Abstract
Recently, men with overactive bladder have been prescribed mirabegron and tamsulosin for the treatment of benign prostatic hyperplasia. Highly efficient and environmentally sustainable spectrophotometric methods have been developed for the accurate determination of mirabegron and tamsulosin in their pure forms as well as within pharmaceutical formulations. This study presents three effective and simple spectrophotometric methods for the simultaneous quantification of mirabegron and tamsulosin. The current protocols have demonstrated validation for linearity across concentration ranges of 3–20 µg/mL for mirabegron and 2–40 µg/mL for tamsulosin, utilizing dual wavelength, ratio difference, and derivative ratio techniques. The coefficients of determination exceeded 0.999. The validation of these methodologies was conducted in accordance with the guidelines set forth by the International council for Harmonization (ICH). Quality control laboratories may utilize existing techniques to identify the binary combination because of their high accuracy and cheap cost. The evaluation of the environmental sustainability of the established approaches was conducted using AGREE, GAPI, MOGAPI and whiteness revealing their notable eco-friendliness. The proposed method was deemed practical after the evaluation carried out with the Blue Applicability Grade Index (BAGI) assessment.
Similar content being viewed by others
Introduction
Benign prostatic hyperplasia (BPH) and overactive bladder syndrome (OAB) are prevalent conditions that often lead to lower urinary tract symptoms (LUTS) and diminished quality of life, particularly among the elderly1,2,3. This medical issue is prevalent among adults aged 40 and older, though it can also impact children and adolescents4. A research investigation overseen in 2011 involving 10,000 individuals from Europe found that approximately 36% of males and 43% of females aged 40 and older displayed symptoms consistent with OAB5. According to the National Overactive Bladder Evaluation (NOBLE) program, nearly 33 million Americans are affected by (OAB). The OAB plays a crucial role in shaping economic policy. In 2005, a study conducted in the US estimated that the yearly costs associated with individuals suffering from OAB exceeded $12 billion USD. This expenditure encompasses indirect costs, including a temporary decline in productivity6. Sometimes, α1-adrenergic receptor blockers (α1B) are used to treat (LUTS) in men who have been diagnosed with (BPH). Even with the (α1B) treatment, symptoms of overactive bladder may persist7. The 2015 guideline from the European Urologic Association advises the use of antimuscarinics or beta-3-adrenergic receptor agonists (β3-ARs) in men diagnosed with BPH to address moderate to severe LUTS, particularly those associated with bladder storage dysfunction. In situations where single treatments fail to provide symptom relief, it is essential to implement a combined therapy approach8.
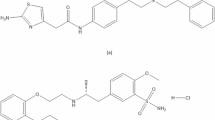
Mirabegron (MIR) is a recently approved medicine by the FDA for the treatment of idiopathic overactive bladder9. Mirabegron functions as a β3 specific receptor agonist, exerting its effects through the relaxation of the detrusor muscle. Regarding the chemical composition of the compound, MIR can be described as (N-[4-[2-[[(2R)-2-hydroxy-2-phenylethyl] amino] ethyl] phenyl] acetamide)10 (Fig. 1a).
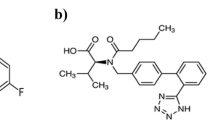
Tamsulosin (TAM), is α1-adrenoceptor antagonist with the chemical formula 5-[(2R)-2-[2-(2-ethoxyphenoxy) ethylamino]propyl]l.2-methoxybenzene-1-sulfonamide11 that is employed in the management of lower urinary tract symptoms linked to benign prostatic hyperplasia (BPH) (Fig. 1b).
Several studies have shown that MIR is an effective and safe adjunct therapy to TAM for alleviating symptoms of OAB resulting from BPH in males, with a low incidence of adverse effects12. Current clinical investigations indicate that the combination of medication such as MIR and TAM for overactive bladder (OAB) has shown improvements in symptom relief without further side effects. The combination treatment of TAM and MIR is anticipated to undergo comprehensive investigation as an alternative to traditional pharmaceuticals for patients who are unable to endure the constraints of existing drug regimens or adverse drug reactions13. Numerous chromatographic studies have been conducted on MIR detection14,15,16,17,18,19,20. and estimation of TAM21,22,23,24,25,26,27,28,29,30, According to the literature, there are HPTLC method31 and HPLC method32 that have been documented for measuring MIR and TAM simultaneously.
Materials and methods
Instrumentation
The UV spectrophotometer was equipped with Jasco spectrum management software. Absorption spectra for test solutions within the wavelength range of 200–400 nm were measured using quartz cells with a diameter of 1 cm.
Materials, reagents and solvents
Reference standards of TAM (99.75%) and MIR (99.88%) were procured from LGC Standards. The pharmaceutical preparations Betmiga 50 mg® and Tamsul 0.4 mg® were supplied from a community pharmacy. Distilled water and ethanol (spectroscopy grade, EL-Gomhouria Company, Egypt) were the solvents used.
Preparation of standard stock solutions
Weighing 10 mg of each reference standard and then accurately transfer it into individual volumetric flask 100 ml produced MIR and TAM stock standard solutions with a concentration 100 µg/mL. The compounds were then dissolved and combined in 20 mL of ethanol, then complete the volume to mark with distilled water.
Preparation of the laboratory prepared mixture
Aliquots of MIR and TAM were transferred from their stock standard solutions into a 10 mL volumetric flask . Then topped to the mark with distilled water and stirred well.
Experiment design
Determining linearity ranges and constructing calibration curves
The objective is to achieve concentrations ranging from 3 to 20 µg/mL for MIR and 2 to 40 µg/mL for TAM in Dual Wavelength (DW), Ratio Difference (RD), and Derivative Ratio (DD1). The absorption spectra of the resulting solutions for both compounds were documented within the range of 200 to 400 nm, employing mixture of distilled water and ethanol as a blank .
Dual wavelength spectrophotometric approach (DW)
The curve of calibration was created by measuring the absorbance changes between 240 and 266 nm and plotting these against different concentrations of MIR. Absorbance values at 230 and 262 nm were obtained from TAM spectra to quantify TAM, and the corresponding differences were plotted against TAM concentrations. The charts were utilized to formulate regression equations.
Ratio difference spectrophotometric approach (RD)
The MIR zero-absorption spectra were acquired, stored, and divided using the 10 µg/mL TAM spectrum as a divisor. Amplitudes of the resultant spectra have been estimated at 220 and 248 nm, and the differences were computed to construct the calibration curve. Zero-absorption spectra of TAM were recorded, saved, and subsequently divided using 7 µg/mL MIR as a divisor. Amplitudes of the resultant spectra have been estimated at 221 nm and 295 nm, and the difference was computed to construct the calibration curve, which was then utilized for regression equation computation.
First derivative ratio spectrophotometric approach (DD1)
In order to create the ratio spectra, the measurements of MIR’s absorption were divided by 10 µg/mL of TAM. One further step was to produce the first derivative of the ratio spectra. Amplitudes of the resultant derivative spectra of MIR have been estimated at 242 nm. To determine TAM in the context of MIR, divide the stored TAM zero-absorption spectra by 7 µg/mL of MIR to obtain the ratio spectra. One further step was to produce the first derivative of the ratio spectra. Amplitudes of the resultant derivative spectra of TAM have been estimated at 302 nm.
Analysis of laboratory-prepared mixture
The spectra of the laboratory-prepared mixtures was processed as outlined in the experiment design section. The concentrations of each analyte were determined using established regression models.
Quantification of the pharmaceutical dosage forms
The formulation of Betmiga® involved careful weighing, crushing, and mixing ten tablets. The average weight of a single tablet is 257.5 mg, which is achieved by incorporating 50 mg of MIR into a volumetric flask 100 mL. Twenty Tamslu® capsules contents were carefully weighed, and their average weights were calculated. The contents of the capsule were carefully combined and the content corresponding to a mass of 5 mg TAM was transferred to a volumetric flask 25 mL. The flasks were filled with 30 mL and 15 mL, respectively, utilizing ethanol. The flasks underwent stirring with an ultrasonic shaker for a duration of 15 min. Following the filtration process with a whatman filter, the contents of the flasks were subsequently diluted using distilled water. Aliquots of Betmiga® and Tamsul® filtrates were transferred into a volumetric flask 25 mL and subsequently diluted to the final volume with distilled water to prepare a solution of MIR and TAM.
Results and discussion
The concurrent use of MIR and TAM is noteworthy for its effectiveness in alleviating symptoms of bladder obstruction in older adults. Three distinct, delicate, simple UV-spectrophotometric approaches have been developed for the simultaneous measurement of MIR and TAM in synthetic mixtures and pharmaceutical formulations, ensuring sufficient accuracy outcomes for competent analytical methodology. The proposed UV approach is efficient, economical, eco-friendly, and precise for routine drug analysis. The spectra presented in Fig. 2 demonstrate significant overlap, indicating that MIR and TAM can be calculated using three advanced platforms. These platforms are effective in selectively eliminating interference or overlap in the combined mixing spectra without prior separation.
Methods optimization
Dual wavelength spectrophotometric approach (DW)
DW effectively reduces interference between MIR and TAM in their mixed formulations due to its simplicity and lack of requirement for specialized software33. Additionally, no further data processing is required. To mitigate interference, two wavelengths were chosen from the mixture spectra, ensuring that the absorbance difference between these wavelengths is directly proportional to the concentration of the target compound, while remaining zero for the interfering compound. The wavelengths used for determining MIR and TAM are detailed in the experiment design and depicted in Fig. 3. Regression equations were established to determine MIR and TAM concentrations by plotting the absorbance difference values at specific wavelengths of zero-order spectra against concentration ranges of 3–20 µg/mL and 2–40 µg/mL, respectively.
For ratio difference approach (RD)
Ratio Difference is a basic computational solution that uses simple mathematical calculations and doesn’t need separation34. This technique relies on the appropriate selection of divisors, ensuring minimal noise and maximum sensitivity, alongside the wavelengths utilized for computations to achieve optimal linearity relationships. MIR can be calculated in conjunction with TAM using the RD technique, achieved by dividing it by a TAM divisor of 10 µg/mL. The amplitudes of the resultant ratio spectrum at 220 and 248 nm were measured to eliminate TAM interference, and the difference in amplitude was subsequently calculated. TAM was measured in relation to MIR by using a MIR divisor of 7 µg/mL for the calculation. The amplitudes of the resultant ratio spectrum at 221 and 295 nm were measured to eliminate MIR interference, and the difference in amplitude was subsequently calculated. Regression equations were developed to ascertain MIR and TAM concentrations by graphing absorbance difference values at designated wavelengths against concentration ranges of 3–20 µg/mL and 2–40 µg/mL, respectively. (see Fig. 4).
First derivative of the ratio spectra method (DD1)
This method relies on the first derivative of ratio spectra, achieved by dividing the zero-order mixture spectra of the analyzed drugs by a specifically selected spectrum of 10 µg/mL TAM to ascertain MIR. Additionally, the zero-order absorption spectra of TAM are divided by a specifically chosen spectrum of 7 µg/mL MIR to determine TAM, after evaluating various concentrations as divisors for both drugs (Fig. 5)35.
Validation of methods
The existing approaches were created in accordance with ICH principles and requirements 36.
Linearity and range
Seven and six concentrations of MIR and TAM within linearity ranges of 3–20 µg/mL and 2–40 µg/mL, respectively, were used to evaluate the linearity of the proposed spectrophotometric methods (Table 1).
Limit of detection (LOD) and limit of quantification (LOQ)
Measurements of detection and quantification were used to assess the sensitivity of the proposed methods. Calculations for LOD and LOQ were based on the following calculations, which relied on using the standard deviation (σ) of the intercept and the slope (S) of the calibration plot given in Table 1.
LOD = 3.3 x σ / slope.
LOQ = 10 x σ / slope.
Accuracy
Three separate drug concentrations, with each concentration tested three times, were used to assess accuracy. After that, we compared the measured concentrations to the actual concentrations and found the percentage recovery (R%). The results showed that the recovery values were within the permitted range, which proves that the approaches are very accurate (Table 1).
Precision
Three distinct concentrations of the proposed drugs, within the linear range, were evaluated by three replicate studies conducted on the same day and again three days later to ascertain the intra-day and inter-day precision of the proposed methods; the findings are shown in Table 1.
Selectivity
The approaches’ selectivity was evidenced by the quantitative measurement of MIR and TAM in their laboratory-prepared combinations, as outlined in (Table 2).
Pharmaceutical formulations application
The three approaches (DW, RD, DD1) were utilized in the application of dosage forms. Analysis of MIR in tablet form and TAM in capsule form demonstrated an average recovery rate of 100.70%, 99.80%, 100.40% and 99.78%,99.86%, 99.62%, correspondingly (Table 3).
The student’s t-test and the F-test were used to compare the suggested methods with the published method statistically. Table 3 shows that there were no significant differences between the two approaches. Methods were systematically compared against one another and published results through various statistical techniques. The test of interval plot was used37. Plots with vertical lines representing confidence intervals imply that the interval’s mean is located near its midpoint. According to the graphs, there are no appreciable variations in the group intervals. (Fig. 6). One useful technique for visualizing data is the boxplot, which illustrates the distribution of data across various sets38. The boxplots for the established and published approaches are shown. The central box depicts the interquartile range, this comprises a line representing the median, upper lines indicating higher numbers, and whiskers showing lower values. The boxplot shows the distribution of data within each group (Fig. 6). The normal probability plot offers an additional method for determining the normality of data distribution (Fig. 6). If a straight line connects the majority of data sets, the data follows a normal distribution37. Tukey’s simultaneous significant difference test is the most important statistical technique39. This tool effectively identifies variations in mean values across different groups. The data interval for each group is illustrated in (Fig. 6) as a horizontal line, with a dot indicating the mean value of the corresponding data group. The overlap of the intervals suggested that the average values of the planned and reported methods were not significantly different.
Assessment of ecological effect and comparison to reported analytical technique
During the development of analytical procedures, evaluating their ecological impact was essential to ensure a transparent and factual assessment for future comparisons of proposed and reported methodologies. The Green Analytical Procedure Index (GAPI)40,41,42,43 was utilized to evaluate the environmental sustainability of the analytical methodologies. The environmental implications of each step in analytical measurement, encompassing preparation, equipment use, and waste generation and management, are evaluated by GAPI, which comprises fifteen unique domains illustrated by five pentacle shapes. The characteristics are illustrated on the pentagram, where the colors green, yellow, and red signify minimum, moderate, and significant environmental impact, respectively. The suggested spectrophotometric techniques exhibit numerous benefits in comparison to the previously documented HPTLC chromatographic approach. The considerations encompass the application of eco-friendly solvents, a reduction in solvent consumption during analysis, lower toxicity levels of solvents, decreased energy demands for the spectrophotometric apparatus, minimized waste generation, and solvent degradation (Table 4).
Using the GAPI statistic to compare many methodologies does not provide a comprehensive result. The MoGAPI program amalgamates the exact total score of the analytical Eco Scale with the benefits of GAPI’s visual effect. As illustrated in Table 4, the software program was implemented to evaluate the ecological sustainability of the recommended analytical and HPTLC published methods44.
AGREE features a clock-shaped diagram segmented into twelve parts, where each part represents one of the twelve principles of Green Analytical Chemistry45,46. The proposed and reported HPTLC methods were assessed using the AGREE tool. The comparison presented in Table 4 accurately outlines the proposed methodology against previously reported methods, employing real data for analysis. The two methodologies demonstrate a red zone (3) on the AGREE scale. A significant issue has arisen due to offline sampling and the transportation of samples to quality control laboratories, stemming from the division between pharmaceutical manufacturing and quality control sites. The proposed spectrophotometric methods exhibit several advantages compared to conventional chromatographic techniques, including fewer stages, lower waste volume, reduced energy consumption of the spectrophotometric device, elimination of toxic solvents, and decreased dependence on organic solvents (Table 4).
The proposal introduces an innovative metric, the Blue Applicability Grade Index (BAGI), aimed at evaluating the feasibility of an analytical method47,48,49. The BAGI framework, when utilized in conjunction with recognized green metrics, elucidates the practical dimensions of White Analytical Chemistry. The color gradient of the pictogram reflects the degree of alignment with the established criteria. For the strategy to be deemed “practical,” it must attain a score of 60 or above. The proposed methodology demonstrates superior performance relative to the previously mentioned HPTLC method, as illustrated in Table 4.
The proposed method was compared to two established methods, including HPTLC and HPLC approaches using the RGB 12 model50, both of them had been published before. It is among the most recent multi-criteria evaluation method available and is in line with White Analytical Chemistry’s (WAC) principles. It considers more than only the influence on the environment. It is composed of 12 algorithms divided into three colored groups and is based on a more flexible approach. Numerous evaluation indicators for important aspects of the analytical process make up each colored category. Table S1 displays the findings of this comprehensive whiteness evaluation.
Conclusion
The suggested approaches provide spectrophotometric procedures that are simple, sensitive, and accurate for MIR and TAM. In recent clinical investigations, the combined therapy of MIR and TAM for over active bladder (OAB) has demonstrated advantages in terms of symptom alleviation without creating additional side effects. Utilizing the spectrophotometric methods that have been provided, it is possible to do an analysis on the overlapping spectra of MIR and TAM, in addition to more complex combinations, utilizing a wide range of tools. The proposed and reported spectrophotometric techniques did not vary statistically significantly, according to a statistical analysis using the t-test and the F-test. In order to assess the impact that these methods have on the environment and determine whether or not they are feasible, the GAPI index, MOGAPI AGREE calculator, BAGI and whiteness evaluation were utilized.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Alexandre, E. C. et al. Mirabegron relaxes urethral smooth muscle by a dual mechanism involving β3-adrenoceptor activation and α1‐adrenoceptor Blockade. Br. J. Pharmacol. 173, 415–428. https://doi.org/10.1111/bph.13367 (2016).
Leron, E., Weintraub, A. Y., Mastrolia, S. A. & Schwarzman, P. Overactive bladder syndrome: evaluation and management. Curr. Urol. 11, 117–125. https://doi.org/10.1159/000447205 (2018).
Abrams, P., Kelleher, C. J., Kerr, L. A. & Rogers, R. G. Overactive bladder significantly affects quality of life. Am. J. Manag Care. 6, S580–S590 (2000).
Milsom, I. et al. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 87, 760–766. https://doi.org/10.1046/j.1464-410x.2001.02228.x (2001).
Stewart, W. F. et al. Prevalence and burden of overactive bladder in the united States. World J. Urol. 20, 327–336. https://doi.org/10.1007/s00345-002-0301-4 (2003).
Scarneciu, I. et al. Overactive bladder: A review and update. Exp. Ther. Med. 22, 1–8. https://doi.org/10.3892/etm.2021.10879 (2021).
Abrams, P. et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the international continence society. Eur. Urol. 61, 37–49. https://doi.org/10.1016/S0090-4295(02)02243-4 (2003).
Gratzke, C. et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur. Urol. 67, 1099–1109. https://doi.org/10.1016/j.eururo.2014.12.038 (2015).
Helou, E. E. et al. The use of mirabegron in neurogenic bladder: a systematic review. World J. Urol. 38, 2435–2442. https://doi.org/10.1007/s00345-019-03040-x (2020).
Bahgat, E. A., Saleh, H., Hendawy, H. A. M. & Darwish, I. M. Challenging electroanalytical method for the determination of mirabegron in biological samples and pharmaceutical formulations. Microchem. J. 200, 110258. https://doi.org/10.1016/j.microc.2024.110258 (2024).
Kamel, E. B. Two green chromatographic methods for the quantification of Tamsulosin and Solifenacin along with four of their impurities. J. Sep. Sci. 45, 1305–1316. https://doi.org/10.1002/jssc.202100980 (2022).
Kang, T. W. & Chung, H. C. Add-on treatment with mirabegron May improve quality of life in patients with benign prostatic hyperplasia complaining of persistent storage symptoms after Tamsulosin monotherapy. Ther. Adv. Urol. 12, 1–7. https://doi.org/10.1177/1756287220974130 (2020).
Shin, W., Yang, A. Y., Yoo, H. & Kim, A. Drug–Drug interactions between Tamsulosin and mirabegron in healthy individuals do not affect pharmacokinetics and hemodynamic parameters significantly. Pharmaceuticals 16, 1457. https://doi.org/10.3390/ph16101457 (2023).
Mishra, S., Surekha, N. & Chauhan, A. Development and validation of stability indicating RP-HPLC method for simultaneous estimation of silodosin and mirabegron in synthetic mixture, in: Ann Pharm Fr, Elsevier, : pp. 243–262. (2024). https://doi.org/10.1016/j.pharma.2023.12.013
Lou, Y. et al. Simultaneous quantification of mirabegron and vibegron in human plasma by HPLC-MS/MS and its application in the clinical determination in patients with tumors associated with overactive bladder. J. Pharm. Biomed. Anal. 240, 115937. https://doi.org/10.1016/j.jpba.2023.115937 (2024).
Kadam, M. M. M., Singh, R. P. & Charde, M. S. Development and validation of stability indicating UHPLC method for the quantitative Estimation of mirabegron and Solifenacin succinate in pharmaceutical dosage form. J. Pharm. Negat. Results. 6727–6737. https://doi.org/10.47750/pnr.2022.13.S07.815 (2023).
Andhale, G., Shankarpure, M., Kadam, J., Shelar, M. & Singh, S. RP-HPLC method development and validation for the simultaneous Estimation of mirabegron and Solifenacin succinate in pharmaceutical dosage form. NeuroQuantology 20, 2652. https://doi.org/10.14704/nq.2022.20.9.NQ44310 (2022).
Rezaei, M. & Ramazani, A. RP-HPLC method development and validation for the quantitative Estimation of mirabegron in extended-release tablets. J. Med. Chem. Sci. 1, 36–40 (2018).
Yehia, A. M., Sami, I., Riad, S. M. & El-Saharty, Y. S. Comparison of two stability-indicating chromatographic methods for the determination of mirabegron in presence of its degradation product. Chromatographia 80, 99–107. https://doi.org/10.1007/s10337-016-3210-1 (2017).
Van Teijlingen, R. et al. Development and validation of LC–MS/MS methods for the determination of mirabegron and its metabolites in human plasma and their application to a clinical Pharmacokinetic study. J. Chromatogr. B. 887, 102–111. https://doi.org/10.1016/j.jchromb.2012.01.018 (2012).
Sangshetti, J. N., Chivte, D. K., Imran, M. & Mahaparale, P. Development of Hplc method for determination of Tamsulosin using quality by design (QBD) approach. Eur. Chem. Bull. 8, 409–414. https://doi.org/10.17628/ecb.2019.8.409-415 (2019).
Afshari, R. A. & Qomi, M. A novel application of three phase Hollow fiber based liquid phase Microextraction (HF-LPME) for the HPLC determination of Tamsulosin from biological fluids. Curr. Pharm. Anal. 12, 258–265 (2016).
Ganthi, H. K. R. et al. Stability indicating HPLC method for quantification of Solifenacin succinate & Tamsulosin hydrochloride along with its impurities in tablet dosage form. Am. J. Analyt Chem. 7, 840–862. https://doi.org/10.4236/ajac.2016.711073 (2016).
Nasare, M. K. et al. Simultaneous determination of finasteride and Tamsulosin in combined dosage form by using RP-HPLC method. J. Liq Chromatogr. Relat. Technol. 37, 1176–1186. https://doi.org/10.1080/10826076.2013.778637 (2014).
Ishaq, B. M., Prakash, K. V. & Mohan, G. K. Simultaneous determination of dutasteride and tamsulosin in pharmaceutical dosage forms by RP-HPLC, in: International Conference on Harmonization (ICH) Analytical Method Validation Guidelines, : p. 16. (2014).
Kumar, G. S. & Kumar, B. S. P. Stability-indicating RP-HPLC method for determination of Tamsulosin Hcl in pharmaceutical dosage form. J. Basic. Clin. Pharm. 3, 255. https://doi.org/10.4103%2F0976-0105.103817 (2012).
Mhamunkar, S. M., Vyavaharkar, R. Y. & Bhoir, S. I. RP-HPLC method development and validation for the simultaneous Estimation of Tamsulosin HCl and Tolterodine tartrate in pharmaceutical dosage form. Int. J. Pharm. Pharm. Sci. 4, 319–322 (2012).
Choi, C. I. et al. Determination of Tamsulosin in human plasma by liquid chromatography/tandem mass spectrometry and its application to a Pharmacokinetic study. J. Chromatogr. B. 909, 65–69. https://doi.org/10.1016/j.jchromb.2012.10.012 (2012).
Thimmaraju, M. K., Rao, V. & Gurrala, S. RP HPLC method for the determination of finasteride and Tamsulosin in bulk and pharmaceutical formulations. Pharm. Lett. 3, 79–86 (2011).
Kumari, R., Dash, P. P., Lal, V. K., Mishra, A. & Murthy, P. N. RP–HPLC method for the Estimation of Tamsulosin hydrochloride in tablet dosage form. Indian J. Pharm. Sci. 72, 785. https://doi.org/10.4103%2F0250-474X.84596 (2010).
Abou El-Alamin, M. M., Toubar, S. S., Mohamed, D. A. & Helmy, M. I. Development of green HPTLC method for simultaneous determination of a promising combination Tamsulosin and mirabegron: stability-indicating assay was examined. BMC Chem. 17, 1–18. https://doi.org/10.1186/s13065-023-01043-9 (2023).
Bahgat, E. A., Saleh, H., Darwish, I. M. & El-Abassy, O. M. Green HPLC technique development for the simultaneous determination of the potential combination of mirabegron and Tamsulosin. Sci. Rep. 15 https://doi.org/10.1038/s41598-025-89216-5 (2025).
Bahgat, E. A., Hashem, H., Saleh, H., Kamel, E. B. & Eissa, M. S. Manipulation and processing of spectral signals for the assay of the newly authorized mixture of bupivacaine/meloxicam using fully green solvents and a comparative green evaluation supporting the greenness and sustainability of the developed smart spectro. J. AOAC Int. 106, 1056–1069. https://doi.org/10.1093/jaoacint/qsad029 (2023).
Elhassan, M. M., Mahmoud, A. M., Hegazy, M. A. & Mowaka, S. Univariate and multivariate determination of Dapagliflozin and saxagliptin in bulk and dosage form. J. AOAC Int. 106, 794–803. https://doi.org/10.1093/jaoacint/qsac143 (2023).
Sebaiy, M. M., Sobhy, M. & Mattar, A. A. Different techniques for overlapped UV spectra resolution of some co-administered drugs with Paracetamol in their combined pharmaceutical dosage forms. Spectrochim Acta Mol. Biomol. Spectrosc. 224, 117429. https://doi.org/10.1016/j.saa.2019.117429 (2020).
Step & ICH Q2 (R2. November) VALIDATION OF ANALYTICAL PROCEDURES: Text and methodology (2023). www.ema.europa.eu/contact
Ibrahim, H., El-Abassy, O. M., Abdellatef, H. E., Hendawy, H. A. M. & El-Sayed, H. M. Simultaneous analysis of two drugs used as supportive treatment for COVID-19: comparative statistical studies and analytical ecological appraisal. BMC Chem. 16, 1–15. https://doi.org/10.1186/s13065-022-00860-8 (2022).
Ibrahim, A. E. et al. Eco-friendly and sensitive HPLC and TLC methods validated for the determination of betahistine in the presence of its process-related impurity. Separations 9, 49. https://doi.org/10.3390/separations9020049 (2022).
Elmasry, M. S., Hassan, W. S., El-Mammli, M. Y. & Badrawy, M. Earth friendly spectrophotometric methods based on different manipulation approaches for simultaneous determination of aspirin and Omeprazole in binary mixture and pharmaceutical dosage form: comparative statistical study. Spectrochim Acta Mol. Biomol. Spectrosc. 266, 120436. https://doi.org/10.1016/j.saa.2021.120436 (2022).
Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: green analytical procedure index. Talanta 181, 204–209. https://doi.org/10.1016/j.talanta.2018.01.013 (2018).
Fawzy, M. G., Hassan, W. E., Mostafa, A. A. & Sayed, R. A. Different approaches for the assessment of greenness of spectrophotometric methodologies utilized for resolving the spectral overlap of newly approved binary hypoglycemic pharmaceutical mixture. Spectrochim Acta Mol. Biomol. Spectrosc. 272, 120998. https://doi.org/10.1016/j.saa.2022.120998 (2022).
Gamal Fawzy, M., Saleh, H., Reda, A. & Bahgat, E. A. A green spectrophotometric method for the simultaneous determination of nasal binary mixture used in respiratory diseases: applying isosbestic point and chemometric approaches as a resolving tool, greenness evaluation. Spectrochim Acta Mol. Biomol. Spectrosc. 121585. https://doi.org/10.1016/j.saa.2022.121585 (2022).
Ayad, M. M., Hosny, M. M., Ibrahim, A. E., El-Abassy, O. M. & Belal, F. F. Development and validation of eco-friendly micellar HPLC method for the simultaneous determination of Hydrochlorothiazide and Valsartan in bulk powder and pharmaceutical dosage forms. J. Iran. Chem. Soc. 17, 1725–1730. https://doi.org/10.1007/s13738-020-01897-z (2020).
MansourF.R., Płotka-Wasylka, J. & Locatelli, M. Modified GAPI (MoGAPI) tool and software for the assessment of method greenness: case studies and applications. Analytica 5, 451–457. https://doi.org/10.3390/analytica5030030 (2024).
El-Sayed, H. M. et al. Safinamide detection based on Prussian blue analogue modified Solid-contact potentiometric sensor. Microchem. J. 191, 108829. https://doi.org/10.1016/j.microc.2023.108829 (2023).
Pena-Pereira, F., Wojnowski, W. & Tobiszewski, M. AGREE - Analytical greenness metric approach and software. Anal. Chem. 92, 10076–10082. https://doi.org/10.1021/acs.analchem.0c01887 (2020).
El-Abassy, O. M., Maged, K., El-Henawee, M. M. & Abd El-Hay, S. S. Development of eco-friendly spectrophotometric methods for analysis of Metformin hydrochloride and linagliptin in presence of Metformin toxic impurity in their pure and dosage forms: validation, practicality and greenness studies. Spectrochim Acta Mol. Biomol. Spectrosc. 309, 123844. https://doi.org/10.1016/j.saa.2024.123844 (2024).
Halim, M. K., Badran, O. M. & Abbas, A. E. F. Sustainable chemometric methods boosted by Latin hypercube technique for quantifying the recently FDA-approved combination of bupivacaine and meloxicam in the presence of bupivacaine carcinogenic impurity: comprehensive greenness, blueness, and whiteness. Microchem. J. 110276. https://doi.org/10.1016/j.microc.2024.110276 (2024).
Ntorkou, M., Kabir, A., Furton, K. G., Tzanavaras, P. D. & Zacharis, C. K. Sol-gel Carbowax 20 M-zwitterionic ionic liquid composite sorbent-based capsule phase Microextraction device combined with HPLC/post-column derivatization for the determination of lanreotide, a human somatostatin analogue in urine. J. Chromatogr. A. 1717, 464674. https://doi.org/10.1016/j.chroma.2024.464674 (2024).
Nowak, P. M., Wietecha-Posłuszny, R. & Pawliszyn, J. White analytical chemistry: an approach to reconcile the principles of green analytical chemistry and functionality. TRAC Trends Anal. Chem. 138, 116223. https://doi.org/10.1016/J.TRAC.2021.116223 (2021).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Omar M El-Abassy: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Resources, Investigation, Formal analysis, Data curation, Conceptualization. Hanaa Saleh: Writing – review & editing, Writing – original draft, Supervision, Methodology, Conceptualization. Islam M. Darwish: Writing – original draft, Software, Validation, Formal analysis, Investigation, Resources, . Eman A. Bahgat : Writing – review & editing, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Abassy, O.M., Saleh, H., Darwish, I.M. et al. Eco-friendly spectrophotometric quantification of the potential combination of mirabegron and tamsulosin. Sci Rep 15, 20252 (2025). https://doi.org/10.1038/s41598-025-06157-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06157-9