Abstract
Somatotopy serves as a fundamental principle underlying sensory information processing, traditionally emphasized in the study of the cerebral cortex. However, little effort has been directed towards unraveling the spatial organization characterizing the earlier stages of somatosensory pathways. In this study, we developed a novel methodology to visualize individual neurons within the trigeminal ganglion—a crucial cluster of cell bodies of sensory neurons innervating the face. Our investigations revealed a reliable sensory response to stimulation of the lower incisor or lip within this ganglion. The responsive neurons were confined to a specific portion of the trigeminal ganglion, consistent with innervation of the lower oral cavity by the mandibular nerve (V3). Contrary to our expectations, we did not observe a discernible map differentiating the lower tooth and the lip. Instead, the spatial representation of the tooth and lip within this portion of trigeminal ganglion exhibited intermingling with no clear border between tooth-responding and lip-responding neurons. These findings contrast with earlier studies that identified tooth and lip responding regions in the somatosensory cortex. Our study sheds light on the complex spatial organization of sensory processing in the trigeminal system, highlighting the need for further research to elucidate the underlying mechanisms and implications for sensory perception and clinical interventions.
Similar content being viewed by others
Introduction
The sensations of teeth play a crucial role in controlling oral behaviors such as biting and chewing. For example, accurate perception of the load on the teeth is essential for the incisors to function as cutting tools, effectively splitting food morsels into smaller pieces with controlled force. The sensory signals applied to the teeth are primarily detected by mechanoreceptors in the periodontal ligaments between the root of the tooth and the alveolar bone1, although they may also involve those in the tooth pulp2,3,4. Indeed, humans lacking periodontal mechanoreceptors or under local anesthesia exhibit deficits in precise manipulative actions involving the application of low forces by the jaw5,6.
The trigeminal ganglion (TG) contains the first group of neurons that receive sensory information from the face, including the oral cavity, and convey it to the brainstem7,8,9,10. The TG constitutes the point of convergence for the three branches of the trigeminal nerve. The ophthalmic branch (V1) innervates the upper third of the facial region, from the forehead to the nose. The maxillary branch (V2) processes sensory information from the middle third of the facial region, including the upper jaw. The mandibular branch (V3) is responsible for sensory processing of the lower third of the facial region and further branches into smaller nerves, including the inferior alveolar nerve, which innervates the lower jaw, periodontal ligaments, and lower lip. Sensory information gathered from each part of the face/oral area, including signals from periodontal mechanoreceptors, is conveyed via pseudounipolar neurons in the TG to the trigeminal nucleus in the brainstem via the trigeminal nerve, then to the thalamus, and finally to the somatosensory cortex.
Topographical organization is a fundamental guiding principle for sensory information processing11. Within the somatosensory system, the somatosensory cortex contains topographic maps of the body surface, often depicted as the “homunculus”8,12,13,14,15. Studies have demonstrated that distinct portions of the somatosensory cortex encode different parts of the oral cavity, such as the teeth, lips, and tongue16,17,18,19,20. However, little is known about how such parts are spatially represented earlier in the somatosensory pathway and only a limited number of studies tackled this question with extracellular unit recording21,22 or anatomical tracing23, primarily on the vibrissa system. In the present study, we employed in vivo calcium imaging12,13,24,25,26,27,28 to investigate the spatial representation of the tooth and lip in the TG. We report that, in contrast to the cerebral cortex, the representation of the tooth and lip in the TG is spatially intermingled.
Methods
Animals
All experimental procedures were approved by the Medical University of South Carolina conducted in conformity with the Institutional Animal Care and Use Committee at each institute, and all methods were performed in accordance with the relevant guidelines and regulations. The experiments are in accordance with ARRIVE guidelines (https://arriveguidelines.org). Thy1-GCaMP6f. mice (GP5.17, Jax #025,393, both male and female, crossed with wild-type C57BL/6) were used in this study. Mice of both sexes, aged > 8 weeks were included. The mice were maintained in group housing (up to five mice per cage) and experiments were performed during the dark period of a 12-h light/12-h dark cycle.
Surgery
Mice were head-fixed under anesthesia (1–5% isoflurane). A headpost was attached to the skull with glue and dental cement for stabilization during imaging. A craniotomy was performed above the left cerebral cortex, and cortical and subcortical tissues were removed via suction until the TG became visible. Then, a 3 × 3 × 8 mm glass cuboid (UQG; Specification, Cover Glasses; Dimension, 3 mm × 3 mm × 8 mm thick) was inserted above the TG. Small pieces of SURGIFOAM™ were placed around the cuboid to prevent bleeding into the imaging surface and to stabilize the glass cuboid. Glue and dental cement were applied around the glass cuboid to further secure it in place. Once the glass cuboid was secured, the animal was injected with an anesthetic (0.2 mL ketamine/xylazine cocktail) and placed under a fluorescence microscope (MVX, Olympus) for one-photon imaging. After imaging, the animals were deeply anesthetized with isoflurane and euthanized via transcardiac perfusion with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). The brain was then removed.
Virus injection into the lower lip
The mice were deeply anesthetized under Isoflurane (1–5% isoflurane). AAV1-CAG-tdTomato (Addgene #59,462-AAV1) was injected into the lower lip using a syringe. After 2–3 weeks, the mice were euthanized via transcardiac perfusion with 4% paraformaldehyde (PFA) in phosphate-buffered saline solution (PBS). The brain structures above the TG was then removed to allow visualization of the TG.
Histology and Immunostaining
The perfused brain was placed in 30% sucrose solution after overnight fixation in 4% PFA. NeuN and GCaMP was immunostained using standard procedures26,29 (Fig. 1e). Slices (thickness, 20 µm) were cut using a cryostat and blocked in a carrier solution (5% bovine serum albumin, Sigma-Aldrich; 0.3% Triton X-100, Sigma-Aldrich; in 0.1 M PBS) for 1 h at room temperature on a shaker. For GCaMP staining, slices were incubated with anti-GFP primary rabbit antibody (1:1000 in carrier solution; Cat. #A11122, ThermoFisher) for 18 h at 4 °C on a shaker. For NeuN staining, slices were incubated with anti-NeuN chicken antibody (1:500 in carrier solution; ABN91, Millipore). After three rinses with 0.1 M PBS for 30 min, sections were incubated with either Alexa-Fluor-488-conjugated anti-rabbit secondary antibody (A21206, ThermoFisher, Lot# 2,668,665; 1:500 in carrier solution) or Alexa-Fluor-568-conjugated anti-chicken secondary antibody (A11041, ThermoFisher, Lot#2,674,372) for 1 h at room temperature on a shaker. After a few additional rinses for 30 min in 0.1 M PBS, slices were mounted on slide glasses for imaging (Fluorescent mounting medium, S302380, Dako).
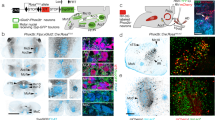
In vivo calcium imaging from the TG. (a) The distal processes of pseudouniploar neurons of the TG innervate the face, including the oral cavity. Thus, stimulation of the tooth will activate the neurons in the TG. (b). Top, Location of trigeminal ganglion (TG) along with cranial nerve II (CN II), V1, V2, V3 branches and cranial nerve V (CN V). Bottom, the locations of the neurons that receive inputs from the lips were labeled with tdTomato. (c). Surgical approach to the TG. Left, The dorsal view of the surgical/imaging site. Right, Schematic view of our imaging approach. A 3 mm × 3 mm × 8 mm glass cuboid was placed through the brain above the TG. (d) The TG was visible using both our surgical microscope (left) and fluorescence microscope (right). (e) Immunostaining for NeuN (red, top) and GCaMP (green, middle). The overlaid image is shown at the bottom. (f) An example of fluorescence images of the TG. Top, average image during the baseline period before sensory stimulation. Middle, average image during sensory stimulation. Bottom , a ΔF/F0 image during sensory stimulation of the oral cavity. Increased signals were observed in some cells. (g) High zoom images of the TG shown in panel f. (h) Three-dimensional representation of ΔF/F0 shown in panel f. (i) Distribution of the diameter of the area exhibiting increased fluorescence. The estimation of the diameter was based on Gaussian fitting.
Stimulation
Sensory stimulation was applied to the facial (labial) surface of the left mandibular incisor and the outer surface of the lower lip in the diastema area (insets in Fig. 2a). These two sites were chosen because they can be accessed without providing sensory stimulation to other parts of the oral cavity. We administered 15 stimulations in 3 s (200 ms intervals), with a baseline period of 3 s before and after the trial. The strength of the simulation is 20–40 mN. When the stimulation was increased to 200 mN, we found that many neurons responded to both lip and tooth stimuli, undermining the goal of studying the selective map in the trigeminal ganglion. This process was repeated for 10 trials per stimulation site.
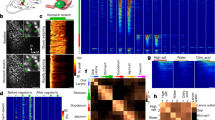
Spatial representation of the tooth and the lip stimulation in the TG. (a) Spatial maps of ΔF/F0 during tooth stimulation (left) and lip stimulation (right) from the same imaging site. The scale bar, 200 µm. Top inset images are the mouse face with somatosensory stimulation sites as red dots. The scale bar, 2 mm. (b) Examples of fluorescent changes in two neurons indicated with arrows in panel a. The left two columns show individual traces overlaid for 10 trials, and the right column shows the average traces. (c) Top, Averaged sensory response of tooth selective neurons and lip selective neurons. Black: tooth selective neurons (n = 84), gray: lip selective neurons (n = 72). Note that non-selective neurons are excluded. Bottom, the cumulative distributions of half rise times and half decay times. (d) ∆F/F0 traces for 69 neurons in panel a showing the responses to tooth stimulation (left) and lip stimulation (right). The cells were sorted based on the difference in activity during tooth and lip stimulation. (e) Distribution of the selectivity index from all imaging sessions (n = 292 from 9 mice). (f) Map of the selectivity index. Each circle corresponds to a cell, and the gray tone of the cells indicates the selectivity index. Left, the map from the experiment shown in panel a. Right, the map from all 9 mice (n = 292). There was no segregation between tooth-selective cells and lip-selective cells. (g) The relationship between the center of gravity for the tooth response and that for the lip response for 9 mice. There was no clear.
Imaging
The imaging experiments were conducted under a fluorescence microscope (MVX-10, Olympus). The excitation light was provided by a blue LED (SOLIS-470, Thorlabs), and the emission light was detected by a monochromatic camera (Thorlabs). The microscope was equipped with a 1 × objective lens (MV PLAPO 1x/0.25 NA). The filter cube contained an excitation bandpass filter of 470/40 nm, a dichroic filter of 495 nm, and an emission bandpass filter of 525/50 nm. The sampling rate of the camera was 20–30 frames per second for an image size of 512 pixel × 512 pixel.
Data processing
The imaging data were analyzed by a custom-written MATLAB program, as previously described24,25,26,27. Imaging data were processed for motion correction and registration. Cells were detected for region-of-interest (ROI) drawing using a custom-made MATLAB program26,30. A fluorescence trace was generated for each ROI and then normalized by the baseline fluorescence (1 s period before the sensory stimulus onset) to produce a ∆F/F0 trace.
For each neuron, the sensory response of each trial was defined as the mean ∆F/F0 for the last 1 s of the sensory stimulation. The responses to the tooth and the lip were analyzed separately. If the sensory response over the 10 trials was significantly larger than 0 (t-test, p < 0.05), the response amplitude of the neuron was defined as the mean sensory response; otherwise, the response amplitude was defined as 0. For each neuron, the selectivity index was calculated as (response amplitude to tooth—response amplitude to lip) / (response amplitude to tooth + response amplitude to lip). We determined the center of gravity of the sensory response by calculating the mean of the cell location weighted by the response amplitude. If the response amplitude of a given neuron to the tooth or the lip was more than 5 times that to the other, the neuron was considered selective (i.e., selectivity index < − 0.66 or selectivity index > 0.66). The half-rise time was defined as the first frame at which the fluorescence signal exceeded 50% of the peak response. The half-decay time was defined as the first frame at which the fluorescence signal fell below 50% of the activity at the time of stimulus offset.
The size of the neuron was determined as follows. First, the mean ΔF/F0 map was calculated for each individual pixel. Then, the ΔF/F0 of a 61 × 61 pixel area centered on an individual cell was fitted to the following two-dimensional Gaussian function with free parameters of A, x0, y0, σx, σy. and rotation angle φ.
\(f \left(x, y\right)=A \text{exp}\left(-\left(\frac{{\left(x-{x}_{0}\right)}^{2}}{2{\sigma }_{x}^{2}} + \frac{{\left(y-{y}_{0}\right)}^{2}}{2{\sigma }_{y}^{2}}\right)\right)\)
This fitting was accomplished using the MATLAB function lsqcurvefit. We then we calculated the sum of σx and σy.
Statistical analysis
Data are described as the median ± s.e.m. unless otherwise noted. The significance of the response amplitude was determined by t-tests using MATLAB. All other statistics were based on non-parametric tests (Mann–Whitney test, Wilcoxon signed-rank test). Significance levels of data are denoted as * p < 0.05, ** p < 0.01, and *** p < 0.001. p > 0.05 was considered insignificant and is denoted as n.s.
Results
To investigate the spatial organization of neurons encoding mechanical stimulation of the tooth, we employed in vivo calcium imaging, a method widely utilized in studying the topographical organization of sensory cortices12,13,31,32,33 (Fig. 1a). Several studies have achieved optical access to the TG by removing all the dorsal structures and exposing it, often followed by a bath application of HEPES buffer, similar to in vitro preparations34,35,36,37. In the current study, we modified a procedure that has been employed for two-photon imaging of the dorsal cerebral cortex38. Specifically, we inserted an elongated glass cuboid (3 mm × 3 mm × 8 mm) directly above the TG after removing the overlying tissues (Fig. 1c). This approach resulted in a broad and clear view of the dorsal surface of the TG (Fig. 1d). We confirm that this view contained the TG regions that receive afferent inputs from the lower lip (Fig. 1b bottom). We injected AAV-tdTomato into the lower lip to find tdTomato expressing cell bodies in the TG. In contrast, conventional virus injection into the incisor teeth did not visualize any cells in the TG, requiring optimization of virus infection approaches.
Similar to numerous studies employing in vivo imaging of the cerebral cortex, our approach preserved tissue health within the cranial cavity, eliminating the need for buffer application during imaging sessions. However, a drawback of this approach is the long distance (> 8 mm) from the cover glass surface to the imaging site. As this length exceeds the working distance of our objective lens (CFI75, Nikon, working distance of 3 mm), conducting two-photon imaging was not feasible. Therefore, in the current experiment, we employed one photon fluorescence imaging. We note that the use of an objective lens with a longer working distance would enable the visualization of neurons in the TG with a two-photon microscope.
We conducted fluorescence imaging of GCaMP6f. in the TG of Thy-1 GCaMP mice (GP5.17)39. Immunostaining against NeuN, a neuron specific marker40, confirmed that the expression of GCaMP was restricted to neurons in these mice (Fig. 1e). We successfully visualized several neurons through the glass cuboid, and some of these neurons exhibited fluorescence changes following manual stimulation of the oral cavity (Fig. 1f,g, Supplementary Movie 1). The size of the activated area was 22.7 ± 0.9 µm (N = 70 neurons from 4 mice) (Fig. 1h,i). Using this in vivo one-photon calcium imaging strategy, we examined the representation of the tooth in the TG and compared it with the representation of the lip. We applied mechanical stimulation to either the facial (labial) surface of the left mandibular incisor (the front tooth located on the lower jaw, adjacent to the midline of the face) or the outside of the lower lip in the diastema area (between the incisors and molars) with 200 ms interval for 3 s (top images in Fig. 2a). These two exposed structures provided easy access, minimizing the risk of inadvertent stimulation of surrounding regions of the oral cavity. In contrast, the molars at the back of the mouth were difficult to approach for precise sensory stimulation. We chose to stimulate 200 ms interval for 3 s because we were unable to detect reliable fluorescence changes in many cells when we applied only single stimulations, presumably due to the lower signal-to-noise ratio of GCaMP6f. compared to more recent versions of GCaMP, such as jGCaMP841.
During sensory stimulation, the neurons exhibited increased fluorescence signals that decayed toward the baseline as the stimulation ended (Fig. 2a,b). Neurons responding to incisor stimulation and those activated by lower lip stimulation were predominantly localized at the entrance of the mandibular nerve35. This is consistent with the innervation of the lower jaw, including these two structures, by the mandibular nerve. While the distribution of the active neurons was relatively sparse, the activity patterns of individual neurons were reproducible across multiple trials (Fig. 2b). For instance, in cell 1 of Fig. 2b, the coefficient of variation (standard deviation/mean) for tooth responses was 0.044, and in cell 2 for lip responses, it was 0.164. Overall, the coefficient of variation of neurons exhibiting more than ΔF/F0 > 10% was 0.171 ± 0.036 (n = 16) for responses to incisor stimulation and 0.201 ± 0.042 (n = 39) for responses to lip stimulation. Notably, these coefficients of variation were substantially smaller than those reported in the cerebral cortex (e.g., 1.59 in ref42 and 0.76 in ref43).
We then compared the spatial patterns of sensory responses in the TG following stimulation of the mandibular incisor and the lower lip (Supplementary Movie 2). Toward this goal, we first examined the response selectivity of individual neurons to incisor and lip stimulation. The majority of responsive neurons exhibited selectivity to either incisor or lip stimulation (Fig. 2d), with 53.4% of neurons responding at least five times more to one stimulus than the other (n = 156/292) (Fig. 2e). We classified these neurons as tooth-selective neurons and lip-selective neurons – note that a significant number of neurons responded to both the lower incisor and the lower lip (Fig. 2e). The temporal dynamics of tooth selective neurons and lip selective neurons were similar (Fig. 2c). The half rise time was not significantly different between these two groups (tooth responses, 643.8 ± 42.2 ms, n = 84; lip responses, 643.8 ± 44.6 ms, n = 72; p > 0.6, Mann–Whitney U-test), although there was difference in the half decay time (tooth responses, 310.8 ± 58.6 ms, n = 84; lip responses, 421.8 ± 80.0 ms, n = 72; p < 0.006, Mann–Whitney U-test; one neuron in each group did not decay less than half during the 3 s post-stimulus imaging period).
Spatially, the tooth-selective neurons and lip-selective neurons did not form clusters; instead, they tended to be intermingled (Fig. 2a,f). When we aligned all imaging sessions based on the center of gravity of tooth responses (Fig. 2f right), we found similar distances to tooth-selective cells and lip-selective cells (374.0 ± 24.0 µm, n = 84 vs. 343.3 ± 20.0 µm, n = 72, p = 0.448, Mann–Whitney U test). Furthermore, no consistent spatial relationship emerged between the center of gravity for tooth responses and that for lip responses (in the horizontal direction, p = 0.82, in the vertical direction p = 0.91, n = 9, Wilcoxon signed-rank test) (Fig. 2g). Critically, the area of neurons activated by either the tooth or the lip stimuli were not statistically different. For example, the distance from the center of gravity of tooth response to tooth selective neurons were similar to that from the center of gravity of lip response to lip selective neurons (374.0 ± 24.0 µm, n = 84 vs. 326.3 ± 20.6 µm, n = 72; p > 0.20, Mann–Whitney U-test). Similarly, the pairwise distances between tooth-selective neurons were similar to those between lip-selective neurons (508.1 ± 15.9 µm, n = 468 vs. 486.6 ± 14.0 µm, n = 389; p > 0.20, Mann–Whitney U-test). Together, these findings collectively indicate a lack of clear topological organization of tooth and lip representations at the level of the TG.
Discussion
In this study, we introduced a novel approach involving the implantation of an elongated glass cuboid to visualize the sensory representation of neurons in the TG. This innovative method enabled us to explore the spatial organization of tooth-responsive neurons and revealed an unexpected finding: the spatial intermingling of neurons responsive to incisor and lip stimulation. The weak sensory stimulation used in our experiment activates primarily low threshold mechanoreceptive afferents44, and the sensory map for other cell-types45 may exhibit distinct spatial patterns. Nonetheless, our findings represent a crucial initial step toward understanding the processing of sensory information related to teeth in the brain, presenting a new avenue for investigating the neural circuitry underlying toothaches—a pervasive issue affecting a vast portion of the global population.
Our approach offers several advantages over traditional methods used to visualize the TG. Unlike previous techniques involving the removal of structures above the TG and the use of HEPES buffer34,35,36,37, our method maintains the TG within a contained area in the cranial cavity, eliminating the need for supplementation of artificial buffer during experiments. Additionally, the direct contact of the glass cuboid with the TG enhances imaging site stability against mouse movement induced by breathing and heartbeat, akin to approaches used in visualizing the cerebral cortex26,28,38,46. Furthermore, our approach holds promise for long-term imaging across multiple days, despite the removal of a significant portion of the brain. Finally, the cost for the hardware is relatively small compared to in vivo two-photon imaging system. An obvious alternative possibility is to employ gradient index (GRIN) lens combined with two-photon microscopy47,48, but the advantage of glass cuboid implantation is that it can achieve a larger field of view (Fig. 1a) compared to GRIN lens that can obtain the field of view of usually less than 500 µm49.
Despite its advantages, our approach has limitations that warrant consideration. First, due to the length of glass cuboid (8 mm), the approach was not compatible with in vivo two-photon imaging because our current objective lens has a working distance of 3 mm. This limitation restricts the signals we obtain to those originating primarily from the surface of the TG, impeding our ability to detect spatial organization in the dorsal–ventral direction. For instance, a recent study showed that tongue-innervating neurons are located on the ventral side of the TG50, and we indeed could only find minimum neural responses to the tongue stimulation in our experiments. To overcome this limitation along the dorsal–ventral axis, future studies will require in vivo two-photon imaging with an objective lens with a working distance of > 8 mm. Second, filling the space with glass that has a high refractive index could cause spherical aberration. Although such aberration did not hinder our single-cell resolution imaging, future studies aiming to investigate smaller structures may need to employ an objective lens with correction collars to compensate for the refractive index mismatch.
We discovered that some neurons responded to both the tooth and the lip, although for most neurons, the response was substantially larger for one stimulus. Previous studies have reported that neurons in the brainstem trigeminal nucleus, the area postsynaptic to the neurons we imaged in the current study, also respond to multiple structures in the oral cavity51. There are two possibilities for the dual coding: first, our mechanical stimulation was transmitted to a different portion of the oral cavity; second, individual neurons in the TG innervate both the lip and the incisor. Although our stimulation protocol was controlled to produce responses in only a subset of neurons, we cannot entirely exclude the first possibility. Determining how the receptive field structures of the oral cavity are formed in the TG will require an approach that traces the individual processes.
Although we cannot completely disregard the possibility that nociceptors in the tooth were stimulated, we believe it unlikely for the following reasons. First, within the tooth, nociceptors are primarily present in the pulp cavity and in dentin52 and these are protected by the tooth enamel, which does not have nociceptors53. Therefore, unless there is some erosion of the crown/enamel, it is reasonable to assume that nociceptive receptors are not activated. Second, it is natural to assume that nociception would not be activated in healthy teeth by forces similar to those during everyday activities like chewing. Previous study has shown that the average voluntary mouse bite force is around 5,000 mN54, which is an order of magnitude larger than our stimulation protocol (20–40 mN) that has been used in sensory stimulation of other parts of the body55. Rather, the force applied to natural teeth is thought to be detected by periodontal mechanoreceptors within the surrounding gingiva that can detect low force levels44,56. Together, the intermingled representation we discovered is unlikely to be due to the involvement of nociceptors.
An obvious limitation in the current study is that we did not stimulate different teeth (e.g., molar vs. incisor) nor different parts of the lip. The small size of the mouth made it challenging to stimulate the unexposed molar without touching the incisor or the lip. As a result, we cannot completely exclude the possibility that there exists a somatotopic map for different parts of the teeth/lips . Future studies that employ the expression of channelrhodopsin in specific parts of the oral cavity might allow us to investigate this possibility.
Earlier studies on vibrissae system have proposed a rough organization of whiskers in the TG21,22, but due to the technical limitation of extracellular unit recording, the precise spatial patterns of sensory responses were not known. Our studies using in vivo calcium imaging provide strong evidence for an intermingled representation of the oral cavity in the TG. Our results on the TG contrast with earlier studies that investigated tooth and lip responses in the somatosensory cortex reporting distinct representations of the lip and tooth as part of the “homunculus” in this brain region16,17,18,19,20. Our finding also contrasts with the visual and auditory systems which maintain retinotopy and tonotopy starting from the first stages of sensory processing throughout most of their respective pathways (e.g., ref57; however, see ref58 for optic nerve fibers). Future investigations will explore how spatially distributed neuronal activity in the TG converges onto specific cortical regions via the thalamus, providing insights into higher-order sensory processing mechanisms.
In summary, our study introduces a novel approach to investigating the spatial organization of neuronal responses in the TG. The spatial intermingling of tooth and lip representations sets the stage for further exploration of the complex neural circuitry underlying oral sensory perception.
Data and code availability
All data and code used for figure generation in this study have been deposited in GitHub. http://github.com/pharmedku/trigeminal.
Materials availability
These studies did not generate unique reagents.
References
Jacobs, R. & van Steenberghe, D. Role of periodontal ligament receptors in the tactile function of teeth: A review. J Periodontal Res 29, 153–167. https://doi.org/10.1111/j.1600-0765.1994.tb01208.x (1994).
Dong, W. K. & Chudler, E. H. Origins of tooth pulp-evoked far-field and early near-field potentials in the cat. J Neurophysiol 51, 859–889. https://doi.org/10.1152/jn.1984.51.5.859 (1984).
Dong, W. K., Shiwaku, T., Kawakami, Y. & Chudler, E. H. Static and dynamic responses of periodontal ligament mechanoreceptors and intradental mechanoreceptors. J Neurophysiol 69, 1567–1582. https://doi.org/10.1152/jn.1993.69.5.1567 (1993).
Dong, W. K., Chudler, E. H. & Martin, R. F. Physiological properties of intradental mechanoreceptors. Brain Res 334, 389–395. https://doi.org/10.1016/0006-8993(85)90239-2 (1985).
Trulsson, M. & Johansson, R. S. Forces applied by the incisors and roles of periodontal afferents during food-holding and -biting tasks. Exp Brain Res 107, 486–496. https://doi.org/10.1007/BF00230428 (1996).
Trulsson, M. & Gunne, H. S. Food-holding and -biting behavior in human subjects lacking periodontal receptors. J Dent Res 77, 574–582. https://doi.org/10.1177/00220345980770041001 (1998).
Purkart, L. et al. Trigeminal ganglion and sensory nerves suggest tactile specialization of elephants. Curr Biol 32, 904-910.e903. https://doi.org/10.1016/j.cub.2021.12.051 (2022).
Petersen, C. C. The functional organization of the barrel cortex. Neuron 56, 339–355. https://doi.org/10.1016/j.neuron.2007.09.017 (2007).
Stüttgen, M. C., Kullmann, S. & Schwarz, C. Responses of rat trigeminal ganglion neurons to longitudinal whisker stimulation. J Neurophysiol 100, 1879–1884. https://doi.org/10.1152/jn.90511.2008 (2008).
Severson, K. S., Xu, D., Yang, H. & O’Connor, D. H. Coding of whisker motion across the mouse face. Elife https://doi.org/10.7554/eLife.41535 (2019).
Kaas, J. H. Topographic maps are fundamental to sensory processing. Brain Res Bull 44, 107–112. https://doi.org/10.1016/s0361-9230(97)00094-4 (1997).
Sato, T. R. & Svoboda, K. The functional properties of barrel cortex neurons projecting to the primary motor cortex. J Neurosci 30, 4256–4260. https://doi.org/10.1523/JNEUROSCI.3774-09.2010 (2010).
Sato, T. R., Gray, N. W., Mainen, Z. F. & Svoboda, K. The functional microarchitecture of the mouse barrel cortex. PLoS Biol 5, e189. https://doi.org/10.1371/journal.pbio.0050189 (2007).
Penfield, W. & Rasmussen, T. The cerebral cortex of man; a clinical study of localization of function (Macmillan, UK, 1950).
Brecht, M. The Body Model Theory of Somatosensory Cortex. Neuron 94, 985–992. https://doi.org/10.1016/j.neuron.2017.05.018 (2017).
Taira, K. The representation of the oral structures in the first somatosensory cortex of the cat. Brain Res 409, 41–51. https://doi.org/10.1016/0006-8993(87)90739-6 (1987).
Cusick, C. G., Wall, J. T. & Kaas, J. H. Representations of the face, teeth and oral cavity in areas 3b and 1 of somatosensory cortex in squirrel monkeys. Brain Res 370, 359–364. https://doi.org/10.1016/0006-8993(86)90494-4 (1986).
Catania, K. C. & Remple, M. S. Somatosensory cortex dominated by the representation of teeth in the naked mole-rat brain. Proc Natl Acad Sci U S A 99, 5692–5697. https://doi.org/10.1073/pnas.072097999 (2002).
Jain, N., Qi, H. X., Catania, K. C. & Kaas, J. H. Anatomic correlates of the face and oral cavity representations in the somatosensory cortical area 3b of monkeys. J Comp Neurol 429, 455–468. https://doi.org/10.1002/1096-9861(20010115)429:3%3c455::aid-cne7%3e3.0.co;2-f (2001).
Remple, M. S., Henry, E. C. & Catania, K. C. Organization of somatosensory cortex in the laboratory rat (Rattus norvegicus): Evidence for two lateral areas joined at the representation of the teeth. J Comp Neurol 467, 105–118. https://doi.org/10.1002/cne.10909 (2003).
Leiser, S. C. & Moxon, K. A. Relationship between physiological response type (RA and SA) and vibrissal receptive field of neurons within the rat trigeminal ganglion. J Neurophysiol 95, 3129–3145. https://doi.org/10.1152/jn.00157.2005 (2006).
Zucker, E. & Welker, W. I. Coding of somatic sensory input by vibrissae neurons in the rat’s trigeminal ganglion. Brain Res 12, 138–156. https://doi.org/10.1016/0006-8993(69)90061-4 (1969).
Maklad, A., Fritzsch, B. & Hansen, L. A. Innervation of the maxillary vibrissae in mice as revealed by anterograde and retrograde tract tracing. Cell Tissue Res 315, 167–180. https://doi.org/10.1007/s00441-003-0816-z (2004).
Sato, T. R. et al. Interhemispherically dynamic representation of an eye movement-related activity in mouse frontal cortex. Elife https://doi.org/10.7554/eLife.50855 (2019).
Niethard, N. et al. Sleep-stage-specific regulation of cortical excitation and inhibition. Curr Biol 26, 2739–2749. https://doi.org/10.1016/j.cub.2016.08.035 (2016).
Itokazu, T. et al. Streamlined sensory motor communication through cortical reciprocal connectivity in a visually guided eye movement task. Nat Commun 9, 338. https://doi.org/10.1038/s41467-017-02501-4 (2018).
Hasegawa, M. et al. Selective suppression of local circuits during movement preparation in the mouse motor cortex. Cell Rep 18, 2676–2686. https://doi.org/10.1016/j.celrep.2017.02.043 (2017).
Abe, K. et al. Enhanced aversive signals during classical conditioning in dopamine axons in medial prefrontal cortex. bioRxiv https://doi.org/10.1101/2023.08.23.554475 (2023).
Sato, T., Tokuyama, W., Miyashita, Y. & Okuno, H. Temporal and spatial dissociation of expression patterns between Zif268 and c-Fos in rat inferior olive during vestibular compensation. NeuroReport 8, 1891–1895. https://doi.org/10.1097/00001756-199705260-00020 (1997).
Pachitariu, M. et al. Suite2p: Beyond 10,000 neurons with standard two-photon microscopy. BbioRxiv 137, 76 (2016).
Ohki, K., Chung, S., Ch’ng, Y. H., Kara, P. & Reid, R. C. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603. https://doi.org/10.1038/nature03274 (2005).
Rothschild, G., Nelken, I. & Mizrahi, A. Functional organization and population dynamics in the mouse primary auditory cortex. Nat Neurosci 13, 353–360. https://doi.org/10.1038/nn.2484 (2010).
Bandyopadhyay, S., Shamma, S. A. & Kanold, P. O. Dichotomy of functional organization in the mouse auditory cortex. Nat Neurosci 13, 361–368. https://doi.org/10.1038/nn.2490 (2010).
Ghitani, N. et al. Specialized mechanosensory nociceptors mediating rapid responses to hair pull. Neuron 95, 944-954.e944. https://doi.org/10.1016/j.neuron.2017.07.024 (2017).
Yarmolinsky, D. A. et al. Coding and plasticity in the mammalian thermosensory system. Neuron 92, 1079–1092. https://doi.org/10.1016/j.neuron.2016.10.021 (2016).
Hu, M., Doyle, A. D., Yamada, K. M. & Kulkarni, A. B. Visualization of trigeminal ganglion sensory neuronal signaling regulated by Cdk5. Cell Rep 38, 110458. https://doi.org/10.1016/j.celrep.2022.110458 (2022).
Moayedi, Y. et al. The cellular basis of mechanosensation in mammalian tongue. Cell Rep 42, 112087. https://doi.org/10.1016/j.celrep.2023.112087 (2023).
Komiyama, T. et al. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature 464, 1182–1186. https://doi.org/10.1038/nature08897 (2010).
Dana, H. et al. Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo. PLoS ONE 9, e108697. https://doi.org/10.1371/journal.pone.0108697 (2014).
Mullen, R. J., Buck, C. R. & Smith, A. M. NeuN, a neuronal specific nuclear protein in vertebrates. Development 116, 201–211. https://doi.org/10.1242/dev.116.1.201 (1992).
Zhang, Y. et al. Fast and sensitive GCaMP calcium indicators for imaging neural populations. Nature 615, 884–891. https://doi.org/10.1038/s41586-023-05828-9 (2023).
Beaman, C. B., Eagleman, S. L. & Dragoi, V. Sensory coding accuracy and perceptual performance are improved during the desynchronized cortical state. Nat Commun 8, 1308. https://doi.org/10.1038/s41467-017-01030-4 (2017).
Jones, L. M., Fontanini, A., Sadacca, B. F., Miller, P. & Katz, D. B. Natural stimuli evoke dynamic sequences of states in sensory cortical ensembles. Proc Natl Acad Sci U S A 104, 18772–18777. https://doi.org/10.1073/pnas.0705546104 (2007).
Trulsson, M. Sensory-motor function of human periodontal mechanoreceptors. J Oral Rehabil 33, 262–273. https://doi.org/10.1111/j.1365-2842.2006.01629.x (2006).
Yang, L. et al. Human and mouse trigeminal ganglia cell atlas implicates multiple cell types in migraine. Neuron 110, 1806-1821.e1808. https://doi.org/10.1016/j.neuron.2022.03.003 (2022).
Dombeck, D. A., Khabbaz, A. N., Collman, F., Adelman, T. L. & Tank, D. W. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 56, 43–57. https://doi.org/10.1016/j.neuron.2007.08.003 (2007).
Jung, J. C. & Schnitzer, M. J. Multiphoton endoscopy. Opt Lett 28, 902–904. https://doi.org/10.1364/ol.28.000902 (2003).
Jung, J. C., Mehta, A. D., Aksay, E., Stepnoski, R. & Schnitzer, M. J. In vivo mammalian brain imaging using one- and two-photon fluorescence microendoscopy. J Neurophysiol 92, 3121–3133. https://doi.org/10.1152/jn.00234.2004 (2004).
Meng, G. et al. High-throughput synapse-resolving two-photon fluorescence microendoscopy for deep-brain volumetric imaging in vivo. Elife https://doi.org/10.7554/eLife.40805 (2019).
Zhang, L., Nagel, M., Olson, W. P., Chesler, A. T. & O’Connor, D. H. Trigeminal innervation and tactile responses in mouse tongue. Cell Rep 43, 114665. https://doi.org/10.1016/j.celrep.2024.114665 (2024).
Sessle, B. J. & Greenwood, L. F. Inputs to trigeminal brain stem neurones from facial, oral, tooth pulp and pharyngolaryngeal tissues: I. Responses to innocuous and noxious stimuli. Brain Res 117, 211–226. https://doi.org/10.1016/0006-8993(76)90731-9 (1976).
Beertsen, W., McCulloch, C. A. & Sodek, J. The periodontal ligament: A unique, multifunctional connective tissue. Periodontol 2000(13), 20–40. https://doi.org/10.1111/j.1600-0757.1997.tb00094.x (1997).
Byers, M. R. Dental sensory receptors. Int Rev Neurobiol 25, 39–94. https://doi.org/10.1016/s0074-7742(08)60677-7 (1984).
Guo, W. et al. Voluntary biting behavior as a functional measure of orofacial pain in mice. Physiol Behav 204, 129–139. https://doi.org/10.1016/j.physbeh.2019.02.024 (2019).
Turecek, J., Lehnert, B. P. & Ginty, D. D. The encoding of touch by somatotopically aligned dorsal column subdivisions. Nature 612, 310–315. https://doi.org/10.1038/s41586-022-05470-x (2022).
Trulsson, M. & Johansson, R. S. Encoding of tooth loads by human periodontal afferents and their role in jaw motor control. Prog Neurobiol 49, 267–284. https://doi.org/10.1016/s0301-0082(96)00016-0 (1996).
Molotkov, D., Ferrarese, L., Boissonnet, T. & Asari, H. Topographic axonal projection at single-cell precision supports local retinotopy in the mouse superior colliculus. Nat Commun 14, 7418. https://doi.org/10.1038/s41467-023-43218-x (2023).
Horton, J. C., Greenwood, M. M. & Hubel, D. H. Non-retinotopic arrangement of fibres in cat optic nerve. Nature 282, 720–722. https://doi.org/10.1038/282720a0 (1979).
Acknowledgements
This work was supported by grants from the Brain and Behavior Research Foundation (Young Investigator Grant, 29268), National Institute on Drug Abuse (COCA pilot grant, P50 DA046373), National Institute of Aging (R03 AG070517), National Institute of Neurological Disorders and Stroke (R21 NS125571, R01 NS131549), BrightFocus (A2021041S) and American Heart Association (19IPLOI34760424) to T.R.S.; JSPS KAKENHI (JP24KJ1644) to R.I.; AMED (JP21wm0425010, JP21gm1510006) and KAKENHI (JP22H02944, JP23K18163), the Collaborative Research Program of Institute for Protein Research, Osaka University, ICR-24-03 to T.H.; JST PRESTO (JPMJPR1883) and KAKENHI (20K23378) to T.K.S.
Author information
Authors and Affiliations
Contributions
A.M., R.I, T.H., T.K.S., and T.R.S. conceived the project and designed the experiments. T.R.S. and T.K.S. prepared the analysis codes. A.M., R.I., E.S., S.T., T.I., and K.A. carried out the experiments. A.M., R.I., E.S., K.A., Y.K., T.H., T.K.S., and T.R.S analysed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Matunis, A., Iwamoto, R., Stacy, E. et al. Intermingled representation of oral cavity in mouse trigeminal ganglion. Sci Rep 15, 22007 (2025). https://doi.org/10.1038/s41598-025-05382-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05382-6