Abstract
Identifying the ureters in patients with advanced endometriosis and severe pelvic adhesive disease can be challenging. Adhesiolysis along the deformed pelvic sidewall may increase the risk of prolonged operative time and incidental ureteral injury. This study aimed to evaluate the safety and surgical outcomes of using Indocyanine Green (ICG) under near-infrared fluorescence for intraoperative ureteral localization and preservation during robot-assisted laparoscopic surgery (RALS) for advanced endometriosis, comparing procedures performed by two junior surgeons to those performed by one senior surgeon. This was a retrospective observational case series conducted by three minimally invasive gynecologic surgeons (X.G., T.K., B.T.) at a single tertiary care center between August 2021 and January 2025. A total of 92 patients underwent RALS using ICG fluorescence; 44 surgeries were performed by two junior surgeons, and 48 by a senior surgeon. The senior group had a higher percentage of patients with complete cul-de-sac obliteration (56.3% vs. 18.2%, p = 0.001). Our primary surgical outcome, total operative time, was 233 min in the junior group and 348 min in the senior group, initially showing a significant difference. However, after adjusting for factors such as history of prior abdominal surgery, cul-de-sac obliteration, and additional procedures (resection of ovarian remnant, bowel shaving, oophorectomy, enterolysis) using multivariable linear regression analysis, no significant difference was observed between the two groups. Other perioperative outcomes—including patient characteristics, estimated blood loss, length of hospital stay, and postoperative pain at weeks 1, 2, and 3—were comparable between the groups. Complication rates did not differ significantly. Notably, there were no cases of temporary or permanent ureteral injury in either group. These findings suggest that with ICG-assisted ureteral mapping, junior surgeons can achieve comparable surgical outcomes to senior surgeons. ICG facilitates intraoperative ureter identification, with the potential to enhance surgical safety by improving surgical precision and supporting the training of junior surgeons in managing complex endometriosis.
Similar content being viewed by others
Introduction
Endometriosis is a common but debilitating disease, with an incidence of 10–15% in women of reproductive age1. It is typically diagnosed and treated surgically through the excision of endometriotic lesions, primarily to alleviate symptoms. However, in advanced-stage endometriosis and conditions such as ovarian remnant syndrome, surgical management becomes more complex due to distorted anatomy from severe adhesions. Notably, ureteral involvement is typically considered a manifestation of deep endometriosis, where lesions infiltrate beyond the peritoneal surface, often involving structures such as the pelvic ureter2. Identifying the pelvic ureter is a crucial step in endometriosis excision surgery, as endometriosis can be located anywhere in the pelvis, increasing the risk of ureteral injuries during excision3.
Minimally invasive surgery is the preferred approach for the resection of endometriosis. The advancement of minimally invasive techniques was significantly transformed with the introduction of the Da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) in 2004. This system enhances surgical precision through 360-degree instrument motion, tremor control, and high-definition 3-D visualization. Recognizing its advantages, the American College of Obstetricians and Gynecologists (ACOG) endorses robot-assisted surgery for complex cases of endometriosis. Hiltunen et al. provided compelling evidence that this approach is effective for resecting deep infiltrative endometriosis, particularly in cases involving the ureters, and significantly improves patient quality of life by reducing endometriosis-related pain4.
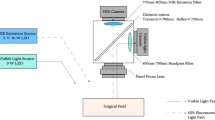
In relation to the surgeon, resection of deep infiltrative endometriosis increases surgeon anxiety. There is a significant correlation between surgical complexity and situational stress5. More than half of iatrogenic ureteral injuries occur during gynecologic procedures6, with one study reporting 35 cases (1.2%) among 2927 surgeries. Some risk factors include endometriosis, pelvic inflammatory disease, prior pelvic surgery, pelvic radiation, and congenital anomalies7. Over the past decade, indocyanine green (ICG) dye has been increasingly used in surgical applications such as endometriosis resection, ureteral perfusion assessment, lymph node dissection, and colorectal procedures8,9. In robotic gynecologic surgery, ICG under near-infrared fluorescence (NIRF) has been shown to enhance real-time ureteral visualization, reduce the risk of iatrogenic injury, shorten surgical time, and alleviate surgeon workload by decreasing both mental and physical demands10,11,12,13,14,15,16,17.
This study investigates the feasibility of using ICG fluorescence for intraoperative ureteral identification in complex endometriosis surgeries, with the objective of minimizing the risk of inadvertent ureteral injury. Specifically, we focus on advanced-stage endometriosis cases to evaluate the applicability and effectiveness of ICG fluorescence in enhancing surgical precision. Additionally, we aim to compare complication rates between surgeons with more than ten years of experience and those with less than five years to assess the impact of surgical expertise on clinical outcomes.
Methods
Study design
A retrospective cohort study was conducted for patients with advanced-stage endometriosis who underwent RALS with intra-ureteral ICG dye injection. Endometriosis was staged by surgeons using the revised American Society for Reproductive Medicine (rASRM) staging classification. Advanced-stage endometriosis was defined as stage III (rASRM score 16–40) or stage IV (rASRM score > 40). Data was securely extracted from the patient electronic medical record. The inclusion criteria comprised patients diagnosed with rASRM stage III or IV endometriosis who underwent RALS with intra-ureteral ICG dye injection. No specific exclusion criteria were initially defined. Patient characteristics included age, body mass index (BMI), prior abdominal surgery, and endometriosis stage with or without complete cul-de-sac obliteration. The total operative time (procedure start to procedure finish—not room-in to room-out time), the estimated blood loss, conversion rates, length of hospitalization, and post-operative pain scores were also collected. We assessed for conversion to laparotomy or other unplanned minimally invasive surgery. Complications were classified using the Clavien-Dindo (CD) system. All procedures were performed between 2021 and 2025 by three fellowship-trained minimally invasive gynecologic surgeons (X.G., T.K., B.T.) using the Da Vinci XI system at Baylor College of Medicine hospital affiliates, including Baylor St. Luke’s Medical Center and Texas Children’s Pavilion for Women. One senior surgeon (X.G.) had more than 10 years (post-MIGS fellowship training) of gynecologic surgical experience, while the junior surgeons (T.K., B.T.) each had less than 4 years (post-MIGS fellowship training) of experience. The study was conducted in accordance with the Declaration of Helsinki and Health Insurance Portability and Accountability Act (HIPAA) rules, and approved by the Institutional Review Board of Baylor College of Medicine (approval number H-51429 and March 8, 2022).
Temporary bilateral ureteral stent placement with ICG
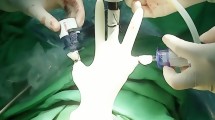
After the patient was prepped and draped in the usual sterile fashion, a 17F sheathed cystoscope, preloaded with a single 5F open-ended 27 cm stent, was prepared. A solution of 25 mg of ICG diluted in 10 mL of normal saline was attached to the Luer-lock of the stent. The cystoscope was primed, and the labia were gently parted to identify the urethral meatus. The cystoscope was then advanced into the bladder, and both ureteral orifices were visualized. The right ureteral orifice was identified, and the stent was advanced retrograde approximately 15 cm into the ureter. Five mL of ICG solution were then injected through the stent, which was left in place for 60 s before being withdrawn. The same process was repeated on the contralateral side: the ureteral orifice was identified, the stent was advanced 15 cm, and ICG was injected and allowed to dwell for 60 s. Afterward, the stent and cystoscope were removed, and all instruments were withdrawn from the urethra.
Data analysis
Data analysis was performed using SPSS software (version 25.0; SPSS Inc., Chicago, IL, USA). Descriptive statistics were generated. Continuous variables were tested for normality with the Kolmogorov–Smirnov test. Variables with a normal distribution were shown as mean ± standard deviation (SD), while variables with a non-normal distribution were shown as median [inter-quartile range (IQR)]. Groups were compared by Student’s T-test for normally distributed variables and Mann–Whitney U-test for non-normal data. Categorical variables were reported as proportions and analyzed using Pearson’s Chi-Square test or Fisher’s Exact test, as appropriate. Multivariable regression analyses were used to adjust for potential confounding variables. Statistical significance was set at p < 0.05.
Results
A total of 92 patients underwent RALS with ICG stent placement from August 2021 to January 2025. Among these, 44 patients had their surgeries performed by two junior surgeons (less than four years post-MIGS fellowship training), while 48 patients were treated by a senior surgeon (greater than 10 years post-MIGS fellowship training).
Significant differences were observed in patients’ characteristics between the two groups (Table 1). The junior group of patients were significantly younger than the senior group (mean age 35.57 vs. 39.29 years, p = 0.005). Additionally, more than 90% of patients in the senior group had a history of at least one abdominal surgery, which is significantly higher than the junior group (91.7% vs. 65.9%, p = 0.004). The revised American Society for Reproductive Medicine (rASRM) endometriosis classification was used. The stage of endometriosis was not different between the two groups (p = 0.11). However, patients in the senior group had a higher rate with complete cul-de-sac obliteration (56.3% vs. 18.2%, p = 0.001).
All patients had procedures of endometriosis excision and cystoscopy; Fig. 1 shows the additional surgical procedures performed. Procedures such as enterolysis (p = = 0.001), bowel shaving (p = 0.01), oophorectomy (p = 0.04) and resection of ovarian remnant (p = 0.001) were more frequently performed in the senior group.
Data regarding surgical outcomes appear in Table 2. The total operative time was significantly shorter in the junior group (median: 233 min vs. 348 min, p = 0.001). Since age, history of abdominal surgery, cul-de-sac obliteration, and additional procedures (enterolysis, bowel shaving, oophorectomy, and resection of ovarian remnant) were not consistent between the two study groups, adjustments were made for these variables. After adjustment, no significant difference in total operative time was observed. The junior group had a shorter hospital stay (median: 1 day vs. 2 days, p = 0.001) and a higher rate of same-day discharge (45.5% vs. 18.8%, p = 0.007). However, there was no difference between the two study groups when patient characteristics were taken into account. The estimated blood loss and post-operative pain at week 1, week 2, and week 3 were always comparable before and after adjustment for patient characteristics. No patients required conversion to laparotomy.
The total complication rate was 6.8% (3 cases) and 12.5% (6 cases) in the junior and senior groups, respectively, showing no significant difference (p = 0.49), (Table 3). It is important to note that no cases of temporary or permanent ureteral injury were observed in either the junior or senior surgeon groups. According to the Clavien–Dindo system, all the patients were CD grade I or grade II. In the junior group, one patient had hematuria, one patient had a fever, and one patient had both pneumothorax and a urinary tract infection (UTI). In the senior group, two patients had urinary retention, two patients had a blood transfusion, one patient had sepsis, and one patient had both a blood transfusion and sepsis.
Discussion
In recent years, there has been a growing emphasis on the surgical management of endometriosis by fellowship-trained surgeons, including newly graduated surgeons, in addressing complex cases of deep infiltrating endometriosis. This includes conditions such as bilateral pelvic sidewall involvement, complete obliteration of the posterior cul-de-sac, and ovarian remnant syndrome—which can lead to ureteral torsion or difficult visualization18,19. The identification of ureters is crucial for safely managing advanced-stage endometriosis and ovarian remnant syndrome. However, this task remains challenging, particularly in cases with severe adhesive disease due to the possible alterations in anatomic location of the ureters6,20. Additionally, the excision of endometriosis around the ureters is complicated by factors such as diminished tactile feedback during robot-assisted surgery21, tissue inflammation or fibrosis, limited visualization of ureteral anatomy, and deep adhesions to the urinary tract22. In a related study, but in performing a parametrectomy, Ianieri, et.al. demonstrated a standardized approach for comparing complications and functional outcomes. Parametrectomy, even when performed by expert surgeons, carries a notable risk of bladder voiding deficits23.
Exacoustos et.al. strongly advocates for the routine use of preoperative sonographic mapping in cases of suspected deep infiltrating endometriosis (DIE). Proper patient counseling based in conjunction with imaging is not only essential for informed consent but also facilitates optimal surgical planning. This also allows for preparation of a multi-disciplinary approach for complex cases, involving general surgeons and/or urologists24. Regardless of surgeon team, ureteral mapping is a paramount part in safely performing complex surgery. A recent systematic review evaluated laparoscopic and robotic-assisted techniques for ureteral reimplantation in the treatment of ureteral endometriosis, concluding that both approaches are safe and effective25. The integration of NIRF with ICG dye has significantly expanded its application across various surgical procedures aiding in precise ureteral mapping13,15,16,17. Numerous studies have demonstrated the effectiveness of ICG in facilitating endometriosis resection, sentinel lymph node mapping, colorectal surgeries, and evaluating vascular patterns in rectosigmoid endometriosis26,27,28,29. The use of fluorescence-guided imaging in robot-assisted ureterolysis has shown potential to enhance procedural efficiency and safety11.
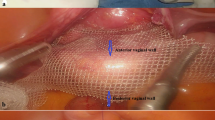
Moreover, resecting deep infiltrative endometriosis is associated with heightened surgeon anxiety. Surgical complexity is closely linked to situational stress and increased mental and physical demands, all of which contribute significantly to overall workload5. This study did not, however, address differences in stress felt between junior and senior surgeons. Our findings indicate that both groups of surgeons achieved similar surgical outcomes with comparable complication rates. Although the senior surgeon performed more Stage IV cases with ICG compared to the junior surgeons—likely due to patient selection—both groups exhibited similar complication rates. Notably, the ureterolysis rates in both groups were high (junior group: 88.6% vs. senior group: 79.2%), and no instances of temporary or permanent ureteral injury were observed in either the junior or senior surgeon groups when ICG-assisted ureteral mapping was utilized. This suggests that with ICG fluorescence for ureteral identification, even junior surgeons were confident enough to perform complex endometriosis excision without causing ureteral injury. These findings indicate that the implementation of ICG ureteral visualization may help reduce junior surgeons’ anxiety and improve safety when performing complex cases.
Regarding total operative time, the median operative times were 233 min in the junior group and 348 min in the senior group. The longer operative time in the senior group is likely due to the higher rate of patients with complete cul-de-sac obliteration, a history of abdominal surgery, and a greater number of procedures performed, such as enterolysis, ovarian remnant resection, oophorectomy, and bowel shaving. This was supported by the absence of a significant difference in total operative time after adjusting for these variables, suggesting that junior surgeons can achieve similar surgical outcomes as senior surgeons under ideal conditions. However, we acknowledge that the inclusion of concurrent procedures (e.g., enterolysis, bowel shaving) may act as confounding variables, as we were unable to obtain the exact console time for each individual procedure. Therefore, using the percentage of concurrent procedures can only partially explain differences in total operative time and cannot provide an exact measure.
Our study provides valuable insights into the use of ICG fluorescence for ureteral identification in robot-assisted surgery for advanced endometriosis. However, several limitations should be acknowledged. First, the retrospective nature of the study introduces several inherent limitations. These include: (1) the lack of randomization, which may result in selection bias, as patient assignment to surgeon groups was not controlled and could have been influenced by factors such as case complexity or surgeon availability; and (2) reliance on existing medical records, which may lead to incomplete or inconsistent data—particularly for variables that were not routinely or uniformly documented, such as analgesic usage. Second, our follow-up period was limited to six weeks post-surgery, during which no adverse effects related to ICG were observed. However, the potential long-term safety and efficacy of ICG use in endometriosis surgery remain unexplored, warranting further prospective, long-term studies to evaluate its sustained impact on surgical outcomes and patient safety.
Despite these limitations, our study has several notable strengths. This is the first study to compare the utilization of fluorescence-guided techniques by surgeons with different experience in RALS for advanced-stage endometriosis cases. This real-time surgical adjunct enhances precision and safety, particularly in cases of deep infiltrative endometriosis and severe pelvic adhesions, even for junior surgeons. A previous study similarly reported the use of endovenous ICG as an effective tool for real-time assessment of bowel perfusion during rectosigmoid resection in patients with deep endometriosis, thereby improving surgical precision and anastomotic safety30. Furthermore, our findings contribute to the growing body of evidence supporting ICG-assisted surgery, offering a foundation for future prospective, multicenter studies to validate its widespread clinical adoption. By sharing our experience, we aim to enhance surgical strategies and optimize patient outcomes in the field of minimally invasive gynecologic surgery.
The integration of ICG fluorescence into surgical workflows represents a significant advancement in robot-assisted ureterolysis, enhancing precision and minimizing risks in cases of deep-infiltrating endometriosis and ovarian remnant syndrome. Our study found no significant differences in complication rates or operative time between junior and senior surgeons, highlighting the potential of ICG-assisted ureteral mapping to shorten the learning curve for graduating fellows and improve the safety of complex endometriosis surgeries, benefiting both surgeons and patients.
For surgeons, the immediate and enhanced visualization of ICG-dyed ureters improves spatial awareness and supports surgical decision-making by providing real-time anatomical reference points. This capability allows for precise identification of ureter structures and better assessment of surgical progress, reducing the risk of complications and ensuring more accurate removal of endometriosis implants. For patients, these advancements may lead to better surgical outcomes, shorter recovery times, and fewer complications. The combination of improved intraoperative precision and real-time feedback could elevate the overall standard of care, enhancing both safety and surgical efficacy in endometriosis excision procedures.
Additionally, this study highlights opportunities to enhance training protocols in endometriosis surgery. The real-time integration of ICG fluorescence can provide trainees with a more comprehensive understanding of ureter anatomy and surgical techniques, potentially shortening the learning curve and increasing surgical proficiency. As highlighted by a recently published study, the integration of ICG fluorescence into surgical workflows has shown significant potential in enhancing surgical precision and improving postoperative outcomes in pediatric surgery, and it is recommended to establish standardized protocols and specialized training to maximize the benefits of ICG31. However, a limitation of our study is that all junior surgeon cases included ICG guidance, and we do not have a direct comparison of their performance without ICG within this cohort. We agree that further prospective, controlled studies are needed before ICG can be universally recommended as a standard training tool.
Further large-scale, multicenter, randomized studies are needed to validate the long-term efficacy of ICG ureteral identification and support its widespread adoption in gynecologic surgery. As surgical methodologies evolve, continued research will be essential in refining this technique, optimizing patient outcomes, and establishing standardized protocols to ensure the highest level of safety and efficacy in minimally invasive gynecologic procedures.
Data availability
The data that support the findings of this study are available on reasonable request to the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Abbreviations
- ICG:
-
Indocyanine green
- RALS:
-
Robot-assisted laparoscopic surgery
- NIRF:
-
Near-infrared fluorescence
- rASRM:
-
Revised American Society for Reproductive Medicine
- BMI :
-
Body mass index
- CD:
-
Clavien–Dindo
- IQR:
-
Inter-quartile range
- UTI:
-
Urinary tract infection
- DIE:
-
Deep infiltrating endometriosis
References
Zhu, Y. et al. Identification of potential diagnostic biomarkers and drug targets for endometriosis from a genetic perspective: a Mendelian randomization study. Gynecol. Obstet. Investig. https://doi.org/10.1159/000543707 (2025).
Di Michele, S. et al. Superficial peritoneal endometriosis vaporization using a CO2 laser: A long-term single-center experience. J Clin Med. 13(6), 1722. https://doi.org/10.3390/jcm13061722 (2024).
Becker, C. M. et al. ESHRE guideline: Endometriosis. Hum Reprod. Open 2022(2), hoac009. https://doi.org/10.1093/hropen/hoac009 (2022).
Hiltunen, J. et al. Robotic-assisted laparoscopy is a feasible method for resection of deep infiltrating endometriosis, especially in the rectosigmoid area. J. Int. Med. Res. 49(8), 3000605211032788. https://doi.org/10.1177/03000605211032788 (2021).
Spagnolo, E. et al. Surgeons’ workload assessment during indocya-nine-assisted deep endometriosis surgery using the surgery task load index: The impact of the learning curve. Front. Surg. 9, 982922. https://doi.org/10.3389/fsurg.2022.982922 (2022).
Chan, J. K., Morrow, J. & Manetta, A. Prevention of ureteral injuries in gynecologic surgery. Am. J. Obstet. Gynecol. 188(5), 1273–1277. https://doi.org/10.1067/mob.2003.269 (2003).
Park, J. H., Park, J. W., Song, K. & Jo, M. K. Ureteral injury in gynecologic surgery: A 5-year review in a community hospital. Korean J. Urol. 53(2), 120–125. https://doi.org/10.4111/kju.2012.53.2.120 (2012).
Safiejko, K. et al. Safety and efficacy of indocyanine green in colorectal cancer surgery: A systematic review and meta-analysis of 11,047 patients. Cancers (Basel). 14(4), 1036. https://doi.org/10.3390/cancers14041036 (2022).
Matsuo, K. et al. Trends in the use of indocyanine green for sentinel lymph node mapping in vulvar cancer. Am. J. Obstet. Gynecol. 229(4), 466–468. https://doi.org/10.1016/j.ajog.2023.07.019 (2023).
Lovell, D. Y., Sendukas, E., Yang, Q. & Guan, X. Surgical enhancement with the placement of temporary bilateral ureteral stents With indocyanine green injection for all stages of endometriosis in vNOTES: Retrospective cross-sectional study. J. Minim. Invasive Gynecol. 32(2), 166–170. https://doi.org/10.1016/j.jmig.2024.09.365 (2025).
Rosati, M. et al. Firefly® system and organ transillumination in robotic gynecologic surgery. JSLS 25(3), e2021.00044. https://doi.org/10.4293/JSLS.2021.00044 (2021).
Raffone, A. et al. The use of near infra-red radiation imaging after injection of indocyanine green (NIR-ICG) during laparoscopic treatment of benign gynecologic conditions: Towards minimalized surgery. A systematic review of literature. Medicina (Kaunas) 58(6), 792. https://doi.org/10.3390/medicina58060792 (2022).
Mandovra, P., Kalikar, V. & Patankar, R. V. Real-time visualization of ureters using indocyanine green during laparo-scopic surgeries: Can we make surgery safer?. Surg Innov. 26(4), 464–468. https://doi.org/10.1177/1553350619827152 (2019).
Raimondo, D. et al. Use of indocyanine green for intraoperative perfusion assessment in women with ureteral endometriosis: A preliminary study. J. Minim. Invasive Gynecol. 28(1), 42–49. https://doi.org/10.1016/j.jmig.2020.04.004 (2021).
Guan, X., Guan, Z., Sunkara, S. & Thigpen, B. Indocyanine green-assisted retrograde ureterolysis in robotic transvaginal NOTES for the management of stage IV endometriosis with obliterated Cul-de-sac. J. Minim. Invasive Gynecol. 30(4), 266–267. https://doi.org/10.1016/j.jmig.2023.02.005 (2023).
Zeng, S. et al. Application of indocyanine green in combination with Da Vinci Xi robot in surgeries on the upper urinary tract: A case series study. J. Clin. Med. 12(5), 1980. https://doi.org/10.3390/jcm12051980 (2023).
White, L. A. et al. Intraureteral indocyanine green augments ureteral identification and avoidance during complex robotic-assisted colorectal surgery. Colorectal Dis. 23(3), 718–723. https://doi.org/10.1111/codi.15407 (2021).
Zapardiel, I., Zanagnolo, V., Kho, R. M., Magrina, J. F. & Magtibay, P. M. Ovarian remnant syndrome: comparison of laparotomy, laparoscopy and robotic surgery. Acta Obstet. Gynecol. Scand. 91(8), 965–969. https://doi.org/10.1111/j.1600-0412.2012.01461.x (2012).
Manzo, C. A. & Celentano, V. Stapled disc excision for the repair of intraoperative rectal injury during robotic surgery for deep infiltrating endometriosis. Langenbecks Arch Surg. 408(1), 153. https://doi.org/10.1007/s00423-023-02890-9 (2023).
Thigpen, B., Koythong, T. & Guan, X. Robotic-assisted laparoscopic ureterolysis for deep infiltrating endometriosis using indocyanine green under near-infrared fluorescence. J. Minim. Invasive Gynecol. 29(5), 586–587. https://doi.org/10.1016/j.jmig.2022.01.018 (2022).
Chandrakar, I., Pajai, S. & Toshniwal, S. Robotic surgery: The future of gynaecology. Cureus. 14(10), e30569. https://doi.org/10.7759/cureus.30569 (2022).
Ianieri, M. M. et al. Are ureterolysis for deep endometriosis really all the same? An anatomical classification proposal for ureterolysis: A single-center experience. Int. J. Gynaecol. Obstet. 162(3), 1010–1019. https://doi.org/10.1002/ijgo.14790 (2023).
Ianieri, M. M. et al. Anatomical-based classification of dorsolateral parametrectomy for deep en-dometriosis. Correlation with surgical complications and functional outcomes: A single-center prospective study. Int. J. Gynaecol. Obstet. 167(3), 1043–1054. https://doi.org/10.1002/ijgo.15781 (2024).
Exacoustos, C., Lazzeri, L. & Zupi, E. Expert sonographers and surgeons are needed to manage deep infiltrating endometri-osis. Ultrasound Obstet. Gynecol. 49(3), 417. https://doi.org/10.1002/uog.17415 (2017).
Di Michele, S., Bramante, S. & Rosati, M. A systematic review of ureteral reimplantation techniques in endometriosis: Laparoscopic versus robotic-assisted approach. J. Clin. Med. 13(19), 5677. https://doi.org/10.3390/jcm13195677 (2024).
Li, Q., Zhang, L., Fang, F., Xu, P. & Zhang, C. Research progress of indocyanine green fluorescence technology in gynecologi-cal applications. Int. J. Gynaecol. Obstet. 165(3), 936–942. https://doi.org/10.1002/ijgo.15249 (2024).
Soriano, C. R. et al. Feasibility of injected indocyanine green for ureteral identification during robotic left-sided colorectal resections. Am. J. Surg. 223(1), 14–20. https://doi.org/10.1016/j.amjsurg.2021.07.012 (2022).
Frumovitz, M. et al. Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): A randomised, phase 3, multicentre, non-inferiority trial. Lancet Oncol. 19(10), 1394–1403. https://doi.org/10.1016/S1470-2045(18)30448-0 (2018).
Raimondo, D. et al. Rectosigmoid endometriosis vascular patterns at intraoperative indocyanine green angiography and their correlation with clinicopathological data. Surg. Innov. 27(5), 474–480. https://doi.org/10.1177/1553350620930147 (2020).
Seracchioli, R., Raimondo, D., Arena, A., Zanello, M. & Mabrouk, M. Clinical use of endovenous indocyanine green during rectosigmoid segmental resection for endometriosis. Fertil Steril. 109(6), 1135. https://doi.org/10.1016/j.fertnstert.2018.02.122 (2018).
Sosnowska-Sienkiewicz, P. et al. Practical guidelines for the use of indocyanine green in different branches of pediatric surgery: A Polish nationwide multi-center retrospective cohort study. Health Sci. Rep. 8(3), e70586. https://doi.org/10.1002/hsr2.70586 (2025).
Acknowledgements
The authors would like to thank the Division of Minimally Invasive Gynecologic Surgery, Baylor College of Medicine, Houston, Texas, U.S.A, for surgery assistance.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, Q.W. and X.G.; methodology, L.R., Q.Y. and X.G.; validation, Q.W. and X.G.; formal analysis, L.R. and Q.Y.; investigation, L.R., Q.Y. and L.C.; resources (surgery), B.T., T.K. and X.G.; data curation, L.R. and Q.Y.; writing—original draft preparation, L.R., Q.Y. and L.C.; writing—review and editing, D.L., B.T., T.K. Q.W. and X.G.; visualiza-tion, Q.Y.; supervision, Q.W. and X.G.; project administration, Q.W. and X.G.; All authors have reviewed and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The need for consent to participate was waived due to retrospective nature of the study by an Institutional Review Board (IRB). This study was IRB-approved from Baylor College of Medicine on March 8, 2022, under the approval number H-51429.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Radilla, L.A.A., Yang, Q., Lovell, D.Y. et al. Fluorescence-guided ureteral identification in robotic surgery for advanced endometriosis: a comparison of junior versus senior surgeons. Sci Rep 15, 19933 (2025). https://doi.org/10.1038/s41598-025-05082-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05082-1
Keyword
This article is cited by
-
Stage-Specific outcomes of robotic transvaginal NOTES hysterectomy in endometriosis: A comparative study
Journal of Robotic Surgery (2025)