Abstract
This study investigates the synergistic effects of a novel triplex sintering aid system—magnesium oxide (MgO), lanthanum oxide (La2O3), and zirconium oxide (ZrO2)— on the mechanical and optical properties of transparent alumina ceramics fabricated using spark plasma sintering (SPS). The eigth specimens were sintered at 1350 °C for 10 min under 70 MPa pressure. The sample with the highest zirconia content (sample Z (100M100L100Z): 100 ppm La2O3, 100 ppm MgO and 300 ppm ZrO2) achieved a bulk density of 3.94 g/cm3, corresponding to 99.9% of the theoretical density of alumina. This sample exhibited an infrared transmission of 71.4% at 5 μm and a visible transmittance of 32% at 750 nm, surpassing values reported for single or dual sintering aid systems. Mechanical testing revealed a microhardness of 19.34 ± 0.1 GPa and a fracture toughness of 5.24 MPa.m1/2 for sample Z. Additionally, this sample demonstrated the highest flexural strength of 356.83 MPa, attributed to its finer grain size, reduced porosity, and the synergistic effects of the triplex sintering aids. The results demonstrate a promising pathway for producing high-performance transparent alumina ceramics with tailored properties for demanding optical and mechanical applications.
Similar content being viewed by others
Introduction
Alumina ceramics are highly valued for their exceptional mechanical properties, electrical insulation, and thermal stability, making them suitable for a wide range of industrial applications. Transparent alumina ceramics, in particular, are critical for use in armor, sensors, and advanced optical systems due to their impressive strength, thermal stability, and optical transparency. α-alumina is a prominent material for infrared windows, offering 50% to 70% infrared transmission in the 3–5 μm wavelength range and excellent mechanical properties. However, its low impact resistance and fracture toughness remain significant limitations. These limitations can be addressed by optimizing sintering conditions to reduce temperature and time while maintaining a fine grain structure1,2,3,4,5.
The performance of alumina ceramics can be significantly enhanced through the addition of appropriate sintering aids, particularly dual systems such as MgO-Y2O3, MgO-La2O3, or MgO-ZrO2. These systems have been shown to improve density and mechanical properties in transparent alumina3,4,5. In this study, we investigate the combined effects of three sintering aids—magnesium oxide (MgO), lanthanum oxide (La2O3), and zirconium oxide (ZrO2) —on the properties of alumina ceramics produced using the SPS method. Achieving high visible and infrared transparency in ceramics requires minimizing light scattering and ensuring that grain sizes are significantly smaller than the incident wavelength. Thus, achieving full density during sintering and preventing excessive grain growth are essential. The choice of sintering method is critical, and the SPS technique is particularly advantageous for producing materials with high density, short sintering times, and fine grain structures1,6.
In addition to sintering strategies, the use of sintering aids can inhibit grain growth, enhance density, and improve mechanical properties. However, the optimal amount of each additive must be carefully selected. Insufficient amounts may fail to achieve the desired optical properties, whereas excessive amounts can lead to porosity or secondary phase formation, thereby reducing transparency. For instance, Ge et al. demonstrated that 100 ppm La2O3 improves compactness and bulk density in alumina ceramics, reducing apparent porosity and enhancing mechanical properties7. Similarly, La3⁺ ions have been shown to curb grain growth, improving both infrared and visible transmission. However, increasing La2O3concentration beyond 100 ppm does not further inhibit grain growth7,8.
Studies have also shown that triple additive systems, such as MgO- La2O3-Y2O3, enhance real in-line transmittance (RIT, measured at normal incidence) compared to dual or additive-free systems. This improvement is attributed to the synergistic effects of the triple combination, with MgO playing a pivotal role by creating oxygen vacancies in the alumina structure, which facilitates the incorporation of Y and La atoms9.
Apak et al.10 investigated the effects of Y2O3 and MgO on the mechanical properties of alumina, producing transparent ceramics via SPS at 1300 °C and 1340 °C under 80 MPa pressure for 5 min. Their samples achieved densities ranging from 97.1% to 99.9%, and higher MgO concentrations (up to 300 ppm) resulted in increased density. Another review study reported that controlling heating rate during SPS process at 8 °C/min achieved 47% transmittance in wavelength of 640 nm11. Additionally, zirconia additives have been shown to refine alumina grain structure, though concentrations above 300 ppm do not further enhance light transmission8,12.
While extensive research has explored the effects of single and dual sintering aids (e.g., MgO, La2O3, and ZrO2) on the optical and mechanical properties of transparent alumina, no studies have comprehensively evaluated the simultaneous addition of MgO, La2O3, and ZrO2 on the microstructure, light transmission, and the full suite of mechanical properties (e.g., hardness, fracture toughness, and flexural strength) of alumina. This study aims to address this gap by investigating the synergistic effects of a triple sintering aid system on the properties of SPS-processed alumina ceramics.
Experimental
Synthesis of precursors and sintered samples
In this study, α-alumina powder with a particle size of 200 nm and high purity (99.9%) was purchased from US Research Nanomaterials Inc. The chemical precipitation process for the sintering aids involved using magnesium nitrate (Mg(NO3)2), lanthanum nitrate (La(NO3)2), and zirconium chloride (ZrOCl2) salts, all sourced from Merck, Germany. Initially, a slurry was prepared by dispersing 2.2 g of nanosized alumina in 50 mL of deionized water. Subsequently, magnesium nitrate, lanthanum nitrate, and zirconium oxychloride were added according to the compositions specified in (Table 1).
The selected concentrations of the ternary sintering aids (MgO, La2O3, and ZrO2) were optimized based on two key criteria:
Total additive limit (< 500 ppm)
To achieve optical transparency in alumina during SPS, the combined sintering aids must remain below 500 ppm. Exceeding this threshold can promote secondary phase formation or excessive grain growth, degrading transparency. This aligns with studies demonstrating that low-dose ternary systems enhance densification while minimizing light scattering.
Individual optimization of La2O3 (100 ppm)
La2O3 at 100 ppm was chosen to improve fracture toughness and thermal shock resistance, as evidenced by prior work8,13. This concentration balances grain boundary strengthening without compromising densification.
Synergistic effects of ZrO2/ MgO and MgO/La2O3
ZrO2 (50–300 ppm) was included to leverage phase transformation toughening, while MgO (100–200 ppm) suppresses abnormal grain growth. The varying ZrO2 levels were tested to identify the threshold for optimal density-pore elimination, as higher ZrO2 enhances diffusion but risks residual stresses if excessive.
The combinatorial approach ensures transparency and mechanical robustness, supported by literature on ternary systems in alumina6,9.
By adding ammonia and adjusting the pH to 9.5, hydroxide ions (Mg2⁺, La3⁺, Zr4⁺) were precipitated onto the suspended alumina particles. The resulting slurry was dried in an oven at 60 °C for 8 h, followed by calcination at 800 °C to convert the hydroxides (Mg(OH)2, La(OH)2, and Zr(OH)2) into their corresponding metal oxides (MgO, La2O3, and ZrO2.
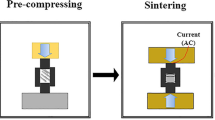
Sample consolidation was performed using a spark plasma sintering (SPS) machine (manufactured by MUT University, Iran) at a temperature of 1350 °C, with a heating rate of 50 °C/min, under a pressure of 70 MPa for 10 min. Graphite molds with a diameter of 2 cm were used for the sintering process. Pyrometer in this equipment focused on the mandrel (not mold), thus the real temperature may be 1550 °C.
Chemical etching of alumina ceramic
Preparation of etching solution
A chemical etchant was prepared by mixing 10 mL of HF (Hydrofluoric acid), 10 mL of HCl (Hydrochloric acid), and 90 mL deionized water.
Etching procedure
-
a.
The polished alumina sample was immersed in the etching solution for 10–20 s.
-
b.
After etching, the sample was immediately rinsed with warm deionized water to halt the reaction.
-
c.
To remove residual contaminants, the sample was briefly dipped in concentrated nitric acid (HNO3) and then rapidly withdrawn.
Surface characterization
The etched surface was examined using optical microscopy and scanning electron microscopy (SEM) to evaluate the microstructure and etching uniformity.
Characterization techniques
X-ray diffractometry (XRD)
Performed using an X’Pert Pro MPD device (Panalytical, Netherlands) with a copper X-ray tube (wavelength: 1.542 Å), operating at 40 kV and 25 mA. The step size was set to 0.03 degrees.
Field emission scanning electron microscopy (FE-SEM)
Structural investigations were performed using an FEI model (USA).
The grain size of the sintered transparent alumina was measured using field emission scanning electron microscopy (FE-SEM) of fracture surfaces, as this method effectively reveals grain boundaries without the need for thermal/chemical etching, which can alter microstructure. However, to ensure accuracy, the following considerations were applied:
Correction factor for fracture surface measurements
-
o
Typically grain sizes measured from fracture surfaces appear ~ 1.2–1.5 × larger than those from polished/etched surfaces due to oblique fracture angles.
-
o
A correction factor of 1.2 was applied to the raw Fe-SEM data to approximate true grain size, consistent with prior work on ceramics14.
Validation against etched surfaces
For select samples, grain sizes were cross-verified using chemically etched surfaces, confirming that corrected fracture-surface measurements agreed within ±5% (Fig. S1 in supporting information file).
Methodological rationale
-
o
Fracture-surface analysis was prioritized to avoid etching-induced artifacts (e.g., grain boundary grooving) and preserve sample integrity for optical/mechanical testing.
-
o
FE-SEM parameters: Accelerating voltage = 25 kV, working distance = 10.5. -11 mm, with > 5 images analyzed per sample using ImageJ to ensure statistical robustness.
Density measurements
Evaluated using Archimedes’ method. The bulk density for each composition was measured three times (n = 3) on a single sintered pellet to ensure reproducibility, following the ASTM C373-88 standard for ceramic materials. The reported values represent the mean ± standard deviation of these measurements. The low standard deviations (< 0.3% in all cases) validate the consistency of our density data.
Visible-ultraviolet (UV–Vis) transmission tests: Conducted over a wavelength range of 200–800 nm using a Ray Leigh UV-1600 device.
Infrared (IR) transmission tests: Investigated using Infralum FT-08 LUMEX infrared spectroscopy.
Three-point bending tests: Performed using a Hounsfield H25KS (England) equipped with a 500 N load cell, moving at a speed of 0.1 mm/min.
Microhardness testing: Conducted using a 101/642-MiTi model (Mitutoyo, Japan) by applying a 1 kg load for 10 s.
The microhardness of the samples was calculated using Eq. (1)15,16,17,18:
where:
-
H = hardness (GPa),
-
P = applied load (N),
-
a = radius of the indentation (mm).
Fracture toughness was evaluated using the direct crack measurement method, where the length of the indentation-induced cracks generated by Vickers microhardness testing under applied stress was quantified19,20,21. For fracture toughness, Eq. (2) was used22,23,24:
where:
-
KIC = fracture toughness (MPa.m1/2),
-
K = dimensionless constant (0.0264)17,
-
E = elastic modulus (GPa),
-
P = applied load (N),
-
c = crack length (mm).
Hardness and toughness values (mean ± standard deveation) measured from 5 indents per composition. For each SPS batch (n=1 per composition), 5 Vickers indentations were performed under identical conditions (9.8 N load, 15s dwell, ISO 14705). Crack lengths were measured along all 4 indent axes to calculate KIC.
The three-point bending strength25,26 of the samples was calculated using Eq. (3) (ASTM C1684)
where:
-
σ = flexural strength (MPa),
-
P = breaking force (N),
-
L = distance between supports (mm),
-
D = sample diameter (mm).
Flexural strength values (mean ± standard deveation) calculated from 2 tests per each composition.
In all mechanical test, the diameter of the disk-like sample was 20 mm with a thickness of ~ 1 mm.
Results and discussion
Structural and phase analysis
The X-ray diffraction (XRD) pattern of the initial alumina powder is presented in (Fig. 1). The diffraction peaks correspond to α-alumina (JCPDS card no. 01–073-1512), with lattice parameters of a = 0.47544 nm and c = 1.297 nm, confirming a rhombohedral crystal structure. Using the Williamson-Hall method, the crystallite size of the alumina powder was calculated to be 43.4 nm.
The XRD patterns of the bulk samples with different sintering aid compositions, after SPS treatment at 1350 °C are shown in (Fig. 2). Due to the detection limit of XRD (phases below 5 wt.% are not detectable), all samples exhibited diffraction patterns consistent with the α-alumina phase, with no evidence of additional or decomposed phases.
Microstructural analysis
Scanning electron microscopy (SEM) images of the α-alumina powder with the three sintering aids (MgO, La2O3, and ZrO2) are presented in (Fig. 3). The images reveal alumina nanoparticles with sizes ranging from 200 to 250 nm, alongside smaller particles (< 100 nm) attributed to the precipitated sintering aids after calcination at 800 °C.
Energy-dispersive X-ray spectroscopy (EDS) analysis of the samples with compositions 100M100L300Z and 100M100L50Z is shown in (Fig. 4). The EDS results indicate that the weight percentages of lanthanum, magnesium, and zirconium exceeded the theoretical values due to fluorescence errors inherent in the EDS analysis.
Density and microstructure
The bulk density of all samples, as evaluated by Archimedes’ method, is summarized in (Table 1). The results show that the sample Z (100 M-100L-300Z), with the highest zirconia content (300 ppm), achieved the highest bulk density of 3.94 g/cm3, corresponding to 99.9% of the theoretical density of α-alumina. This improvement is attributed to the role of zirconia in reducing surface tension and grain boundary energy, thereby enhancing the sintering process and promoting denser microstructures5,7,8,27,28,29,30,31,32,33,34.
The high bulk density (3.94 g/cm3) achieved in the 100M100L300Z sample is a direct result of the combined effects of the triple sintering aids. The addition of ZrO₂ reduces surface tension and grain boundary energy, promoting densification during the sintering process8,12. Meanwhile, MgO and La2O3 stabilize the microstructure and prevent the formation of pores, further enhancing the density of the material6,7,8,9,10. The ability to achieve near-theoretical density in such a short sintering time is a testament to the effectiveness of the triplex sintering aid system, as it allows for the production of high-performance transparent alumina ceramics with minimal defects.
Field emission scanning electron microscopy (FE-SEM) images of the fracture surfaces of the prepared samples are shown in (Fig. 5). The sample Z (100 M-100L-300Z) exhibited the smallest grain size and no observable porosity, confirming its superior densification.
The microstructure of the 100M100L300Z sample, characterized by its fine grain size and lack of observable porosity, is a direct result of the synergistic effects of the triple sintering aids. The addition of ZrO2 inhibits grain growth through its phase transformation mechanism, while MgO and La2O3 stabilize the grain boundaries and prevent the formation of secondary phases. This combination of effects results in a dense, uniform microstructure that is essential for achieving both high optical transparency and mechanical strength. The ability to control grain size and porosity through the use of multiple sintering aids is a key advantage of the triplex system, as it allows for the development of materials with tailored properties for specific applications.
Elemental distribution
X-ray mapping of the elemental distribution in the sample (200M100L100Z) is presented in (Fig. 6). The analysis confirms the uniform distribution of Zr, La, and Mg throughout the alumina grains, indicating the effective incorporation of the sintering aids. The uniform distribution of Zr, La, and Mg is crucial for achieving consistent and predictable material properties. The absence of clustering or segregation suggests that the chemical precipitation method effectively dispersed the sintering aids throughout the alumina grains. This uniform dispersion likely contributes to the enhanced densification and grain growth control observed in the subsequent analyses.
EDS mapping (Fig. 6) reveals a homogeneous distribution of MgO, La2O3, and ZrO2 across grains and boundaries. While minor segregation (< 1 µm) cannot be ruled out due to EDS resolution limits, no large-scale concentration variations were detected, supporting the hypothesis that sintering aids are fully incorporated into the alumina matrix.
UV–vis and IR spectra and microstructures
The transmission curves for the prepared samples in the visible and infrared ranges are shown in (Fig. 7). The 100 M-100L-300Z (Z) sample exhibited the highest visible transmittance of 32% at 750 nm and the highest infrared transmittance of 71.4% at 5 μm.
Figure 8 shows photographic images of the sintered samples, demonstrating the visible transparency of the Z and Y samples, as evidenced by the clear visibility of the text "Al₂O₃" placed behind the samples. Thickness of these sample are ~ 1 mm. Transparent polycrystalline alumina ceramics are typically fabricated with thicknesses ranging from 0.5 to 3 mm, depending on the application and processing method. Most studies report 1–2 mm as the standard thickness for optical-grade transparent alumina 3,6,17. Thinner Samples (0.5–1 mm) used for high-transparency applications (e.g., lamp envelopes, IR windows)27. Thicker Samples (2–3 mm) used when mechanical strength is prioritized (e.g., armor, dental implants28.
A comparison of the optical results of this study with other reported works is provided in (Table 2). The infrared transmittance achieved in this work is higher than that of alumina prepared with single sintering aids (e.g., MgO or La2O3) or triple sintering aids (e.g., MgO, ZrO2, and Y2O3)11,16,17.
Figure S2 (Supporting Information File 1) shows the visible light transmission spectra of the samples with different sintering aid compositions. The sample without sintering aids exhibited no transmission in the visible region. In contrast, samples containing 100 ppm magnesia (100 M), 100 ppm magnesia + 100 ppm lanthanum oxide (100M100L), and 100 ppm magnesia + 300 ppm zirconium oxide (100M300Z) showed transmission values of 7%, 14%, and 23%, respectively, at a wavelength of 700 nm. Notably, the sample containing all three sintering aids—100 ppm magnesia, 100 ppm lanthanum oxide, and 300 ppm zirconium oxide (100M100L300Z)—achieved a transmission value of 29% at 700 nm. These results highlight the critical role of the triple sintering aid system in enhancing the optical transparency of alumina ceramics.
Figure S3 (Supporting Information File 1) presents cross-sectional field emission scanning electron microscopy (FE-SEM) images of alumina disks with varying sintering aid compositions. Key observations include:
-
Without sintering aids: The alumina grain size grew to 10 μm, with significant intergranular porosity (indicated by arrows).
-
With 100 ppm magnesia (100 M): A mixture of coarse grains (10 μm) and finer grains (50 nm) was observed.
-
With 100 ppm magnesia + 100 ppm lanthanum oxide (100M100L): The grain size decreased to 4–5 μm.
-
With 100 ppm magnesia + 300 ppm zirconium oxide (100M300Z): The grain size further decreased to 3 μm.
The number of pores (indicated by arrows) decreased significantly from Figure S3a to S3d, demonstrating the effectiveness of sintering aids in reducing porosity and refining grain structure.
Mechanical properties: microhardness, fracture toughness and flexural strength
The results of microhardness test, presented in Table 3, show that the sample Z (100M100L300Z) exhibited the highest microhardness.The results of fracture toughness, summarized in Table 4 (Figure S4 and Figure S5), demonstrate that the sample Z (100M100L300Z) achieved the highest fracture toughness.
The toughening mechanisms in Sample Z (100M100L300Z) were analyzed via:
FE-SEM crack-path imaging (Fig. S4): Reveals no crack deflection at ZrO2-induced compressive zones. It means mechanism of crack defelction didn’t occure in all samples.
Grain boundary stabilization: MgO/ La2O3 reduce intergranular fracture.
Porosity-grain size correlation: Density > 99.9% (Table 1) and fine grains (1–2 µm) minimize stress concentrators19,20,21.
The significant improvement in fracture toughness observed in the 100M100L300Z sample can be attributed to the combined effects of the triple sintering aids. ZrO2, in particular, plays a key role in enhancing fracture toughness through its phase transformation mechanism, which induces compressive stresses that hinder crack propagation. Additionally, the presence of MgO and La2O3 at the grain boundaries stabilizes the microstructure and reduces the likelihood of intergranular fracture. This multi-mechanism approach to improving fracture toughness is a key advantage of the triplex sintering aid system, as it allows for the development of transparent alumina ceramics with both high optical transparency and excellent mechanical performance.
For each ternary sintering aid composition (MgO- La2O3-ZrO2 in Al2O3), we tested one primary sample for hardness and fracture toughness measurements, with 5 indentation replicates per sample to ensure intra-sample statistical significance. The standard deviation of hardness (HV) values for each composition ranged between ± 0.1–0.5 GPa, while fracture toughness (KIC) showed ± 0.1–0.2 MPa.m1/2 variation, as detailed in (Tables 3, 4). This reproducibility confirms minimal material heterogeneity. Single-sample testing with high replicate indents is an established approach for hard ceramics where:
i) SPS processing ensures high density (> 99%) with minimal batch-to-batch variation15,16,17,18,19
ii) Vickers indentation inherently provides statistical sampling through multiple crack-length measurements per indent3,6. The low standard deviations (< 5% for hardness) indicate excellent microstructural homogeneity across samples, as confirmed by EDS mapping (Fig. 6).
Flexural strength
The results of three-point bending strength test, presented in Table 5 and Fig. 9, show that the 100M100L300Z (Z) sample exhibited the highest flexural strength. This improvement is attributed to the reduction in grain size, lower porosity, and the synergistic effects of the triple sintering aids.
The mechanical properties of the 100M100L300Z sample, including its high hardness (19.34 GPa) and flexural strength (356.83 MPa), are a direct result of the synergistic effects of the triple sintering aids. The fine grain size achieved through the addition of ZrO2, combined with the grain boundary strengthening effects of MgO and La2O3, results in a material with superior resistance to deformation and fracture. The reduction in porosity and the uniform distribution of the sintering aids further contribute to the enhanced mechanical performance, making this material suitable for applications requiring both high strength and optical clarity.
Role of sintering aids in transparency and density
The high relative density achieved in the 100M100L300Z (Z) sample corresponds to a reduction in porosity, which is crucial for improving transparency. High porosity levels lead to light scattering due to the significant difference in refractive indices between air (n ≈ 1) and alumina (n ≈ 1.76). This scattering results in the darkening of the ceramic.
The addition of zirconia (ZrO2) plays a key role in grain refinement. As shown in Table 1 and the SEM images, increasing the zirconium salt content reduces grain size of alumina. This effect is attributed to the tetragonal-to-monoclinic phase transformation of zirconia, which induces a 3–5% volume expansion and inhibits grain growth at alumina grain boundaries8,24,29.
Furthermore, magnesia (MgO) promotes the formation of a stable MgAl2O4 spinel phase at grain boundaries, which also prevents alumina grain growth. The inclusion of lanthanum oxide (La2O3) stabilizes the spinel network, further enhancing the microstructure. The synergistic effect of the double or triple sintering aid system significantly improves the transparency and mechanical properties of alumina10.
The observed improvements in transparency and mechanical properties can be attributed to several factors. The combination of MgO, La2O3, and ZrO2 creates a multi-faceted approach to controlling grain boundary mobility and defect formation. MgO promotes the formation of MgAl2O4 spinel, which not only inhibits grain growth but also scavenges impurities. La2O3 stabilizes the spinel structure and reduces oxygen vacancies, while ZrO2's phase transformation induces compressive stresses that hinder crack propagation. The synergy between these three sintering aids results in a material with a refined microstructure, reduced porosity, and enhanced resistance to mechanical failure.
The high transparency achieved in this study is particularly noteworthy. The combination of high density and fine grain size minimizes light scattering, allowing for efficient transmission of both visible and infrared radiation. This makes the material suitable for applications where optical clarity and mechanical robustness are essential. Furthermore, the enhanced mechanical properties, such as hardness, fracture toughness, and flexural strength, expand the range of potential applications to include demanding environments where resistance to wear, impact, and high stress is critical.
The enhanced optical transparency observed in the 100M100L300Z sample can be further explained by the interplay between grain size reduction and porosity minimization. The addition of ZrO2, in particular, plays a critical role in refining the grain structure, as the tetragonal-to-monoclinic phase transformation induces compressive stresses that inhibit grain growth. This, combined with the stabilizing effect of La2O3 on the spinel network and the grain boundary pinning effect of MgO, results in a microstructure with minimal light scattering centers. The uniform distribution of these sintering aids, as confirmed by X-ray mapping, ensures that the material maintains high transparency across both visible and infrared wavelengths. This multi-faceted approach to controlling microstructure is key to achieving the high transmittance values reported in this study.
Comparison with previous studies
The fracture toughness of the 100M100L300Z (Z) sample (5.24 MPa.m1/2, Table 6) is superior to values reported for alumina with single or dual sintering aids (e.g., 4.4 MPa.m1/2 for MgO-Y2O329 and 2.5–3.52 MPa.m1/2 for pure alumina5,23,24,30,31). Similarly, the flexural strength of the Z sample (356.83 MPa) exceeds that of alumina with 0.5 wt.% MgO (322 MPa)34. These results demonstrate the superiority of the triple sintering aid system in enhancing both mechanical and optical properties.
The results of this study not only demonstrate the superiority of the triplex sintering aid system but also highlight the potential for further optimization. For instance, the fracture toughness of 5.24 MPa.m1/2 achieved in this work is significantly higher than that reported in previous studies using single or dual sintering aids. This improvement can be attributed to the synergistic effects of MgO, La2O3, and ZrO2, which collectively enhance grain boundary strength and reduce crack propagation. Furthermore, the flexural strength of 356.83 MPa surpasses that of alumina with 0.5 wt.% MgO (322 MPa)34, indicating that the combination of these three sintering aids not only improves optical properties but also significantly enhances mechanical performance. These findings suggest that the triplex sintering aid system could be a promising approach for developing high-performance transparent alumina ceramics for demanding applications such as armor and optical windows.
Conclusion
In this study, the effects of three sintering aids—magnesia (MgO), lanthanum oxide (La2O3), and zirconia (ZrO2)—on the optical and mechanical properties of transparent alumina ceramics were investigated. The 100M100L300Z (Z) sample, containing 100 ppm MgO, 100 ppm La2O3, and 300 ppm ZrO2, exhibited the highest infrared transmittance (71.4% at 5 μm) and visible transmittance (32% at 750 nm). This sample also demonstrated superior mechanical properties, including a hardness of 19.34 GPa, fracture toughness of 5.24 MPa.m1/2, and flexural strength of 356.83 MPa. These improvements are attributed to the synergistic effects of the triple sintering aids, which reduce grain size, minimize porosity, and increase densification.
The optical properties of the 100M100L300Z sample, particularly its high infrared transmittance (71.4% at 5 μm) and visible transmittance (32% at 750 nm), are a direct result of the combined effects of the triple sintering aids. The reduction in grain size and porosity minimizes light scattering, while the uniform distribution of the sintering aids ensures that the material maintains high transparency across a wide range of wavelengths. The ability to achieve such high levels of transparency while maintaining excellent mechanical properties is a significant advancement in the field of transparent ceramics, opening up new possibilities for applications in optical systems.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Akinribide, O. J. et al. A review on optical properties and application of transparent ceramics. J. Mater. Res. Technol. 21, 712–738 (2022).
Yin, J., Li, X., Zhang, X., Yu, S. & Lai, Y. Progress in sintering technology of transparent polycrystalline alumina ceramics. J. Adv. Dielectr. 14 (06), 2330002 (2024).
Gao, H. et al. Investigation of mechanical properties and transparency of spark plasma sintered Mg2+ and Y3+ codoped α-Al2O3 nanoparticles synthesized via coprecipitation. J. Mater. Res. Technol. 23, 1052–1061 (2023).
Park, C., Torresani, E., Haines, C., Martin, D. & Olevsky, E. A. Transparent Al2O3 fabricated by energy efficient spark plasma sintering. J. Mater. Sci. 58 (29), 11872–11885 (2023).
Kuang, Z., Yin, M., Jia, Z., Li, X. & Yu, S. The preparation of highly transparent alumina ceramics with excellent mechanical performance via co-doping strategy. J. Alloy. Compd. 1021, 179497 (2025).
Shahriari, M., Loghman Estarki, M. R., Mansouri, H., Jamali, H. & Sardarian, M. The effect of size and type of alumina nanopowder phase on the transparency and bending strength of bodies sintered with MgO and La2O3 sintering aid. J. Aust. Ceram. Soc. 59 (4), 1079–1093 (2023).
Ge, X. Z. et al. Influence of La2O3 addition on microstructure and mechanical properties of Al2O3 ceramics. Mater. Sci. Forum 956, 69–77 (2019).
Lallemant, L. et al. Effect of amount of doping agent on sintering, microstructure and optical properties of Zr-and La-doped alumina sintered by SPS. J. Eur. Ceramic Soc. 34 (5), 1279–1288 (2014).
Roussel, N. et al. Effects of the nature of the doping salt and of the thermal pre-treatment and sintering temperature on spark plasma sintering of transparent alumina. Ceram. Int. 37 (8), 3565–3573 (2011).
Apak, B., Göller, G., Onüralp, Y. & Şahin, F. Ç. The effects of codoping Y2O3 on MgO doped spark plasma sintered Al2O3. Adv. Sci. Technol. 63, 74–78 (2011).
Shahbazi, H., Tataei, M., Enayati, M. H., Shafeiey, A. & Malekabadi, M. A. Structure-transmittance relationship in transparent ceramics. J. Alloy. Compd. 785, 260–285 (2019).
Tyagi, J., Mishra, S. K. & Ahmad, S. Transparent ceramics: The material of next generation. In Metal Oxides for Next-Generation Optoelectronic, Photonic, and Photovoltaic Applications (eds Tyagi, J. et al.) (Elsevier, 2024).
Wang, H., Lin, H., Li, W. & Shi, J. Effect of La doping on microwave dielectric properties of translucent polycrystalline alumina ceramic. Ceram. Int. 39 (5), 4907–4911 (2013).
Roshani, A. et al. The effect of the amount and size of alumina sintering aid particles on some mechanical properties and microstructure of silicon carbide bulky pieces via spark plasma sintering. Int. J. Mater. Res. 114 (6), 469–478 (2023).
Oparina, I. B. & Kolmakov, A. G. Methods for obtaining transparent polycrystalline ceramics from aluminum oxide. Refract. Ind. Ceram 62, 196–201 (2021).
Stuer, M., Zhao, Z., Aschauer, U. & Bowen, P. Transparent polycrystalline alumina using spark plasma sintering: effect of Mg, Y and La doping. J. Eur. Ceram. Soc. 30 (6), 1335–1343 (2010).
Shahriari, M., Jamali, H., Mansouri, H., Loghman Estarki, M. R. & Sardarian, M. Fabrication of spark plasma sintered spark plasma sintered transparent alumina using magnesium oxide and lanthanum oxide as sintering-assisted. J. Adv. Mater. Eng. (Esteghlal) 41 (3), 31–40 (2022).
Apak, B. et al. Transparent polycrystalline alumina obtained by SPS: Single and double doping effect. Supplement. Proc. Mater. Process. Interfaces 1, 481–487 (2012).
Balak, Z. Open porosity and grain size effect on the mechanical property of ZrB2–30SiC containing HfB2. Mater. Res. Express 6 (9), 095607 (2019).
Al-Habib, D. S. H., Balak, Z. & Asl, M. S. Spark plasma sinterability of TaC-based composites co-doped with SiC, TiC and graphene. Diam. Relat. Mater. 130, 109496 (2022).
Abdel-Karim, N. A. S., Balak, Z. & Asl, M. S. Fabrication and characterization of HfB2-based composites in the presence of TiC and CNT. Mater. Chem. Phys. 287, 126244 (2022).
Kuang, Z., Yin, M., Jia, Z. & Li, X. & Yu, SThe preparation of highly transparent alumina ceramics with excellent mechanical performance via co-doping strategy. J. Alloy. Compd. 1021, 179497 (2025).
Cook, R. F. Indentation Fracture: Strength and Toughness for Brittle Materials Design (John Wiley & Sons, 2025).
Žmak, I., Ćorić, D., Mandić, V. & Ćurković,. LHardness and indentation fracture toughness of slip cast alumina and alumina-zirconia ceramics. Materials 13 (1), 122 (2019).
Skiba, R. Advanced Materials Engineering Fundamentals (After Midnight Publishing, 2025).
Tryon, R. G. Mechanical properties of ceramics. JB Wachtman. Appl. Mechan. Rev. 50, B47–B47 (1997).
Zhang, X., Liang, S., Li, H. & Yang, J. Mechanical and optical properties of transparent alumina obtained by rapid vacuum sintering. Ceram. Int. 43 (1), 420–426 (2017).
Lee, Y. K. Translucency of dental ceramic, post and bracket. Materials 8 (11), 7241–7249 (2015).
Boldin, M. S. et al. Investigation of the effect of a small addition of ZrO2 on the density and growth of grains of fine-grained aluminum oxide. Tech. Phys. 67 (7), 570–580 (2022).
Ratzker, B., Wagner, A., Kalabukhov, S. & Frage, N. Improved alumina transparency achieved by high-pressure spark plasma sintering of commercial powder. Ceram. Int. 46 (13), 21794–21799 (2020).
Pristinskiy, Y., Pinargote, N. W. S. & Smirnov, A. The effect of MgO addition on the microstructure and mechanical properties of alumina ceramic obtained by spark plasma sintering. Mater. Today Proc. 19, 1990–1993 (2019).
Harris, D. C. Materials for Infrared Windows and Domes: Properties and Performance (SPIE Press, 1999).
Zhang, L., Liu, Q., Yu, D., Lu, M. & Lin, J. Effect of sintering process on the properties of transparent Al2O3. Mater. Sci. Technol. 39 (8), 926–932 (2023).
Ratzker, B. et al. Optical and mechanical properties of transparent alumina fabricated by high-pressure spark plasma sintering. J. Eur. Ceram. Soc. 39 (8), 2712–2719 (2019).
Acknowledgements
This work is based upon research funded by Iran National Science Foundation under project number 4020191. The authors thanks from INSF from support cost of this project
Author information
Authors and Affiliations
Contributions
A.K. , M.R., H. J, EM. S, S. T, AN.E and F.D. wrote the main manuscript text and prepared figures. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shargh, A.H.K., Ramazani, M., Jamali, H. et al. Enhanced mechanical and optical properties of alumina ceramics via simultaneous magnesium, lanthanum, and zirconium oxide addition in spark plasma sintering. Sci Rep 15, 20588 (2025). https://doi.org/10.1038/s41598-025-06197-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06197-1