Abstract
Type 2 diabetes significantly increases cardiovascular risk. Glucagon-like peptide-1 receptor agonists (GLP-1 RA) reduce major adverse cardiovascular events, heart failure hospitalizations, and death. However, the comparison among GLP-1 RAs on cardiovascular outcomes is limited. We compared all-cause death and cardiovascular events in patients using semaglutide or dulaglutide in patients with type 2 diabetes. This retrospective observational study used the TriNetX database of deidentified electronic medical records from January 1, 2018, to December 31, 2020. We identified 4,691,652 patients with type 2 diabetes, among whom 231,075 initiated semaglutide and 189,103 initiated dulaglutide. After propensity score matching, 171,105 patients were included in each group. The primary outcome measure was all-cause death during the 3-year follow-up period.; secondary outcomes were acute myocardial infarction, stroke, and acute heart failure.Over 3 years, the risk for all-cause death in patients who received semaglutide relative to those who received dulaglutide was significantly lower (4.2% vs. 5.6%, p < 0.001; hazard ratio [HR] 0.75; 95% confidence interval [CI] 0.72–0.78). Similarly, patients who received semaglutide were less likely to have acute myocardial infarction (5.2% vs. 5.6%; HR, 0.94; 95% CI, 0.91–0.97; p < 0.001), stroke (5.8% vs. 6.4%; HR, 0.90; 95% CI, 0.87–0.93; p < 0.001), and acute heart failure (5.3% vs. 6.1%; HR, 0.88; 95% CI, 0.85–0.91; p < 0.001).In this multicenter, retrospective, observational study, semaglutide was associated with lower 3-year risks of all-cause death, acute myocardial infarction, stroke, and acute heart failure compared with dulaglutide in patients with type 2 diabetes.
Similar content being viewed by others
Introduction
Type 2 diabetes is one of the most significant risk factors for cardiovascular disease, including death and heart failure1,2,3,4. Previous clinical trials have demonstrated that glucagon-like peptide-1 receptor agonists (GLP-1 RAs) improve cardiovascular outcomes, including heart failure, as well as all-cause death5,6,7,8. A meta-analysis of these GLP-1 RA cardiovascular outcome trials found that GLP-1 RAs reduced major adverse cardiovascular events (MACE) by 12%, heart failure hospitalizations by 9%, and all-cause death by 12%9. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7), a Phase 3b trial of semaglutide, demonstrated that semaglutide was superior to dulaglutide in improving glycemic control and weight loss10. Previous studies using network meta-regression analysis also demonstrated that semaglutide improved glycemic control and weight loss compared to dulaglutide11. In a previous study that matched populations from the Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN 6) and Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND) cardiovascular outcome trials using the propensity score method, semaglutide reduced 3-point MACE (cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke) by 26% compared to dulaglutide, although the reduction was not statistically significant (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.54–1.01)12. However, the evidence of head-to-head comparison among GLP-1 RA on cardiovascular outcomes in real-world settings remains insufficient.
The purpose of this study is to compare the risk of all-cause death and cardiovascular outcomes, including heart failure, in patients with type 2 diabetes treated with semaglutide versus dulaglutide in a real-world setting using TriNetX, a global healthcare data and analytics platform.
Methods
Study population
We conducted a multicenter retrospective observational study using TriNetX (TriNetX, LLC, Cambridge, Massachusetts, USA), a global healthcare data and analytics platform. The TriNetX platform provides real-world data analysis using electronic health records (EHRs) for more than 250 million patients from over 130 healthcare organizations across North America, South America, Europe, the Middle East, Africa, and Asia Pacific. The TriNetX database aggregates de-identified, longitudinal health data from millions of patients across multiple healthcare settings, including hospitals, outpatient centers, and primary care clinics. Each healthcare organization maintains local control of its data, while TriNetX securely accesses and aggregates de-identified data across institutions. By only aggregating the results for analysis, TriNetX enhances privacy. Available data includes patient demographics (such as age, sex, race, and ethnicity), diagnoses (standardized by the International Statistical Classification of Diseases and Related Health Problems [ICD] − 10 codes), procedures (using Current Procedural Terminology [CPT] codes), laboratory results, medications prescribed, and clinical outcomes. Because the TriNetX platform collects data from the EHR, disease diagnosis is not based on diagnostic criteria but on the recording of disease names in the EHR. We identified disease and health problems using the ICD − 10 code. We included patients aged ≥ 18 years with type 2 diabetes (ICD-10; E11) from January 1, 2018, to December 31, 2021. The exposure group was defined as the initiation of semaglutide treatment after their diagnosis of type 2 diabetes. The control group was defined as initiation of dulaglutide treatment after their diagnosis of type 2 diabetes. The start of the observation period was defined as the respective initiation day.
Outcome measures
The primary outcome measure was all-cause death during the 3-year follow-up period. Secondary outcome measures were acute myocardial infarction (ICD-10: I21), stroke (cerebral infarction or nontraumatic intracerebral hemorrhage) (ICD-10: I61, I63), or acute heart failure (ICD-10: I50.21, I50.23, I50.31, I50.33, I50.41, I50.43, I50.811, I50.813).
Data collection and definitions
Covariates considered confounding factors for exposure and outcome were extracted from the database with reference to previous studies5,6,7,8. Each factor was extracted from 1 month before day 0 of the respective medication initiation. Diseases recorded in the EHR during the observation period were included as comorbidities, whereas previously recorded diseases that were discontinued at the beginning of the observation period were not included as comorbidities. We extracted the following 30 factors: age, sex, body mass index (BMI), race (Asian, American Indian or Alaska Native, Black or African American, Native Hawaiian or Other Pacific Islander, White, Other Race, unknown), diagnoses (heart failure, ischemic heart diseases, old myocardial infarction, atrial fibrillation and flutter, hypertension, dyslipidemia, chronic kidney disease, cerebral infarction), and medication (be-ta blockers, diuretics, mineralocorticoid receptor antagonists [MRA], angiotensin converting enzyme [ACE] inhibitors, angiotensin II receptor blockers [ARB], sacubitril, statins, metformin, sulfonylureas, sodium-glucose co-transporter 2 [SGLT2] inhibitors, insulin, laboratory (systolic blood pressure, diastolic blood pressure, glomerular filtration rate [GFR], hemoglobin, albumin, LDL cholesterol, hemoglobin A1c [HbA1c]). A list including definitions of covariates is reported in Supplementary Table 1.
Ethics approval
We conducted this study in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. As a federated network, research studies using TriNetX do not require ethical approval. To comply with legal frameworks and ethical guidelines guarding against data re-identification, the identity of participating healthcare organizations and their individual contribution to each dataset are not disclosed. The study analyzed data using TriNetX, and did not require ethical review, primarily because it dealt only with anonymized data, the results available for reference were representative of the study population, and it did not deal with individual patient-level data13,14. No protected health information or personal data are made available to the users of the platform. This study was approved by the Ethics Committee of Omi Medical Center (local identifiers: 2024-0096) because it included patient data from our institution, although the data were completely anonymized, as mentioned above. Due to the retrospective nature of the study, the Ethics Committee of Omi Medical Center waived the need of obtaining informed consent.
Statistical analysis
Covariates between patients who received semaglutide and those who received dulaglutide were evaluated. Categorical variables were presented as numbers (%), and continuous variables were presented as means with standard deviations (SDs). To reduce potential confounding effects in the comparison between patients who received semaglutide and those who received dulaglutide, we estimated a propensity score (PS) using a logistic regression model adjusted for all covariates. One-to-one pair matching between patients who received semaglutide and those who received dulaglutide was performed using greedy nearest neighbor matching with calipers of width equal to 0.1 SD of the logit of the PS. To measure covariate balance, we checked the standardized mean difference (SMD) of before and after matching. When the SMD was < 0.1015, there was negligible imbalance between the two groups. The cumulative incidence of the outcome measures that occurred during 3-year period was estimated using the Kaplan–Meier method, with intergroup differences assessed using the log-rank test. The risks for patients who received semaglutide relative to those who received dulaglutide for the outcome measures were estimated by calculating the crude hazard ratios (HRs) and their 95% confidence intervals (CIs).
To measure the differences in treatment effects, subgroup analyses were performed for age (18 to 64 years, 65 to 74 years, over 75 years), sex (female, male), BMI (≤ 30 kg/m2, 30 kg/m2 < ), HbA1c (< 7.0%, 7.0 to 7.9%, 8.0% ≤), GFR (< 30 mL/min/1.73m2, 30 to 44.9 mL/min/1.73m2, 45 to 59.9 mL/min/1.73m2, 60 mL/min/1.73m2 ≤), previous myocardial infarction or cerebral infarction, heart failure, and hypertension. The TriNetX platform required additional cohorts to be set up for subgroup analysis. After PS matching, the difference in treatment effects between patients who received semaglutide and those who received dulaglutide on all-cause death in each subgroup was analyzed, and the crude HR was calculated with the corresponding 95% CIs.
As an additional analysis, we performed a subgroup analysis stratified by SGLT2 inhibitor use, with acute heart failure as the outcome.
Cohort definitions and statistical analyses were performed on January 2, 2025, using the Query Builder and Analytics Functions on the TriNetX platform. All tests were two-tailed, and differences were considered statistically significant at p < 0.05.
Results
Study population
From January 1, 2018, and December 31, 2021, 4,691,652 patients aged ≥ 18 years were diagnosed with type 2 diabetes on the TriNetX platform. Among the 657,057 patients who received GLP-1 RAs, 231,075 received semaglutide, and 189,103 received dulaglutide. After propensity score matching, each group included 171,105 patients (Fig. 1).
Baseline clinical characteristics
The characteristics of patients aged ≥ 18 years with type 2 diabetes according to the semaglutide and dulaglutide groups were summarized in Table 1.
Before PS matching, patients who received semaglutide, compared with those who received dulaglutide, had a higher BMI, were less likely to receive ACE inhibitors, statins, metformin, sulfonylureas, and insulin. They also had higher hemoglobin, albumin, and lower HbA1c levels. After PS matching, the SMDs were all less than 0.10 and the covariate balance between the groups was improved (Table 1).
Clinical outcomes
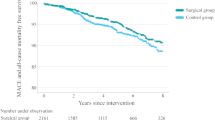
The 3-year cumulative incidence of all-cause death was significantly lower in patients who received semaglutide than in those who received dulaglutide (4.2% [4,073/171,105] vs. 5.6% [6,789/171,105]; log-rank P < 0.001). The risk for all-cause death in patients who received semaglutide relative to those who received dulaglutide was significant (HR, 0.75; 95% CI, 0.72–0.78;) (Fig. 2). Similarly, patients who received semaglutide were less likely to have acute myocardial infarction (5.2% [5,764] vs. 5.6% [7,224]; HR, 0.94; 95% CI, 0.91–0.97; log-rank P < 0.001), stroke (5.8% [6,686] vs. 6.4% [8,557]; HR, 0.90; 95% CI, 0.87–0.93; log-rank P < 0.001), and acute heart failure (5.3% [5,884] vs. 6.1% [7,854]; HR, 0.88; 95% CI, 0.85–0.91; log-rank P < 0.001) (Fig. 2).
Subgroup analyses
Figure 3 showed the results of the subgroup analyses for age, sex, BMI, HbA1c, GFR, previous myocardial infarction or cerebral infarction, heart failure, and hypertension. With the exception of the subgroup with a GFR of less than 30 mL/min/1.73m2, the 3-year cumulative incidence of all-cause death was significantly lower in patients who received semaglutide than those who received dulaglutide.
Supplementary Table 2 showed the results of subgroup analysis stratified by SGLT2 inhibitors, with acute heart failure as the outcome. Semaglutide was associated with a lower risk of acute heart failure in patients not receiving SGLT2 inhibitors, and the trend was consistent with those receiving SGLT2 inhibitors.
Discussion
In this multicenter, retrospective, observational study using a global healthcare research network, the 3-year risks of all-cause death, acute myocardial infarction, stroke, and acute heart failure were significantly lower in patients with type 2 diabetes who received semaglutide than in those who received dulaglutide. The findings of this study suggest that the clinical use of semaglutide may be expanded in pharmacological therapy for the prevention of cardiovascular events in patients with type 2 diabetes.
Approval of GLP-1 RAs began with exenatide and liraglutide, followed by dulaglutide in 2014 and semaglutide in 2017. The half-life has increased with each drug development, from 2.4 h for exenatide and approximately 12 h for liraglutide to approximately 110 h for dulaglutide and 6–7 days for once-weekly semaglutide16. While GLP-1 RAs have undergone continuous improvement, glycemic control, cardiovascular benefits, and renal benefits vary among different drugs16. A matched study from SUSTAIN 6 and REWIND demonstrated that semaglutide reduced to 3-point MACE by 26% compared to dulaglutide, although the reduction was not statistically significant12. This matched study included 1,648 patients in the semaglutide group and 1,649 in the placebo group from the SUSTAIN 6 trial, as well as 4,949 patients in the dulaglutide group and 4,952 in the placebo group from the REWIND trial. A network meta-analysis comparing cardiovascular outcomes and mortality by GLP1-RA showed the following outcomes: MACE, cardiovascular death, death from any cause, myocardial infarction, stroke, and heart failure hospitalization17. Oral semaglutide significantly reduced the risk of all-cause death compared to dulaglutide (HR, 0.56; 95% CI, 0.33–0.93). There was no significant difference between once-weekly semaglutide and dulaglutide. Additionally, no significant differences were observed between semaglutide and dulaglutide for myocardial infarction, stroke, or heart failure hospitalization. The clinical trials on which this network meta-analysis was based included approximately 3,000 participants for semaglutide and 15,000 for dulaglutide. Our study included approximately 170,000 participants in each group after propensity score matching and demonstrated that semaglutide was associated with a reduced risk of all-cause death, acute myocardial infarction, stroke, and acute heart failure compared to dulaglutide. A major strength of our study is that we were able to collect a sufficiently large population in a real-world setting.
In the development of once-weekly semaglutide, resistance to DPP-4 degradation was enhanced by substituting alanine at position 8 with Aib (2-aminoisobutyric acid). Additionally, modifications were made to improve albumin binding and prolong its half-life in circulation. These structural changes resulted in a long-acting and highly efficacious drug18. In the development of dulaglutide, alanine at position 8 in the native GLP-1 sequence was substituted with valine, and the peptide was fused to the Fc domain of immunoglobulin G to confer resistance to DPP-4 degradation. Due to its large molecular weight, renal clearance was minimized, enabling once-weekly dosing19,20. No direct comparison between semaglutide and dulaglutide has been conducted regarding resistance to DPP-4 degradation or plasma half-life. Conversely, clinical trials have directly compared semaglutide and dulaglutide in terms of HbA1c reduction and weight loss. The SUSTAIN 7 trial compared the effects of once-weekly semaglutide and dulaglutide on HbA1c reduction and weight loss10. Semaglutide 0.5 mg was compared with dulaglutide 0.75 mg, and semaglutide 1.0 mg with dulaglutide 1.5 mg. At all doses, semaglutide significantly reduced HbA1c levels and body weight compared to dulaglutide. The Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10) trial compared oral semaglutide and dulaglutide in terms of HbA1c reduction and weight loss21. Oral semaglutide at doses of 3 mg, 7 mg, and 14 mg was compared with dulaglutide 0.75 mg. Oral semaglutide 14 mg significantly reduced HbA1c levels and body weight compared to dulaglutide 0.75 mg. Similarly, oral semaglutide 7 mg also led to reductions in HbA1c levels and body weight from baseline. The population enrolled in the SUSTAIN 7 trial was 55–56 years of age, 43–46% female, with an HbA1c of 8.2–8.3%, and a BMI of 33.1–33.7 kg/m². The population in the PIONEER 10 study was 57–61 years of age, 22–32% female, with an HbA1c of 8.2–8.4%, and a BMI of 25.8–26.3 kg/m². In contrast, the PS–matched population in our study had a mean age of 59.3 years, 50.4–50.8% were female, with an HbA1c of 8.6–8.7%, and a BMI of 35.1–35.2 kg/m². As shown above, our study population was older, had a higher proportion of women, higher HbA1c levels, and higher BMI compared to previous clinical trials comparing semaglutide and dulaglutide. The SUSTAIN 7 and PIONEER 10 trials did not evaluate hard outcomes such as death or cardiovascular events; however, both demonstrated improvements in glycemic control and body weight. In contrast, the SUSTAIN 6 trial showed improvements not only in glycemic control and obesity but also in hard outcomes, suggesting that these factors may be interrelated6. The findings of this study demonstrated that semaglutide was significantly associated with a reduction in all-cause death and cardiovascular events compared with dulaglutide. Based on the results of this study, along with findings from the SUSTAIN 6 and 7 trials and the PIONEER 10 trial, our data suggest that the population for whom semaglutide may be appropriate could be expanded to include older patients with elevated HbA1c and higher BMI.
Results from subgroup analyses of the Evaluate Renal Function with Semaglutide Once Weekly (FLOW), the Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity (SELECT), the Effect of Semaglutide 2.4 mg Once Weekly on Function and Symptoms in Subjects with Obesity-related Heart Failure with Preserved Ejection Fraction (STEP-HFpEF), and the Semaglutide Treatment Effect in People with Obesity and Heart Failure with Preserved Ejection Fraction and Diabetes Mellitus (STEP-HFpEF DM) trials showed that semaglutide tended to be more effective in younger patients, those with higher BMI, higher GFR, and lower HbA1c levels22,23,24. The results of the subgroup analysis in this study were similar. While all of the aforementioned clinical trials were placebo-controlled, the present study was a head-to-head comparison using dulaglutide as the active comparator. In real-world settings, semaglutide exhibited trends consistent with those observed in previous clinical trials when compared with the active comparator, further reinforcing the existing evidence supporting its use. Based on these results, it is hypothesized that semaglutide may be particularly effective in younger patients with milder diabetes who are overweight or obese. Furthermore, the results of the SELECT and STEP-HFpEF trials indicate that the effects of semaglutide may extend to non-diabetic patients. Further studies on semaglutide in patients with milder conditions are expected.
Strengths and limitations
In this study, we used the TriNetX platform to integrate and analyze anonymized longitudinal electronic health record (EHR) data. A key strength of this study is its validation of clinical trial findings in a real-world setting using a large sample size. Although the beneficial effects of GLP-1 receptor agonists (GLP-1 RAs) are often described as class effects, differences exist among agents in terms of glycemic control and weight reduction. Therefore, comparing their cardiovascular protective effects is an important research question. The direct comparison between semaglutide and dulaglutide represents a major strength of this study. Furthermore, we used propensity score matching to minimize confounding. Baseline characteristics between groups were well balanced, supporting the robustness of the statistical findings.
The present study has several limitations. First, this study collected information from electronic healthcare records, and the diagnoses were based on ICD-10 codes. Thus, it is not a definitional diagnosis; for example, using the modified Framingham criteria to diagnose heart failure. Second, each factor, collected as a confounding factor and presented in patient characteristics, was data from 1 month before the index day to day 0. Because of the difference of up to 1 month, the data may not reflect the patient’s condition on the index day. Third, the TriNetX platform used in this study could not differentiate between once-weekly semaglutide and oral semaglutide. Therefore, it should be noted that the effectiveness of semaglutide in this study represents the average effect of both formulations. Based on the results of SUSTAIN 7 and PIONEER 10, it is possible that the findings of this study may not be directly generalizable to patients receiving oral semaglutide at doses of 3 mg and 7 mg. Finally, there was a limitation regarding the PS matching used in the outcome analysis. Although we included as many confounders as possible in PS matching, we did not adjust for unknown or unmeasured confounders. Therefore, the results of this study must be confirmed in clinical trials or observational studies designed to thoroughly exclude bias.
Conclusions
In this multicenter, retrospective, observational study, semaglutide was associated with lower 3-year risks of all-cause death, acute myocardial infarction, stroke, and acute heart failure compared with dulaglutide in patients with type 2 diabetes. Additional prospective observational studies and randomized controlled trials are required to validate these findings.
Research insights
What is currently known about this topic?
Type 2 diabetes substantially increases the risk of cardiovascular events, and glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have been shown to reduce major adverse cardiovascular outcomes. However, comparisons between different GLP-1 RAs—specifically semaglutide and dulaglutide—are limited.
What is the key research question?
Does semaglutide or dulaglutide confer a lower risk of all-cause mortality and cardiovascular events (acute myocardial infarction, stroke, and acute heart failure) in patients with type 2 diabetes over a 3-year period?
What is new?
In this large, real-world, propensity score–matched analysis, semaglutide was associated with significantly lower risks of all-cause death, acute myocardial infarction, stroke, and acute heart failure compared with dulaglutide.
How might this study influence clinical practice?
The findings suggest semaglutide may be a preferred choice of GLP-1 RA for reducing cardiovascular risk in type 2 diabetes, offering clinicians valuable evidence to guide therapeutic decisions.
Data availability
Researchers can request access to data from the TriNetX research network through the TriNetX platform (https://live.trinetx.com). However, this may involve associated costs, require a data-sharing agreement, and no patient-identifiable information can be accessible. If you have any request, please contact the corresponding author (Takao Kato).
Abbreviations
- GLP-1 RA:
-
Glucagon-like peptide-1 receptor agonist
- MACE:
-
Major adverse cardiovascular events
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- HER:
-
Electronic health record
- ICD:
-
International statistical classification of diseases and related health statistical classification of diseases and related health problems
- CPT:
-
Current procedural terminology
- BMI:
-
Body mass index
- MRA:
-
Mineralocorticoid receptor antagonist
- ACE inhibitor:
-
Angiotensin converting enzyme inhibitor
- ARB:
-
Angiotensin II receptor blocker
- SGLT2 inhibitor:
-
Sodium-glucose co-transporter 2
- GFR:
-
Glomerular filtration rate
- HbA1c:
-
Hemoglobin A1c
- STROBE:
-
Strengthening the reporting of observational studies in epidemiology
- SD:
-
Standard deviation
- PS:
-
Propensity score
- SMD:
-
Standardized mean difference
References
Nichols, G. A., Gullion, C. M., Koro, C. E., Ephross, S. A. & Brown, J. B. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 27 (8), 1879–1884 (2004).
Nichols, G. A., Hillier, T. A., Erbey, J. R. & Brown, J. B. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 24 (9), 1614–1619 (2001).
Cubbon, R. M. et al. Diabetes mellitus is associated with adverse prognosis in chronic heart failure of ischaemic and non-ischaemic aetiology. Diab Vasc Dis. Res. 10 (4), 330–336 (2013).
Packer, M. Heart failure: the most important, preventable, and treatable cardiovascular complication of type 2 diabetes. Diabetes Care. 41 (1), 11–13 (2018).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl. J. Med. 375 (4), 311–322 (2016).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl. J. Med. 375 (19), 1834–1844 (2016).
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl. J. Med. 381 (9), 841–851 (2019).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394 (10193), 121–130 (2019).
Kristensen, S. L. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 7 (10), 776–785 (2019).
Pratley, R. E. et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 6 (4), 275–286 (2018).
Lingvay, I., Bauer, R., Baker-Knight, J., Lawson, J. & Pratley, R. An indirect treatment comparison of semaglutide 2.0 mg vs dulaglutide 3.0 mg and 4.5 mg using multilevel network Meta-regression. J. Clin. Endocrinol. Metab. 107 (5), 1461–1469 (2022).
Evans, L. M. et al. A population-adjusted indirect comparison of cardiovascular benefits of once-weekly subcutaneous semaglutide and dulaglutide in the treatment of patients with type 2 diabetes, with or without established cardiovascular disease. Endocrinol. Diabetes Metab. 4 (3), e00259 (2021).
Horiuchi, Y. et al. Sodium-Glucose Cotransporter-2 inhibitors in heart failure with malnutrition, frailty, sarcopenia, or Cachexia. J. Clin. Med. 13 (6), 1670 (2024).
Modzelewski, K. L., Pipilas, A. & Bosch, N. A. Comparative outcomes of empagliflozin to Dapagliflozin in patients with heart failure. JAMA Netw. Open. 7 (5), e249305 (2024).
Austin, P. C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun. Stat. - Simul. Comput. 38 (6), 1228–1234 (2009).
Zheng, Z. et al. Glucagon-like peptide-1 receptor: mechanisms and advances in therapy. Signal. Transduct. Target. Ther. 9 (1), 234 (2024).
Alfayez, O. M., Almohammed, O. A., Alkhezi, O. S., Almutairi, A. R. & Al Yami, M. S. Indirect comparison of glucagon like peptide-1 receptor agonists regarding cardiovascular safety and mortality in patients with type 2 diabetes mellitus: network meta-analysis. Cardiovasc. Diabetol. 19 (1), 96 (2020).
Lau, J. et al. Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) analogue semaglutide. J. Med. Chem. 58 (18), 7370–7380 (2015).
Glaesner, W. et al. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab. Res. Rev. 26 (4), 287–296 (2010).
Sharma, D., Verma, S., Vaidya, S., Kalia, K. & Tiwari, V. Recent updates on GLP-1 agonists: current advancements & challenges. Biomed. Pharmacother. 108, 952–962 (2018).
Yabe, D. et al. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): an open-label, randomised, active-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 8 (5), 392–406 (2020).
Lincoff, A. M. et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl. J. Med. 389 (24), 2221–2232 (2023).
Butler, J. et al. Semaglutide versus placebo in people with obesity-related heart failure with preserved ejection fraction: a pooled analysis of the STEP-HFpEF and STEP-HFpEF DM randomised trials. Lancet 403 (10437), 1635–1648 (2024).
Perkovic, V. et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl. J. Med. 391 (2), 109–121 (2024).
Acknowledgements
The authors are greatly indebted to Arisa Kitagawa, Sanae Aratani, and Nanase Suyama for her continued support of our research efforts.
Author information
Authors and Affiliations
Contributions
T. Kishimori and T. Kato analyzed and interpreted the data and wrote the manuscript. A. T., R. Y., J. K., and Y. I. contributed data analysis and interpretation. T. M. and T. Y. provided guidance on data presentation. T. Kato, A. W., and M. O. revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kishimori, T., Kato, T., Wada, A. et al. Comparative cardiovascular outcomes of semaglutide to dulaglutide in patients with type 2 diabetes. Sci Rep 15, 21333 (2025). https://doi.org/10.1038/s41598-025-06245-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06245-w