Abstract
This study presents a novel nanocomposite hydrogel film, composed of physically crosslinked polyacrylamide (PAM) and carbon nanotube (CNT) flakes, as a potential drug delivery system. Field emission scanning electron microscopy (FESEM) analysis elucidated the microstructure and interconnected pore network of the hydrogel, highlighting their direct influence on drug loading capacity and release profile. The hydrogel exhibited outstanding swelling behavior and structural stability under physiological conditions. These attributes rendered the hydrogel suitable as an effective drug carrier for release of doxorubicin (DOX), as a model drug. The DOX-loaded hydrogel demonstrated a sustained release profile at pH 5.5. Biocompatibility and cytotoxicity of the hydrogel were assessed through MTT assays using human embryonic kidney 293 (HEK-293) and MCF-7 human breast cancer cell lines over 24- and 48-hour intervals. The results confirmed the biocompatibility of the hydrogel, while the DOX-loaded hydrogel effectively inhibited MCF-7 proliferation cells, validating its therapeutic potential. Moreover, toxicity evaluations, including brine shrimp lethality and hemolysis assays, confirmed the low toxicity and excellent hemocompatibility of the hydrogel. These findings underscore the potential of the PAM/CNT nanocomposite hydrogel as a versatile and safe drug delivery system, with promising applications in advanced therapeutic strategies.
Similar content being viewed by others
Introduction
Hydrogels are three-dimensional polymeric networks that are physically or chemically crosslinked and capable of swelling in aqueous environments without dissolving1,2. Owing to their high water retention capacity and porous structure, hydrogels have gained significant attention in drug delivery systems (DDSs), serving as effective carriers for controlled and sustained release3,4,5,6,7,8. In addition, their high biocompatibility, combined with favorable physicochemical properties, has made them one of the most reliable biomaterials for biomedical applications4,8. In addition to their excellent biocompatibility, hydrogels possess favorable physicochemical properties, establishing them as one of the most reliable biomaterials for biomedical applications. In recent years, hydrogels have emerged as a promising platform for overcoming the limitations of conventional DDSs, particularly by reducing systemic toxicity associated with traditional therapeutic approaches4,9. Among various types of hydrogels, CNT-based hydrogels have attracted considerable interest due to the remarkable physical and chemical properties of CNTs10. CNTs have emerged as advanced nanomaterials with broad applications across diverse fields, including water purification, drug delivery11and the fabrication of conductive nanocomposite hydrogels12.The conjugated π-system on the surface of CNTs promotes π-π stacking interactions with aromatic therapeutic molecules, such as DOX, a widely recognized anticancer agent13. These interactions enable the efficient loading of DOX onto CNT surfaces, allowing for the controlled release of DOX at the target site and thereby enhancing its therapeutic efficacy. Additionally, the high aspect ratio and needle-like shape of CNTs facilitate cellular uptake, further improving the efficiency of drug delivery14. The proven biocompatibility and biodegradability of CNT-based hydrogels further enhance their potential as promising drug delivery vehicles15. For instance, Dong et al.16 developed a sustained DDS for DOX using a thermosensitive hydrogel incorporated with Chitosan-CNTs. This system exhibited a dual-stage drug release profile and enabled the controlled release of DOX under near-infrared (NIR) irradiation, exploiting the photothermal effects of CNTs to induce a gel–sol transition for targeted drug delivery. This system was applied for controlled and sustained release of DOX, along with Rhodamine B (RB), with a slower release rate observed for DOX, attributable to its dual release mechanism from both CNTs and the hydrogel17. Similarly, NIR irradiation has been employed to precisely control the release of both hydrophilic and hydrophobic drugs from hydrogels containing MWCNTs18. Incorporating CNTs into temperature-sensitive hydrogels enhances their responsiveness to NIR stimuli, thereby facilitating the controlled release of drugs through photothermal conversion19. Additionally, the incorporation of CNTs into hydrogels enhances their swelling properties, reduces the drug release rate, and improves the controllability of the process compared to hydrogels without CNTs20. Despite the promising potential of CNTs, their inherent toxicity, particularly due to their elongated structure, continues to present a significant challenge in biomedical applications21. Various strategies have been explored to mitigate CNT toxicity, including degradation through biological or chemical agents. Degradation of CNTs into smaller fragments reduces their toxicity and enhances their suitability for DDSs22,23,24.
In our previous work25we introduced a novel approach for synthesizing PAM/CNT flakes (degraded CNTs) nanocomposite hydrogel films. The degraded CNTs, in the form of flakes, function as crosslinkers within the hydrogel network, forming hydrogen bonds with polymer chains. This approach not only creates porous networks and a variety of microstructures within the hydrogel, but also enhances its biocompatibility, making it an ideal candidate for biomedical applications26. This study presents a highly porous PAM/CNT nanocomposite hydrogel with negligible toxicity, high biocompatibility, and exceptional swelling capacity (> 1000%), demonstrating efficient controlled drug release for potential biomedical applications. The porous structure of the hydrogel was extensively characterized using FESEM, and its swelling behavior was assessed over a 72-hour period. The ability of the hydrogel to deliver DOX at both acidic (pH 5.5) and physiological (pH 7.4) pH levels was systematically investigated. The biocompatibility of the hydrogel was validated through assays using HEK-293 cells, while cytotoxicity studies with the MCF-7 human breast cancer cell line demonstrated the efficient delivery and anticancer efficacy of DOX-loaded hydrogels. Furthermore, the non-toxicity of the hydrogel was confirmed through brine shrimp bioassays and hemolysis tests. These findings underscore the potential of the PAM/CNT nanocomposite hydrogel as a viable and promising platform for sustained drug delivery and cancer therapy.
Materials and methods
Materials
Multi-walled carbon nanotubes (MWCNTs) (> 95% purity, 5–15 nm outer diameter,10–30 μm length) were obtained from EXIR Co. (Wein, Austria). Acrylamide (AM, 99%), sulfuric acid (H₂SO₄, 98%), nitric acid (HNO₃, 70%), and sodium persulfate (NaPS, 98%) were purchased from Sigma-Aldrich. Deionized (DI) water was utilized throughout the hydrogel synthesis. The 3-(4,5-dimethylthiazol-2-yl) − 2,5-diphenyltetrazolium bromide (MTT) assay reagent was also obtained from Sigma-Aldrich (St. Louis, USA).
Preparation of the nanocomposite hydrogel film
The nanocomposite hydrogel film was synthesized following our previously reported method25. Briefly, 0.002 g of O-MWCNT (oxidized multi-walled carbon nanotube) was dispersed in 7 mL of distilled water, sonicated for 45 min, and subsequently deoxygenated by nitrogen gas purging for one hour. The resulting suspension was transferred to a nitrogen-purged round-bottom flask under continuous stirring. A solution of 0.002 g NaPS in 1 mL of water was then added, followed by the addition of 1.2 g AM dissolved in 2 mL of water. The reaction mixture was stirred and heated to 87.5 °C, yielding a viscous black mixture. This mixture was cast into a Petri dish and left to dry at room temperature for 48 h, ultimately forming the nanocomposite hydrogel film.
Characterization
The morphology and microstructures of the synthesized, freeze-dried, and incompletely formed hydrogels were analyzed using field emission scanning electron microscopy (FESEM, TESCAN MIRA 3, Czech Republic). Prior to imaging, the samples were coated with a thin layer of gold via sputter coating to enhance conductivity. UV-visible spectra of the hydrogels were recorded using a Shimadzu UV-vis spectrophotometer to analyze their optical properties.
Hydrogel swelling studies
The swelling behavior of the hydrogel films were evaluated by immersing samples in distilled water at room temperature and in buffered solutions with pH values of 5.5 and 7.4 (at 37 °C) for 76 h. At predetermined intervals, the hydrogels were removed from the solution, excess surface water was carefully blotted, and the samples were weighed.The swelling ratio (SR) was calculated using the following Eq. 18,27:
where Ws and Wd represent the weights of the swollen and dry hydrogels, respectively.
In vitro DOX release studies
Among the hydrogel films, the optimized nanocomposite hydrogel was selected for in vitro DOX release experiments25. The bulk hydrogel was pulverized into particles (250–425 μm) and subsequently immersed in distilled water for 24 h to achieve complete swelling. DOX loading was performed by immersing a certain amount of the hydrogel in a 2 mg/mL DOX solution, followed by the removal of unbound DOX. After diluting the loaded hydrogels, they were placed in a dialysis bag (MWCO 3500 Da) and immersed in 5 mL of PBS (Phosphate Buffered Saline) (pH 7.4, 0.01 M) or acetate buffer (pH 5.5,0.01 M) at 37 °C. At predetermined intervals, the incubation medium (5 mL) was replaced with fresh buffer, and DOX concentration was quantified via UV-vis spectroscopy at 480 nm. Calibration curves for DOX at pH 7.4 and 5.5 were determined by measuring the absorption of DOX at 480 nm (Fig. S5 and S6). All experiments were conducted in triplicate to ensure reproducibility.
Cell culture
The MCF-7 human breast cancer cell line and human embryonic kidney 293 (HEK-293) cell line were obtained from the American Tissue Culture Collection (ATCC, Manassas, VA). Cells were cultured in RPMI (Roswell Park Memorial Institute) 1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS), 1% penicillin, and 1% streptomycin. The cultures were maintained in a humidified incubator at 37 °C with 5% CO2.
MTT assay
The MTT assay was employed to validate the cytotoxicity and biocompatibility of the hydrogel films in vitro28. For this evaluation, HEK-293 and MCF-7 human breast cancer cell lines (ATCC, Manassas, VA) were used. The method used to evaluate the biocompatibility of the hydrogels was consistent with previously described methodologies in the literature15.
Toxicity test
The toxicity of the hydrogel films was assessed using brine shrimp lethality bioassay. Briefly, brine shrimps (Artemia salina) were hatched in a 250 mL glass vessel containing artificial seawater (32 g/L) under constant oxygen supply at room temperature for 48 h. Subsequently, ten Artemia salina nauplii were counted and transferred into each well of a 96-well plate. The hydrogel film samples in different concentrations (125, 250, 500, 1000 and 2,000 µg/mL) were added into wells containing active nauplii in triplicate. After 24 h, the survival of nauplii was observed and counted using an optical microscope, and the mortality rate was determined.
Hemolysis test
The hemolysis test was performed to evaluate the biocompatibility of the hydrogel films. First, 1 mL of human red blood cells (RBC) was obtained by centrifuging human blood and removing the serum. Then, the RBCs were washed three times with sterile PBS and subsequently diluted with PBS. Afterward, 0.5 mL of the hydrogel solution was added to the RBC suspension. Deionized water was used as a positive control, and PBS served as a negative control. The samples were incubated at 37 °C for 4 h, followed by centrifugation at 1300 rpm for 15 min. The absorbance of the supernatant was measured at 540 nm. The percentage of hemolysis was calculated using the following Eq. 229:
where A sample, A negative and A positive represent the absorbance of the sample, negative control, and positive control, respectively, at 540 nm.
Results and discussion
Synthesis of the nanocomposite hydrogel film
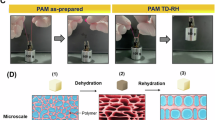
Nanocomposite hydrogel film was synthesized through the in situ degradation of O-MWCNTs, particularly those with a higher degree of defects, coupled with the free radical polymerization of AM monomers25. The crosslinking of PAM was created by hydrogen bonding between the degraded O-MWCNTs and the amide groups of PAM chains. The synthesis mechanism for forming the nanocomposite hydrogel film is illustrated in Fig. 1, which closely resembles the figure presented in previous study25with the inclusion of a hydrogel film image in this version. The in-situ degradation of O-MWCNTs in an aqueous AM solution, combined with the simultaneous polymerization of AM monomers, results in the formation of a viscous suspension containing both PAM chains and O-MWCNT flakes of diverse sizes. As water evaporates, the flakes interact with PAM chains, forming physical crosslinks through hydrogen bonding. Hydrophobic interactions between PAM chains and the CNTs further stabilize the network, ultimately resulting in the formation of a hydrogel matrix. The structural characteristics and mechanical properties of the synthesized hydrogel have been extensively analyzed in our previous work25. For instance, selected ATR, Raman analyses, and mechanical tests results are presented in Figures S1–S3 of the Supplementary Material.
Hydrogel morphology studies
To evaluate the feasibility of applying hydrogels in drug release, a thorough evaluation of the porous networks and their precise morphology is essential. Therefore, SEM was utilized to study the microstructures and micro-networks of the hydrogel films. Figure 2 shows the SEM micrographs of the outer surface morphology of the synthesized and freeze-dried hydrogels. As observed in Fig. 2a, the hydrogel surface exhibits microstructures resembling parallel lines, which form surface reliefs. The appearance of these microstructures on the hydrogel surface validates the formation of a 3D network structure within the hydrogel matrix25. Figure 2b shows agglomerates of unreacted particles, which are eliminated as impurities during the initial swelling stage in water.
The morphologies of the micro-network structures on the outer surface of freeze-dried hydrogel are shown in Fig. 2c–f. Spider web-like microstructures are observed covering the entire surface of the hydrogel. These structures arise from phase separation between the crosslinks and water30. Additionally, the micro-network structures contribute to enhancing the mechanical properties of hydrogels31. The main porous network is visible under the micro-network structures (Fig. 2d–f). Cross-sectional SEM images of the freeze-dried hydrogel and incompletely formed hydrogels at varying magnifications are presented in Fig. 3. As observed in Fig. 3a and d (and Fig. S4a and 4b), poorly formed pore walls lead to incomplete hydrogels with poor mechanical properties. This incomplete formation is attributed to the non-uniform distribution of CNTs, causing CNT agglomerates within the reaction mixture. Additionally, another type of incomplete network is observed in Fig. 3c and f, where the pores appear highly irregular and non-uniform. This irregularity may result from excessive CNT destruction during hydrogel production. Both incomplete hydrogels exhibit poor physical and morphological properties, making them unsuitable for many applications such as drug delivery.
Figure 3b and e display the SEM images depicting the honeycomb-like structure of the porous network within the hydrogel. The pores are arranged in a highly uniform and regular pattern, characterized by a hexagonal geometry, with thin walls separating them. Moreover, since the average pore diameter within the hydrogel network is a few micrometers, the hydrogel is classified as a microporous material. This microporous structure, with its interconnected network and distinct morphology, offers significant advantages for applications in drug delivery and tissue engineering, where controlled transport and cellular interactions are essential32.
Figure 4 shows the embedded micro-network structures within the pores of the hydrogel network (Fig. 4a–c) and an incompletely formed hydrogel (Fig. 4d–f) at different magnifications. As depicted in Fig. 4a–c, the micro-network structures comprise continuous, branched microfibers that interconnect to form network pores, with dimensions ranging from several nanometers to a few hundred nanometers. In contrast, the incomplete hydrogel sample, shown in Fig. 4d-f, reveals fractured microfibers, resulting in a weakened network structure. The formation of these embedded micro-network structures within the hydrogel is primarily attributed to phase separation between the crosslinks and water, which significantly enhances the mechanical properties of the hydrogel. Additionally, the porous nature of these microstructures, may facilitate drug entrapment and controlled release. Notably, the stability of these porous and micro-network structures is maintained after several months25. SEM analysis further confirms the promising potential of the nanocomposite hydrogel film for drug delivery applications, owing to its robust porous and micro-network architecture.
SEM images of the outer surface of the hydrogel films. (a) The synthesized hydrogel film, (b) Some agglomerates on the surface of the synthesized hydrogel film, (c–f) The freeze-dried hydrogel films with different magnifications (Figures in panels a and c are reproduced from ref25, licensed under a Creative Commons Attribution 3.0 Unported Licence).
The cross-sectional SEM images of the porous networks within the freeze-dried hydrogel (b and e) and the incompletely formed hydrogels (a, c, d and f) with magnifications of 7,000× (top row) and 10,000× (bottom row). (Figure in panel e is reproduced from ref25, licensed under a Creative Commons Attribution 3.0 Unported Licence).
Swelling behavior
The swelling behavior of the nanocomposite hydrogel films was evaluated in distilled water (at room temperature) and in buffered solutions at pH 5.5 and 7.4 (at 37 °C), utilizing the methodology outlined in our previous publication25. The results demonstrated excellent swelling performance and stability of the hydrogels. Figure 5 shows the swelling ratios of the hydrogel samples in distilled water and at pH 5.5 and 7.4 over a period of 76 h. Initially, a slight decrease in swelling was observed in the range of 0.5–3 h for the samples in distilled water. This swelling drop is attributed to the hydrophobic nature of the hydrogel, resulting from the presence of free CNTs within the network. Between 3 and 6 h, the hydrogel reached its equilibrium swelling ratio of 11.95. After 6 h, the swelling ratio remained stable at 10.69, indicating equilibrium. The slight reduction in the equilibrium swelling ratio compared to the initial swelling is attributed to partial dissolution of the hydrogel, resulting in a reduction in both mass and swelling. As shown in Fig. 5, the swelling behavior at pH 7.4 closely follows the swelling curve in distilled water, as expected. The slightly higher swelling at pH 7.4 is likely due to the repulsion between the negatively charged carboxylate groups. In contrast, the reduced swelling observed at pH 5.5 can be attributed to hydrogen bonding and a decrease in ion concentration within the network25.
The detailed swelling data are summarized in Table S1, demonstrating that the nanocomposite hydrogel film exhibits remarkable stability and high swelling capacity, with only a minor reduction in swelling ratio after 76 h. This stability is likely due to effective crosslinking within the hydrogel network. Overall, the swelling studies confirmed that the hydrogel is a suitable candidate for drug delivery applications, owing to its excellent swelling behavior and maintained structural integrity.
In vitro drug release
The optimized nanocomposite hydrogel, selected for its exceptional swelling behavior, stability, and porous structure, was evaluated for its potential in drug delivery applications. DOX was chosen as the model drug due to its extensive use in chemotherapy, well-characterized release properties, and its suitability for evaluating hydrogel performance in drug loading and release. DOX was loaded into the hydrogel by immersing it in a 0.2 mg/mL DOX solution for 24 h, followed by rinsing with PBS solution. The carboxyl groups on the O-MWCNT flakes facilitated DOX loading via hydrogen bonding and electrostatic interactions33. Additionally, π-π stacking interactions between the O-MWCNTs and DOX molecules, along with hydrogen bonding and hydrophobic interactions between DOX and the PAM chains, further promoted drug entrapment13. The interconnected microporous structure of the hydrogel also enhanced its drug delivery potential32. The entrapment efficiency (EE) and the loading capacity (LC) of DOX in the hydrogel were determined to be 83.4% and 5.11% respectively.
The release behavior of DOX from the loaded hydrogels was systematically studied under various pH conditions for 72 h at 37 °C (Fig. 6). At pH 5.5, mimicking the acidic environment of cancerous tissues34the drug release exhibited biphasic behavior: an initial linear release phase within the first 6 h, followed by a sustained release phase extending up to 72 h. The initial release phase is attributed to the rapid release of DOX molecules located on the surface of the hydrogel, combined with the overall process of drug diffusion. The subsequent release phase is mainly influenced by the differential release rates of DOX from two sources within the hydrogel matrix: DOX involved using hydrogen bonding and DOX adsorbed onto O-MWCNTs through π–π stacking interactions. Based on the calibration curves from Supplementary Fig. S5 and S6, the total release of DOX from the hydrogel in an acidic medium was calculated to be 74.3%, significantly higher than the release observed in a neutral medium (PBS, pH 7.4), which was 26.4%. This difference arises from the higher solubility of DOX in acidic environments, facilitating a faster and more complete release at lower pH levels. In contrast, the limited release at pH 7.4, which mimics physiological conditions and normal tissue, suggests robust interactions between DOX and the hydrogel network, predominantly governing drug diffusion. Additionally, hydrogel erosion under acidic conditions, leading to crosslink degradation, contributes to the enhanced release of DOX at lower pH values. These findings demonstrate the high potential of the hydrogel for controlled drug delivery.
MTT assay
Cytotoxicity tests of the hydrogel films with different concentrations (12.5, 25, 50, 100, 200 and 400 µg/mL) were assessed on HEK-293 cells for 24 and 48 h. As depicted in Fig. 7, at the highest concentration (400 µg/mL), a minimal toxicity of 8% was observed after 24 h, corresponding to 92% cell viability compared to the control (100% viability). After increasing the incubation time to 48 h, toxicity rose slightly to 13%, with 87% cell viability at the same concentration. Overall, cell viability showed a decreasing trend with both extended incubation time (from 24 to 48 h) and increased hydrogel concentration (12.5 to 400 µg/mL). Notably, these results show that even at the highest concentration examined, the hydrogel films exhibited no significant cytotoxicity, as cell viability remained above 87% after 48 h. Consequently, these observations indicate promising biocompatibility of the hydrogel films, suggesting their potential application in DDSs35. Furthermore, these results are consistent with previous reports in the scientific literature demonstrating the biocompatibility of CNT-based hydrogel films on HeLa cells15.
The anticancer activity of free DOX, hydrogel films and DOX loaded hydrogel films was evaluated against the MCF-7 cancer cell line using the MTT assay. Different concentrations (12.5, 25, 50, 100, 200 and 400 µg/mL) of free DOX, hydrogel films, and DOX loaded hydrogel films were tested at 24 and 48-hour intervals (Fig. 8a and b). The results indicated that hydrogel films alone did not exhibit significant anticancer activity at the tested concentrations (Fig. 8a and b). However, at 400 µg/mL, hydrogel films showed 40% cytotoxicity after 48 h (Fig. 8b). In contrast, free DOX and DOX-loaded hydrogels exhibited notable anti-proliferative effects at all tested concentrations, demonstrating efficacy after 24 and 48 h of incubation (Fig. 8a and b).
The notable biocompatibility of hydrogel films (Fig. 7) implies that the cytotoxicity observed in DOX-loaded hydrogel carriers primarily arises from the encapsulated DOX. At a concentration of 400 µg/mL, DOX-loaded hydrogel films exhibited 57% and 63% cytotoxicity after 24 and 48 h of incubation, respectively (Fig. 8a and b). In contrast, free DOX demonstrated 71% cytotoxicity (29% cell viability) under identical conditions, indicating time-independent cytotoxicity at these concentrations. The lower anticancer activity of DOX-loaded hydrogels, relative to free DOX, is attributed to slower cellular internalization and sustained DOX release36. These results indicate the potential of DOX-loaded hydrogel films to deliver significant anticancer activity against MCF-7 cells, demonstrating notable efficacy after 24 and 48 h of incubation.
It would be valuable to compare the effects of DOX delivery via hydrogels with other DDSs, such as nanoparticles and liposomes, in order to evaluate their advantages and limitations. Hydrogels, as polymeric networks with high water retention, enable controlled and sustained DOX release. Moreover, hydrogels can be engineered to respond to external stimuli, such as pH or temperature, making them suitable for particularly localized drug delivery37. In contrast, nanoparticles, as small solid carriers, offer high drug loading capacity and efficient targeted delivery. However, they may face challenges such as rapid clearance by the immune system and potential toxicity due to drug accumulation in organs38. Liposomes, formed by lipid bilayers, are widely used for DOX encapsulation, enabling the delivery of both hydrophilic and hydrophobic drugs while reducing systemic toxicity and enhancing therapeutic efficacy39,40,41. Despite these advantages, liposomes may also experience rapid degradation and clearance, limiting their effectiveness in sustained drug release39,40. Hydrogels, nanoparticles, and liposomes exhibit distinct release mechanisms, with hydrogels ensuring a slower, sustained release, while nanoparticles and liposomes exhibit faster drug release due to their smaller size and higher degradation rates42,43. Given their potential to offer prolonged and environmentally responsive drug release, hydrogels represent a promising alternative for enhancing therapeutic outcomes, particularly in localized cancer treatments.
In vitro cytotoxicity analysis and hemolysis test
The cytotoxicity of hydrogel films was assessed using brine shrimp lethality bioassay. Nauplii were hatched from brine shrimp eggs and subsequently exposed to the hydrogel films. Survival rates of the nauplii at different concentrations of the hydrogel were recorded after 24 h. At all tested concentrations, the number of alive nauplii exceeded 60.0% (Fig. 9a). The survival rate of nauplii was higher at lower hydrogel concentrations compared to higher concentrations. As shown in Fig. 9a, specifically, concentrations in the range of 0.125–0.5 mg/mL demonstrated minimal toxicity. These observations further confirm the non-toxic nature of the hydrogel samples under the tested conditions. The reduced nauplii survival at higher concentrations may arise from hydrogel cytotoxicity, reduced dissolved oxygen, or the formation of a viscous layer on the gills of nauplii44. These findings confirm the safety of hydrogel films for drug delivery applications.
Hemolysis assays were conducted to assess the hemocompatibility of the hydrogels, as a reliable indicator of blood safety45,46. The hemolytic activity was evaluated at a hydrogel concentration of 2 mg/mL over a period of 4 h. As shown in Fig. 9b, minimal disruption of the RBC membrane was observed at hydrogel concentrations ranging from 0.125 to 0.5 mg/mL, while hemolysis remained below 5% even at higher concentrations (1–2 mg/mL). These results suggest that no significant hemoglobin release occurs due to the interaction of RBCs with the hydrogels46. Furthermore, a distinct comparison between the control and hydrogel graphs can be seen in Fig. 9b. Overall, the obtained results indicate that the hydrogels do not show hemolytic activity for RBCs, confirming their biocompatibility and potential as safe carriers for biomedical applications47.
In this study, we synthesized a nanocomposite hydrogel consisting of acrylamide and CNTs, following our previously established methodology25. The network structure of the hydrogel was systematically evaluated using SEM. The analysis revealed a highly porous and interconnected network with distinct microstructures (Figs. 2, 3 and 4), which can be attributed to the uniform dispersion of CNT flakes and effective crosslinking within the hydrogel matrix. This unique architecture significantly influences the swelling behavior. Specifically, we observed a substantial swelling ratio of 1082% at pH 7.4 and 1021% at pH 5.5 (Fig. 5), indicating the hydrogel’s capacity to respond to pH variations. The hydrophilic nature of the amide groups in the polyacrylamide network, along with the carboxyl functionalities in the CNTs, significantly enhances the hydrogel’s water absorption capacity. The drug release study exhibited pH-dependent behavior, with sustained DOX release (74.31%) over 72 h at pH 5.5, compared to a substantially lower release (25.72%) at pH 7.4 (Fig. 6). This variation is attributed to the increased solubility of DOX in acidic environments, which facilitates more efficient drug release. These findings highlight the potential of this hydrogel as a pH-responsive DDS for targeted drug release in acidic tumor microenvironments. Moreover, biocompatibility assessments demonstrated that the hydrogel exhibits non-toxic properties. HEK cell viability remained consistently high, even at elevated concentrations, over both 24- and 48-hour periods (Fig. 7). Brine shrimp lethality assays indicated negligible toxicity at higher concentrations, while hemolysis tests confirmed the absence of hemolytic activity. These results underscore the hydrogel’s safety for biomedical applications. Furthermore, the hydrogel exhibited significant cytotoxicity against cancer cells, with marked reductions in cell viability observed after 24 and 48 h of incubation (Fig. 8). This in vitro cytotoxicity against cancer cells positions the hydrogel as a promising candidate for controlled drug release systems and targeted cancer therapies.
Table 1 compares the most significant reports in the literature on drug release using CNT-based hydrogels with the present study. In all these systems, the incorporation of CNTs into the hydrogel matrix has enhanced their physicochemical properties. For instance, CNTs has enhanced electrical conductivity in responsive hydrogels27mechanical properties in injectable hydrogels48infrared absorption in NIR-responsive hydrogels16,18and both mechanical reinforcement and crosslinking formation in the current hydrogel25. All of these hydrogel systems demonstrated controlled drug release profiles. Specifically, stimulus-responsive hydrogels regulated drug release through photothermal effects16,18 or electrical stimulation27. Additionally, injectable hydrogels employed multiple factors to control drug release48. Moreover, in this study, drug release is primarily governed by the acidic pH. Furthermore, biocompatibility assessments were conducted on various cell lines, along with additional evaluations distinguishing their effects from those on cancer cells.
The present study exhibits several distinct advantages over previous works, as detailed in Table 1:
-
1.
Minimal precursor composition and simple one-step synthesis: Hydrogel formation requires only two precursors and follows a straightforward, one-step method, reducing concerns about material toxicity and solvent-related issues while enhancing time efficiency. The use of an extremely low concentration of carbon nanotubes (0.16 wt%) and their partial degradation during hydrogel formation and crosslinking significantly minimizes toxicity.
-
2.
Highly porous structure with multiple micro-networks: The hydrogel features a well-developed porous structure with interconnected micro-networks both inside the pores and on the surface.
-
3.
Exceptional swelling capacity: The hydrogel demonstrated the highest swelling ratio, exceeding 1000% in all tested environments, including distilled water and buffer solutions with pH 5.5 and 7.4.
-
4.
pH-responsive sustained drug release: The hydrogel enabled the controlled release of doxorubicin, which was significantly higher in acidic conditions; nearly three times greater than in physiological pH environments.
-
5.
Excellent biocompatibility and selective cytotoxicity: The hydrogel exhibited no toxicity at any tested concentration, as confirmed by hemolysis assays. Moreover, DOX-loaded hydrogel showed significant cytotoxicity against cancer cells at 400 µg/mL, resulting in 57% cell death after 24 h and 63% after 48 h of incubation.
Conclusions
In this study, a biocompatible PAM/O-MWCNT nanocomposite hydrogel film was successfully synthesized via free radical polymerization and utilized for the in vitro release of DOX. Morphological analysis using FESEM confirmed the presence of microstructures and interconnected micro-networks within the hydrogel matrix, which play a crucial role in drug release. The porous hydrogel exhibited excellent swelling behavior and structural stability in buffer solutions (pH 5.5 and 7.4) at 37 °C over a 72-hour period. The drug release capability of DOX was evaluated in buffer solutions at pH 5.5 and 7.4 at 37 °C for 72 h, demonstrating a sustained release profile, particularly at acidic pH. The drug loading efficiency (EE) and loading capacity (LC) were determined to be 83.4% and 5.11%, respectively, indicating efficient DOX incorporation facilitated by hydrogen bonding and π–π stacking interactions. The MTT assay confirmed the non-toxicity of the hydrogel to HEK-293 cells, showing high cell viability (> 87%) even at a concentration of 400 µg/mL after 48 h. Notably, the DOX-loaded hydrogel demonstrated significant anticancer activity against MCF-7 cells, highlighting its therapeutic potential. Biocompatibility evaluations, including the brine shrimp lethality and hemolysis assays, confirmed the non-toxic nature and hemocompatibility of the hydrogel, further supporting its safety for biomedical applications. Overall, these findings highlight the promising potential of the synthesized nanocomposite hydrogel for drug delivery applications, offering a biocompatible and efficient platform for controlled drug release.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Ahmed, E. M. & Hydrogel Preparation, characterization, and applications: A review. J. Adv. Res. 6, 105–121 (2015).
Shahzadi, I. et al. Formation of biocompatible mgo/cellulose grafted hydrogel for efficient bactericidal and controlled release of doxorubicin. Int. J. Biol. Macromol. 220, 1277–1286 (2022).
Rahman, M. S. et al. Morphological characterization of hydrogels. Cellulose-based Superabsorbent Hydrogels, 819–863 (2019).
Kesharwani, P., Bisht, A., Alexander, A., Dave, V. & Sharma, S. Biomedical applications of hydrogels in drug delivery system: an update. J. Drug Deliv. Sci. Technol. 66, 102914 (2021).
Bernhard, S. & Tibbitt, M. W. Supramolecular engineering of hydrogels for drug delivery. Adv. Drug Deliv. Rev. 171, 240–256 (2021).
Vigata, M., Meinert, C., Hutmacher, D. W. & Bock, N. Hydrogels as drug delivery systems: A review of current characterization and evaluation techniques. Pharmaceutics 12, 1188 (2020).
Yu, Y. et al. Recent advances in thermo-sensitive hydrogels for drug delivery. J. Mater. Chem. B. 9, 2979–2992 (2021).
Cheng, Y., Zhang, H., Wei, H. & Yu, C. Y. Injectable hydrogels as emerging drug-delivery platforms for tumor therapy. Biomaterials Sci. 12, 1151–1170 (2024).
Ghasemiyeh, P. & Mohammadi-Samani, S. Hydrogels as drug delivery systems; pros and cons. Trends Pharm. Sci. 5, 7–24 (2019).
Vashist, A. et al. Advances in carbon nanotubes–hydrogel hybrids in nanomedicine for therapeutics. Adv. Healthc. Mater. 7, 1701213 (2018).
Sable, H. et al. Strategically engineering advanced nanomaterials for heavy-metal remediation from wastewater. Coord. Chem. Rev. 518, 216079 (2024).
Ahmad, N. et al. Nanoparticles incorporated hydrogels for delivery of antimicrobial agents: developments and trends. RSC Adv. 14, 13535–13564 (2024).
Yaghoubi, A. & Ramazani, A. Anticancer DOX delivery system based on cnts: functionalization, targeting and novel technologies. J. Controlled Release. 327, 198–224 (2020).
Heister, E. et al. Triple functionalisation of single-walled carbon nanotubes with doxorubicin, a monoclonal antibody, and a fluorescent marker for targeted cancer therapy. Carbon 47, 2152–2160 (2009).
Kouser, R., Vashist, A., Zafaryab, M., Rizvi, M. A. & Ahmad, S. Biocompatible and mechanically robust nanocomposite hydrogels for potential applications in tissue engineering. Mater. Sci. Engineering: C. 84, 168–179 (2018).
Dong, X. et al. Thermosensitive hydrogel loaded with chitosan-carbon nanotubes for near infrared light triggered drug delivery. Colloids Surf., B. 154, 253–262 (2017).
Wei, C. et al. Simultaneous fluorescence imaging monitoring of the programmed release of dual drugs from a hydrogel-carbon nanotube delivery system. Sens. Actuators B. 273, 264–275 (2018).
García Verdugo, K. F. et al. Nanocomposite hydrogels based on Poly (vinyl alcohol) and carbon nanotubes for NIR-Light triggered drug delivery. ACS Omega. 9, 11860–11869 (2024).
Zhong, Q. et al. Smart DEA–QCGM–CNT hydrogels with temperature-and NIR-responsive behavior achieved by the synergy between CNT and QCGM for wound dressing. Mater. Adv. 3, 2568–2582 (2022).
Saeednia, L., Yao, L., Cluff, K. & Asmatulu, R. Sustained releasing of methotrexate from injectable and thermosensitive chitosan–carbon nanotube hybrid hydrogels effectively controls tumor cell growth. ACS Omega. 4, 4040–4048 (2019).
Francis, A. P. & Devasena, T. Toxicity of carbon nanotubes: A review. Toxicol. Ind. Health. 34, 200–210 (2018).
Peng, Z. et al. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: A review. Environ. Int. 134, 105298 (2020).
Chen, M., Qin, X. & Zeng, G. Biodegradation of carbon nanotubes, graphene, and their derivatives. Trends Biotechnol. 35, 836–846 (2017).
Rosca, I. D., Watari, F., Uo, M. & Akasaka, T. Oxidation of multiwalled carbon nanotubes by nitric acid. Carbon 43, 3124–3131 (2005).
Yaghoubi, A., Ramazani, A. & Ghasemzadeh, H. Synthesis of physically crosslinked PAM/CNT flakes nanocomposite hydrogel films via a destructive approach. RSC Adv. 11, 39095–39107 (2021).
Wang, Q., Zhang, Y., Ma, Y., Wang, M. & Pan, G. Nano-crosslinked dynamic hydrogels for biomedical applications. Materials Today Bio, 100640 (2023).
Park, S. Y., Kang, J. H., Kim, H. S., Hwang, J. Y. & Shin, U. S. Electrical and thermal stimulus-responsive nanocarbon-based 3D hydrogel sponge for switchable drug delivery. Nanoscale 14, 2367–2382 (2022).
Wang, F. Z. et al. Biocompatible polymeric nanocomplexes as an intracellular stimuli-sensitive prodrug for type-2 diabetes combination therapy. Biomaterials 73, 149–159 (2015).
Javanbakht, S. & Mohammadi, R. Double cross-linking oxidized sodium alginate with Ag-based metal-organic framework and borax as an antibacterial spray-filming hydrogel for bacterial barrier. Carbohydr. Polym. Technol. Appl. 9, 100629 (2025).
Dong, W. et al. Superior mechanical properties of double-network hydrogels reinforced by carbon nanotubes without organic modification. Int. J. Mol. Sci. 14, 22380–22394 (2013).
Li, T. et al. Hybrid double-network hydrogels with excellent mechanical properties. New J. Chem. 44, 16569–16576 (2020).
Li, J. & Mooney, D. J. Designing hydrogels for controlled drug delivery. Nat. Reviews Mater. 1, 1–17 (2016).
Yang, W. J. et al. Nanogel-incorporated injectable hydrogel for synergistic therapy based on sequential local delivery of combretastatin-A4 phosphate (CA4P) and doxorubicin (DOX). ACS Appl. Mater. Interfaces. 10, 18560–18573 (2018).
Taghikhani, A., Babazadeh, M., Davaran, S. & Ghasemi, E. Facile Preparation of a pH-sensitive biocompatible nanocarrier based on magnetic layered double hydroxides/cu MOFs-chitosan crosslinked к-carrageenan for controlled doxorubicin delivery to breast cancer cells. Colloids Surf., B. 243, 114122 (2024).
Carlos Ruiz-Ruiz, J., Ramón-Sierra, J., Arias-Argaez, C., Magaña-Ortiz, D. & Ortiz-Vázquez, E. Antibacterial activity of proteins extracted from the pulp of wild edible fruit of Bromelia pinguin L. Int. J. Food Prop. 20, 220–230 (2017).
Sun, Z., Song, C., Wang, C., Hu, Y. & Wu, J. Hydrogel-based controlled drug delivery for cancer treatment: a review. Mol. Pharm. 17, 373–391 (2019).
Narayanaswamy, R. & Torchilin, V. P. Hydrogels and their applications in targeted drug delivery. The Road. Nanomed. Precision Medicine, 1117–1150 (2020).
Arshad, R. et al. Nanomaterials as an advanced nano-tool for the doxorubicin delivery/Co-Delivery—A comprehensive review. J. Drug Deliv. Sci. Technol. 83, 104432 (2023).
Olusanya, T. O., Ahmad, H., Ibegbu, R. R., Smith, D. M., Elkordy, A. A. & J. R. & Liposomal drug delivery systems and anticancer drugs. Molecules 23, 907 (2018).
Makwana, V., Karanjia, J., Haselhorst, T., Anoopkumar-Dukie, S. & Rudrawar, S. Liposomal doxorubicin as targeted delivery platform: current trends in surface functionalization. Int. J. Pharm. 593, 120117 (2021).
Aloss, K. & Hamar, P. Recent preclinical and clinical progress in liposomal doxorubicin. Pharmaceutics 15, 893 (2023).
Zhao, N., Woodle, M. C. & Mixson, A. J. Advances in delivery systems for doxorubicin. J. Nanomed. Nanatechnol. 9, 519 (2018).
Bisht, A. et al. A comprehensive review on doxorubicin: mechanisms, toxicity, clinical trials, combination therapies and nanoformulations in breast cancer. Drug Delivery Translational Research, 1–32 (2024).
Gull, N. et al. In vitro study of chitosan-based multi-responsive hydrogels as drug release vehicles: A preclinical study. RSC Adv. 9, 31078–31091 (2019).
Dhanka, M., Shetty, C. & Srivastava, R. Injectable methotrexate loaded Polycaprolactone microspheres: physicochemical characterization, biocompatibility, and hemocompatibility evaluation. Mater. Sci. Engineering: C. 81, 542–550 (2017).
Pawar, V., Dhanka, M. & Srivastava, R. Cefuroxime conjugated Chitosan hydrogel for treatment of wound infections. Colloids Surf., B. 173, 776–787 (2019).
Sethi, S. et al. Cross-linked Xanthan gum–starch hydrogels as promising materials for controlled drug delivery. Cellulose 27, 4565–4589 (2020).
Gangrade, A. & Mandal, B. B. Injectable carbon nanotube impregnated silk based multifunctional hydrogel for localized targeted and on-demand anticancer drug delivery. ACS Biomaterials Sci. Eng. 5, 2365–2381 (2019).
Acknowledgements
This work is funded by “Iran National Science Foundation (INSF) under project No. 4028330” and the “University of Zanjan”. We gratefully acknowledge the “Iran National Science Foundation (INSF)” and the “University of Zanjan”. In addition, the present research has been done in line with Alireza Yaghoubi PhD thesis.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by A.Y., A.R., and H.G. A.Y. wrote the first draft of the manuscript and was responsible for the project. A.R. and M.S were responsible for submission. A.R, H.G. and M.S. were responsible for guidance and supervision. E.M was responsible for biological tests and clinical data. A.R. was responsible for funding acquisition. All authors reviewed the manuscript and agreed on the final version of the article to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yaghoubi, A., Ramazani, A., Sillanpaa, M. et al. Biocompatible porous PAM/CNT nanocomposite hydrogel films for sustained drug delivery and cancer therapy. Sci Rep 15, 22387 (2025). https://doi.org/10.1038/s41598-025-05473-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05473-4