio in papillary thyroid cancer. Otolaryngol. Head Neck Surg. 165(4), 519–527 (2021).
Zhang, T. et al. Risk factors of cervical lymph node metastasis in multifocal papillary thyroid cancer. Front. Oncol. 12, 1003336 (2022).
Chung, S. R. et al. Sonographic diagnosis of cervical lymph node metastasis in patients with thyroid cancer and comparison of European and Korean guidelines for stratifying the risk of malignant lymph node. Korean J. Radiol. 23(11), 1102–1111 (2022).
American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C, Cooper DS, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19(11), 1167–1214 (2009).
Kim, D. H. et al. Predictive value of ipsilateral central lymph node metastasis for contralateral central lymph node metastasis in patients with thyroid cancer: Systematic review and meta-analysis. Head Neck 43(10), 3177–3184 (2021).
Shen, W., Pan, X. J. & Li, Q. H. Utility and significance of clinical risk factor scoring model in predicting central compartment lymph node metastasis (CLNM) in patients with papillary thyroid cancer (PTC). Pak. J. Med. Sci. 38(1), 214–218 (2022).
Moo, T. A. & Fahey, T. J. 3rd Lymph node dissection in papillary thyroid carcinoma. Semin. Nucl. Med. 41(2), 84–88 (2011).
Haugen, B. R. et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26(1), 1–133 (2016).
Lambin, P. et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 48(4), 441–446 (2012).
Li, F. et al. Using ultrasound features and radiomics analysis to predict lymph node metastasis in patients with thyroid cancer. BMC Surg. 20(1), 315 (2020).
Conti, A. et al. Radiomics in breast cancer classification and prediction. Semin. Cancer Biol. 72, 238–250 (2021).
Bandara, M. S. et al. Ultrasound based radiomics features of chronic kidney disease. Acad. Radiol. 29(2), 229–235 (2022).
Wu, G. et al. Structural and functional radiomics for lung cancer. Eur. J. Nucl. Med. Mol. Imaging 48(12), 3961–3974 (2021).
Carbonell, G. et al. Precision of MRI radiomics features in the liver and hepatocellular carcinoma. Eur. Radiol. 32(3), 2030–2040 (2022).
Xiong, Z. et al. Ultrasound radiomics based XGBoost model to differential diagnosis thyroid nodules and unnecessary biopsy rate: Individual application of SHapley additive exPlanations. J. Clin. Ultrasound 52(3), 305–314 (2024).
Chen, P. et al. BRAF V600E and lymph node metastases in papillary thyroid cancer. Endocr. Connect. 9(10), 999–1008 (2020).
Chen, W. et al. Unilateral papillary thyroid carcinoma treated with contralateral central lymph node dissection: A nomogram to aid in decision-making. Medicine (Baltimore) 99(38), e22200 (2020).
Mayerhoefer, M. E. et al. Introduction to radiomics. J. Nucl. Med. 61(4), 488–495 (2020).
Qu, H. et al. Clinical risk factors for central lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis. Clin. Endocrinol. (Oxf.) 83(1), 124–132 (2015).
Ni, X. et al. A risk stratification model for metastatic lymph nodes of papillary thyroid cancer: A retrospective study based on sonographic features. Front. Endocrinol. (Lausanne) 13, 942569 (2022).
Londero, S. C. et al. Papillary thyroid carcinoma in Denmark, 1996–2008: Outcome and evaluation of established prognostic scoring systems in a prospective national cohort. Thyroid 25(1), 78–84 (2015).
Oh, H. S. et al. Young age and male sex are predictors of large-volume central neck lymph node metastasis in clinical N0 papillary thyroid microcarcinomas. Thyroid 27(10), 1285–1290 (2017).
Lee, J. Y. et al. Validation of ultrasound risk stratification systems for cervical lymph node metastasis in patients with thyroid cancer. Cancers (Basel) 14(9), 2106 (2022).
Liu, W. et al. A proposed heterogeneous ensemble algorithm model for predicting central lymph node metastasis in papillary thyroid cancer. Int. J. Gen. Med. 15, 4717–4732 (2022).
Wei, T. et al. Predictive factors of contralateral paratracheal lymph node metastasis in unilateral papillary thyroid carcinoma. Eur. J. Surg. Oncol. 41(6), 746–750 (2015).
Ji, Y. B. et al. Predictive factors and pattern of central lymph node metastasis in unilateral papillary thyroid carcinoma. Auris Nasus Larynx 43(1), 79–83 (2016).
Chen, J. et al. Ultrasound validation of predictive model for central cervical lymph node metastasis in papillary thyroid cancer on BRAF. Future Oncol. 16(22), 1607–1618 (2020).
Chen, Q. et al. The total number of prelaryngeal and pretracheal lymph node metastases: Is it a reliable predictor of contralateral central lymph node metastasis in papillary thyroid carcinoma?. J. Surg. Res. 214, 162–167 (2017).
Sadowski, B. M., Snyder, S. K. & Lairmore, T. C. Routine bilateral central lymph node clearance for papillary thyroid cancer. Surgery 146(4), 696–703; discussion 703–695 (2009).
Funding
This study has received funding by Liaoning Natural Science Foundation: 2022-YGJC-52.
Author information
Authors and Affiliations
Contributions
(I) Conception and design: Liang Sang, He Ma, Zhihong Wang (II) Administrative support: Liang Sang, He Ma (III) Provision of study materials or patients: Zhihong Wang (IV) Collection and assembly of data: Minghui Zhang, Shuangqingyue Zhang, Yan Wang, Zijun Yu, Ziyi Yang (V) Data analysis and interpretation: Minghui Zhang, Shuangqingyue Zhang(VI) Manuscript writing: All authors (VII) Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Ethical approval
The ethics batch number of this research protocol is AF-SOP-07-1.2-01. I confirm that all experimental protocols were approved by the First Hospital of China Medical University licensing committee.
Informed consent
The requirement for informed consent was waived by the name of the IRB or a reference to the relevant legislation from the First Hospital of China Medical University licensing committee.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, M., Zhang, S., Wang, Y. et al. Prediction of contralateral central lymph node metastasis in unilateral papillary thyroid carcinoma based on radiomics. Sci Rep 15, 21948 (2025). https://doi.org/10.1038/s41598-025-04588-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04588-y