Abstract
The global prevalence of alopecia areata (AA) is estimated to be around 2%. However, the global disease burden of AA and current research hotspots in this field have received limited attention. The disability adjusted life years (DALYs), incidence rates, and prevalence rates of AA from 1990 to 2019 were collected from the Global burden of disease (GBD) 2019 database and analyzed using R software to elucidate the temporal trend in disease burden associated with AA. VOSviewer software was employed to cluster keywords within the field of AA for identification of research hotspots. On a global scale, areas with low socio-demographic index (SDI) exhibited the highest increase in DALYs, incidence rates, and prevalence rates for AA from 1990 to 2019, while regions with high SDI observed the most substantial decrease. However, it is noteworthy that high SDI regions continued to bear the highest burden of AA. AA imposes a higher disease burden on women than men within the same age bracket. Young individuals (aged between 25 and 39 years) experience a greater disease burden compared to other age cohorts. Bibliometric analysis reveals that recent research focus in the field of AA primarily revolves around clinical trials and evaluating various treatment modalities such as Janus kinases (JAK) inhibitors and platelet rich plasma. The disease burden of AA may still be on the rise worldwide. This study further validates the gender- and region-specific impacts of AA and its associated burden, offering valuable guidance for prevention strategies and resource allocation. JAK inhibitors and platelet rich plasma are currently favored by researchers, and further high-quality studies are required to assess their long-term efficacy and safety more comprehensively.
Similar content being viewed by others
Introduction
The pathogenesis of Alopecia areata (AA), a non-cicatricial alopecia, is characterized by T cell-mediated inflammatory autoimmune mechanisms and is influenced by genetic, environmental, and immune factors1,2,3. The overall quality of life (QoL) was significantly lower in patients with alopecia areata, and they exhibited a high prevalence of psychiatric comorbidities4,5. The systematic epidemiological analysis of AA reveals a global prevalence of approximately 2%, with significant variations in its subtypes, age of onset, regional distribution, and environmental factors6. The prevention and treatment of AA have received significant investments in healthcare resources; however, there is a scarcity of studies systematically evaluating the impact of AA on disease burden and rational allocation of healthcare resources across different regions and populations.
The course of AA is unpredictable and can spontaneously resolve or persistently recur, posing challenges for its treatment7. With the deepening understanding of the pathogenesis of AA, certain immunosuppressants have gained attention8. Simultaneously, autologous derivatives such as platelet concentrates and mesenchymal stem cells have also garnered favor9,10. However, only a few treatments have been evaluated through randomized controlled trials, with most lacking substantial evidence.
The Global burden of disease (GBD) database, published by the Institute for Health Metrics and Evaluation at the University of Washington, represents the most extensive and comprehensive estimation of health losses to date. The GBD database was utilized in this study to comprehensively assess the global epidemiological and disease burden data of AA from 1990 to 2019. Additionally, bibliometric analysis was employed to identify current research trends in the field of AA, thereby offering a more comprehensive and systematic understanding for both public health and clinical research perspectives.
Methods
Data source
The data utilized in this study was sourced from the GBD 2019 database (accessible at http://ghdx.healthdata.org/gbd-results-tool), which was constructed and disseminated by the Institute for Health Metrics and Evaluation at with the University of Washington. The GBD study 2019 assessed the burden of different diseases and provided comparable data in 204 countries and regions from 1990 to 2019 for the public11.
The publicly available data of AA was collected from the GBD 2019 database, and it was extracted separately based on various classifications including age, gender, Socio-demographic Index (SDI), region, and year. The age groups and regional categorization were based on the classification of the GBD 2019 database. In this classification, individuals below 20 years old were divided into three categories: under 5 years old, 5–14 years old, and 15–19 years old. The disease burden data of AA for each year from 1990 to 2019 are presented separately by both-sex, male, and female. The SDI was divided into 5 categories according to previous literature: low, low-middle, middle, high-middle, and high SDI12,13. The SDI is a comprehensive measure of national and regional development status, with values ranging from 0 to 1. A higher SDI indicates a better development status in terms of health outcomes14. According to the classification described above, the number and age-standardized rate (ASR) on disability-adjusted life years (DALYs), incidence and prevalence for AA were assessed to evaluate the disease burden.
Disease burden
The disease burden of AA was estimated by DALYs, incidence and prevalence across age, gender, region, and SDI, which were presented in absolute number and ASR with 95% uncertainty interval (UI). According to the World Health Organization World Standard Population Distribution, the ASR was calculated to compare differences between groups of different age demographics. DALYs is calculated as the sum of years of life lived with disability (YLD) and years of life lost (YLL), which is a metric defined as the total healthy life years lost from morbidity to death. All data of disease burden were obtained from the GBD Results Tool (http://ghdx.healthdata.org/gbd-results-tool), including number and ASR of DALYs, incidence and prevalence.
Data analysis
The absolute number and ASR (per 100,000 population) along with their 95% UI were used to illustrate the burden of the DALYs, incidence and prevalence for alopecia areata, and the DALYs, incidence and prevalence were compared across different sex, age, SDI, and location. The estimated annual percentage change (EAPC) and its 95% CI were used to indicated the temporal trend from 1990 to 2019. EAPC was an indicator that describes the trend in the ASR, which was used to assess the trend in the ASR of AA from 1990 to 2019. A regression line was fitted by converting the ASR to logarithmic form, namely: y = α + βx + ɛ, where y = ln (ASR) and x = year. EAPC was calculated as 100×(exp(β)-1), and its 95% confidence interval (CI) can be obtained from the regression line15. The ASR demonstrates an upward trend when both the EAPC and the lower boundary of the 95% CI exceed 0. In contrast, the ASR exhibits a downward trend when both the EAPC and the upper boundary of the 95% CI are less than 0. Otherwise, the ASR is considered stable if the 95% CI of the ASR includes 0. The heat map was used to depict the distribution of DALYs, incidence and prevalence rate in different age groups and SDI groups for AA, and the global map was draw to describe the ASR of DALYs, incidence and prevalence across different regions (R package: ggplot2, pheatmap). According to temporal trends in ASRs of DALYs, incidence and prevalence for AA, we made a hierarchy cluster analysis to classify the countries and territories into 4 groups as follows: (a) significant decrease; (b) minor decrease; (c) minor increase; (d) remained stable. The “factoextra” package was used to conduct the hierarchy cluster analysis. All analysis and visualization were performed by R software (version 3.6.3).
Bibliometric analysis of AA
The Web of Science Core Collection is thoroughly searched from its inception to December 1, 2023. The search strategy was as follows: TS= (alopecia areata) And publication date = All years (1990–2023). The keywords extracted from the study of alopecia areata were visually analyzed using VOSviewer software (version 1.6.18). Subsequently, clustering analysis was employed to classify these keywords into distinct groups based on their frequency of occurrence. Additionally, color coding was utilized to depict the temporal order and frequency of keywords, facilitating a comprehensive examination of research trends and novelty.
Results
Global burden and changes for AA
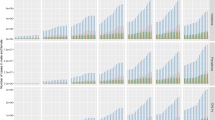
The number of DALYs for AA in 2019 resulted in a total of 600,570.37 (95% UI: 378,239.38 –891,060.98), with males accounting for 208,625.19 (34.74%) and females constituting the remaining 65.26% of the population. Compared to 1990, there was a significant increase of 49.51% in DALYs number observed in 2019.
However, the global ASR of DALYs for AA decreased from 7.74 (4.86–11.47) in 1990 to 7.51 (4.73—11.14) in 2019, with an EAPC of -0.12 (95% UI: − 0.12 to − 0.11). The ASR of DALYs for both male and female AA exhibited a declining trend, with the EAPC and its 95%CI being less than 0. In terms of SDI regions, there was a declining trend in DALYs for countries and regions with high, high-middle and middle SDI (EAPC and its 95%CI were all less than 0), while an increasing trend was observed in DALYs for countries and regions with low and low-middle SDI (EAPC and its 95%CI were all greater than 0). The region with the highest number of DALYs in 2019 is East Asia, which recorded 129,127.76 DALYs (95%UI: 81,512.47–191,628.05), accounting for approximately 21.5% of the global total. High-income North America had the highest ASR of DALY at 11.43 per 100,000 population (95%UI: 7.22–16.95), while South Asia had the lowest rate at 6.23 per 100,000 population (95% UI:3.94 –9 0.22). (See Fig. 1; Table 1). A more granular examination of Fig. 2A reveals distinct geographic disparities in the temporal trends of AA-related DALYs, with statistically significant increases observed across Oceania, North Africa and Middle East, Western Sub-Saharan Africa, Southern Latin America, Central Asia, East Asia, Southeast Asia, Eastern Europe, and Central Europe (EAPC and its 95%CI were all greater than 0). Notably, Western Sub-Saharan Africa exhibited the most pronounced escalation in disease burden. Conversely, the analysis identifies Andean Latin America as the region demonstrating the most substantial decline in DALYs over the study period, underscoring heterogeneous epidemiological trajectories across global populations.
The estimated global incidence of AA in 1990 and 2019 was 21,742,836.45 (95% UI: 20,996,994.59–22,478,104.50) and 32,426,829.18 (95% UI: 31,370,861.58 to 33,473,493.05), respectively. The ASR of global AA incidence decreased from 418.64 (95% UI: 405.28–432.10) in 1990 to 405.70 (95% UI:392 0.72–418 0.82) in 2019. The global incidence of AA is declining with EAPC of – 0.13 (95%UI: – 0.13 to − 0.12). Both male and female AA incidence exhibited a declining trend, with the EAPC and its 95%CI being less than 0. In terms of SDI regions, the incidence rates of high, high-middle, and middle SDI countries and regions exhibit a declining trend (with EAPC and its 95%CI all less than 0), while the incidence rate of low SDI countries and regions demonstrates an increasing trend (with EAPC and its 95%CI all greater than 0). There is no significant change observed in low-middle SDI countries and regions (See Table 2). Regarding the incidence of alopecia areata across specific regions, its temporal trend demonstrated substantial consistency with corresponding DALY patterns (see Fig. 2B).
The global prevalence of AA was estimated to be as high as 12,273,004.26 (95% UI: 11,834,801.61–12,715,922.91) in 1990 and 18,398,715.17 (95% UI: 11,834,801.61–12,715,922.91) in 2019. The ASR of prevalence attributed to AA decreased from 237.28 (229.09 to 244.97) in 1990 to 229.93 (222.03–237.62) in 2019. There was a decreasing trend observed for global prevalence due to AA with an estimated annual percentage change of − 0.13 (− 0.13 to − 0.12). The prevalence of AA in both males and females exhibited a declining trend, with the EAPC and its 95%CI being less than 0. In terms of regions categorized by SDI, there was a decreasing trend in the incidence rates of high, high-middle, and middle SDI countries and regions (EAPC and its 95%CI were all less than 0). Conversely, low SDI countries and regions showed an increasing trend in prevalence rates (EAPC and its 95%CI were all greater than 0). No significant changes were observed in low-middle SDI countries/regions. The United States exhibited the highest ASR of prevalence and DALYs among the 204 countries and regions, with rates of 355.58 (95% UI: 345.23 −365.14) and 11.48 (95% UI: 7.25−16.94), respectively (See Table 3; Fig. 2C).
Global burden of AA by age and sex
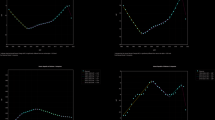
The period from 1990 to 2019 witnessed higher rates of DALYs, incidence, and prevalence among females compared to males (see Fig. 3). From 1990 to 2019, there was a downward trend in DALYs, incidence, prevalence and the global burden of disease across all age groups. This decline was particularly pronounced among individuals aged over 70 years old (see Fig. 4). The age group with the highest burden is 25 to 39 years old, exhibiting the highest DALYs, incidence, prevalence in terms of both number and rate (refer to Fig. 5). Additionally, it can be observed that the burden generally decreases with increasing age.
Global number and age-standardized rate (per 100,000) of DALYs (A), incidence (B) and prevalence (C) for AA by sex from 1990 to 2019. The bar and black error line represent the cases with its 95% UI. The solid line and shading indicate the ASRs with its 95% UI. Rate is per 100,000 population. ASR, age-standardized rate; DALYs, disability-adjusted life years; UI, uncertainty interval.
Global number and rate (per 100,000) of DALYs (A), incidence (B) and prevalence (C) for AA by age groups in 2019.The bar represents the cases. The solid line and shading indicate the rate with its 95% UI. Rate is per 100,000 population. DALYs, disability-adjusted life years; UI, uncertainty interval.
Global burden of AA by SDI
In various SDI countries and regions, it is evident that the burden of high SDI countries and regions is more significant across all age groups compared to other SDI. Women residing in high SDI countries and regions exhibit the highest incidence, prevalence, and DALYs burden (refer to Supplementary Fig. 1, 2, and3), particularly those aged between 25 and 39 years (refer to Fig. 6). The areas were ranked in descending order based on the ASR of DALYs in 2019 as follows: high SDI areas had a rate of 9.57 (95%UI: 6.05 ~ 14.26), high-middle SDI areas had a rate of 7.62 (95% UI: 4.79 ~ 11.28), middle SDI area had a rate of 7.47 (95% UI: 4.71 ~ 11.04), low-middle SDI area had a rate of 6.70 (95% UI: 4.20 ~ 9.96), and low SDI areas had a rate of 6.55(95%UI : 4 0.15 ~ 9.74). From the year 1990 to 2019, the highest decline in ASR of DALY rates was observed in the high SDI area with an EAPC of -0.816 (95% UI: -0.192 to -0.124), while the fastest increase occurred in the low SDI area with an EAPC of 0.037 (95% UI: 0.033 to 0.400).
Analysis of Keywords and Research Hotspots on AA.
The keywords from the 4429 articles were extracted and VOSviewer software was used to analyze cooccurrence. Among 44,622 keywords, 617 keywords with a cumulative frequency greater than 25 were selected for analysis. Finally, 365 keywords were used for the cooccurrence analysis. 365 keywords are divided into 4 clusters according to their relevance, Cluster 1(red, 116 items), cluster 2 (green, 112 items), cluster 3 (blue, 107 items), cluster 4 (yellow, 30 items) (Fig. 7A).
The analysis of keywords in publications of AA. (A) The words were divided into five clusters: Cluster 1 (red area) primarily pertains to clinical trials and studies on AA; Cluster 2 (green area) focuses on the pathogenesis of AA; Cluster 3 (blue region) is about the epidemiological characteristics and risk factors of AA; Cluster 4 (yellow area) centers around the diagnosis of AA.The circle with a large size represented the keywords that appeared at a high frequency; (B) Distribution of keywords was presented according to the appearance for the average time. The blue colour represented early appearance and yellow colour recent appearance. This figure was generated using VOSviewer software (version 1.6.18).
Cluster 1 (red area) primarily pertains to clinical trials and studies on AA, including the efficacy, clinical trial, safety, treatment, double blind, janus kinase inhibitor, platelet rich plasma, side effect, adverse effect. Cluster 2 (green area) focuses on the pathogenesis of AA, encompassing cell, expression, pathway, gene, cytokine, serum, receptor, immune cell, oxidative stress. Cluster 3 (blue region) is about the epidemiological characteristics and risk factors of AA, including age, prevalence, cohort study, female, child, burden, syndrome. Cluster 4 (yellow area) centers around the diagnosis of AA, encompassing diagnosis, feature, examination, scalp biopsy, follicle.
Then we utilized VOSviewer to assign colors to keywords based on their appearance time, enabling us to directly visualize the recent research hotspots of AA. The color blue represents relatively early keyword appearances, while yellow indicates more recent occurrences. Research trends over the years have focused on alopecia tool (Cluster 1), platelet rich plasma (Cluster 1), adverse event (Cluster 1), janus kinase inhibitor (Cluster 1), tofacitinib (Cluster 1),baricitinib (Cluster 1), dupilumab (Cluster 1), jak inhibitor (Cluster 1), drug administration (Cluster 1), biomarker (Cluster 2), pathway (Cluster 2), activator (Cluster 2), oxidative stress (Cluster 2), transcription (Cluster 2), nationwide population (Cluster 3), comorbidity (Cluster 3), higher risk (Cluster 3), hazard ratio (Cluster 3), frontal fibrosing alopecia (Cluster 4), nonscarring alopecia (Cluster 4). These terms with high frequency that have emerged in recent years represent current research hotspots and the future research trend (Fig. 7B). The recent focus in the field of AA has been primarily on clinical trials and the evaluation of various treatment modalities, particularly JAK inhibitors and autologous derivative product.
Discussion
According to statistics, the United States spent a staggering $46.5 billion exclusively on hair salon care in 2019 (https://www.statista.com/statistics/1221846/hair-salon- market-size-usa/). Multiple studies indicate that out-of-pocket costs for AA patients range from $500 to $3,300 per year or even up to $5,000 per month if they require drugs not covered by medicare16,17,18. Despite the substantial impact of AA on patients’ quality of life, global epidemiological investigations have yet to establish standardized criteria, and comprehensive assessment systems for evaluating the disease’s economic burden remain underdeveloped. This knowledge gap directly impedes the optimization of diagnostic and therapeutic strategies, particularly in achieving breakthroughs in critical areas such as elucidating pathogenesis mechanisms (e.g., dysregulation of JAK-STAT signaling pathways) and developing innovative treatments. To address these limitations, this study systematically delineates the epidemiological characteristics of AA across different genders, geographical regions, and SDI quintiles using the latest GBD 2019 database. Concurrently, through bibliometric analysis, we conduct a comprehensive assessment of current international research trends and focal points, thereby providing empirical support for guiding future scientific investigations. Our findings reveal a significant global increase in DALYs, incidence rates, and prevalence of AA from 1990 to 2019. While high-SDI nations demonstrate declining incidence rates, they paradoxically maintain some of the world’s highest disease burden metrics. Conversely, low-SDI countries exhibit escalating AA-related health burdens. Notably, younger populations and female individuals appear disproportionately affected by AA-associated health impacts. Regarding therapeutic advancements, JAK inhibitors (JAKis) and platelet concentrate emerge as the most promising treatment modalities within current research paradigms, though the evidentiary foundation supporting these interventions requires substantial strengthening through rigorous clinical validation.
Characteristics of AA distribution across populations of different gender, geographic regions, and SDI levels
From 1990 to 2019, the global number of AA incident cases increased by over 10.68 million, representing a growth rate exceeding 49.41%. This absolute increase must be interpreted within the context of global demographic transitions, as the proportion of working-age individuals (15–49 years) - the population most susceptible to AA and primary healthcare consumers - expanded by approximately 23% during the same period. Notably, despite expanding population denominators, ASRs of DALYs, incidence, and prevalence for AA demonstrated declining trends.
Socioeconomic disparities significantly exacerbate healthcare inequalities. In low-income countries, inadequate health insurance coverage forces most AA patients to rely on out-of-pocket payments, contrasting sharply with high-SDI regions where populations benefit from enhanced healthcare accessibility (exemplified by substantially higher dermatoscope utilization rates in developed nations versus low-SDI countries) and improved disease awareness (evidenced by marked increases in primary care diagnostic accuracy following AA inclusion in medical training programs)19,20. While disease burden reductions were most pronounced in high-SDI regions, these areas paradoxically maintained the world’s highest AA burden in 2019, with high-SDI nations exhibiting age-standardized prevalence rates 1.5-fold greater than low-SDI counterparts - a disparity potentially and partially attributable to diagnostic biases arising from over-medicalized screening practices in affluent healthcare systems.
From 1990 to 2019, low Socio-demographic Index (SDI) regions exhibited rising age-standardized rates (ASRs) of disability-adjusted life years (DALYs), incidence, and prevalence of alopecia areata (AA) compared to high-SDI areas, with sub-Saharan Africa demonstrating an AA-related years lived with disability growth rate far exceeding global averages. Racial disparities in AA susceptibility are strongly linked to genetic determinants, as evidenced by heightened vulnerability observed in African Americans and Asian populations relative to White counterparts in the United States and United Kingdom21,22. The HLA-DQB1*03 allele, located on chromosome 6p and functioning as a polymorphic histocompatibility antigen critical to immune regulation, has emerged as a potential pan-AA susceptibility marker, showing higher carrier frequencies in African-descent populations compared to White groups23.
Beyond genetic predisposition, environmental and socioeconomic interactions amplify disease risk - particularly in marginalized communities through exposure to endocrine-disrupting chemicals in substandard hair products (common in regions with unregulated cosmetic supply chains) and chronic psychosocial stressors. Systemic racism exacerbates physiological impacts, as evidenced in African American cohorts demonstrating elevated cortisol levels from sustained discrimination-related stress, which disrupts hair follicle stem cell differentiation, accelerates telogen phase entry, and potentially synergizes with androgen pathways through cortisol-mediated enhancement of 5α-reductase activity. This dual mechanism not only aggravates androgenetic alopecia progression but may indirectly intensify AA pathogenesis via dysregulated follicular cycling24,25. Compounding these biological factors, healthcare inequities disproportionately limit diagnostic access in underserved populations, with reduced utilization of specialty care services further obscuring true disease burden in vulnerable demographics. The convergence of genetic susceptibility, environmental exposures, psychosocial stressors, and structural healthcare barriers creates multiplicative risk amplification in specific racial and socioeconomic groups, suggesting current epidemiological data may substantially underestimate AA’s actual impact in low-SDI regions22,26.
Emerging evidence highlights a disproportionate alopecia areata (AA) burden among women aged ≥ 45 years, with cohort studies in menopausal women demonstrating that diminished estrogen receptor β (ERβ) expression impairs hair follicle stem cell proliferation – a mechanism strongly associated with postmenopausal AA incidence surges27,28. Our findings indicate that females in high-SDI countries exhibit the highest age-standardized incidence, prevalence, and DALY rates, particularly among those aged 25–39 years. This disparity may reflect occupational stressors and delayed childbearing patterns prevalent in developed nations. Autoimmune mechanisms further contribute to sex-based burden differences, as women undergo profound endocrine-immune shifts during reproductive milestones (puberty, pregnancy, lactation, menopause). Pregnancy-induced estrogen surges disrupt Th1/Th2 cytokine equilibrium, compromising immune tolerance and promoting T-cell activation – pivotal pathophysiological drivers of AA3,29,30.
Contemporary epidemiological observations suggest a cyclical interplay between hair loss and psychological distress among adolescent populations, particularly in resource-advantaged regions where youth cohorts exhibit elevated stress profiles linked to educational and occupational pressures. This self-reinforcing dynamic appears most pronounced in societies characterized by competitive socioeconomic environments, with age-specific stress metrics reflecting generational shifts in psychosocial burdens over recent decades31,32. Given the disproportionate burden among young adults, we advocate integrating AA screening into university mental health assessments and establishing early intervention protocols for high-risk demographics, particularly targeting 25-29-year-old women in developed regions. This dual approach has the potential to address both the neuroendocrine triggers of AA exacerbation and systemic gaps in youth-focused dermatological care26.
Based on the findings of our analysis, it is imperative for public health policymakers to possess a comprehensive understanding of the variations in the disease burden associated with AA across different geographical regions, ethnicities, genders, and age groups. Enhancing financial coverage for both diagnosis and treatment of AA would contribute significantly towards mitigating the burden inequality prevalent among diverse racial and socioeconomic cohorts.
Current emerging treatment trends in alopecia areata
In response to the aforementioned disease burden characteristics, the field of alopecia areata research is actively investigating effective therapeutic strategies. Analysis of high-frequency terms in this domain using VOSviewer software reveals that treatment modalities represented by JAK kinase inhibitors (JAKis) and platelet concentrates have emerged as the most promising research directions due to their demonstrated clinical efficacy. Notably, these therapeutic breakthroughs potentially correlate with the relative mitigation of disease burden in high-SDI countries, possibly reflecting the critical influence of healthcare resource distribution on disease prevention and control. Furthermore, high-quality clinical studies investigating JAKis applications in alopecia areata have been conducted, with some demonstrating encouraging outcomes. However, the evidence base for platelet concentrate therapy remains insufficient, and its long-term efficacy and safety require further validation through additional rigorous investigations9,33.
JAK plays a crucial role in hematopoiesis, immune response, and host defense34. In AA, IFN-γ has been demonstrated to induce IL-15 production in hair follicles via the JAK1/2 signaling pathway. Additionally, IL-15 stimulates T cells to produce IFN-γ through the JAK1/3 signaling pathway, thereby amplifying the inflammatory response surrounding hair follicles8,35. Consequently, JAK inhibitors represent a promising therapeutic option for treating AA. Baricitinib, an oral, reversible and selective inhibitor of JAK1/JAK28. In Phase 2, BRAVE-AA1, and BRAVE-aa2 trials, Baricitinib demonstrated superior efficacy compared to placebo in promoting hair growth among adult patients with severe AA after a 36-week treatment period36. Based on these findings, as of June 2022, both the EMA and FDA have granted approval for a JAKis as the first and currently sole labeled therapy for adult AA. In the two subsequent Phase III trials (BRAVE-AA1 and BRAVE-AA2), patients treated with Baricitinib at doses of 4 mg and 2 mg, respectively, demonstrated an improvement in hair regrowth rate at week 52. Specifically, SALT scores ≤ 20 were observed in 40.9% and 21.2% of patients in the BRAVE-AA1 trial, while in the BRAVE-AA2 trial, these scores were achieved by 36.8% and 24.4% of patients37. Ritlecitinib is a selective JAK3/TEC kinase inhibitor that attenuates autoimmune responses and promotes hair follicle regeneration by inhibiting the JAK-STAT pathway and reducing pro-inflammatory cytokines. Phase II and III clinical trials are investigating its use in alopecia areata (AA). Ritlecitinib has been shown to significantly reduce serum levels of IFN-γ, IL-15, and other inflammatory markers, thereby improving conditions conducive to hair growth38. A 24-week double-blind phase (DBP) of a Phase 2a study (NCT02974868) found Ritlecitinib and Brepocitinib effective and well-tolerated in patients with ≥ 50% scalp hair loss due to AA. Common adverse events were mild to moderate, including infections and skin/nervous system disorders39. The multicenter, randomized, double-blind, placebo-controlled Phase III ALLEGRO trial (NCT03732807) enrolled 718 patients ≥ 12 years with moderate-to-severe AA. Preliminary findings indicated that 23% of patients in the Ritlecitinib 50 mg group achieved a SALT score of ≤ 20 at week 24, compared to 1.5% in the placebo group. Ritlecitinib demonstrates significant efficacy and a favorable safety profile in AA treatment40.
While JAKis exhibit promising potential in reversing immune-mediated hair loss in AA, there is a risk of inducing off-target damage due to the redundancy of JAK proteins involved in cytokine, growth factor, and hormone receptor signaling8,41. However, adverse events associated with JAKis usage in AA patients have been limited and mild thus far, demonstrating a favorable safety profile42. In comparison to currently available systemic immunosuppressants, baricitinib appears to offer a more advantageous benefit-to-risk ratio for treating AA43,44. Furthermore, as larger-scale clinical trials continue to be conducted, the likelihood of additional FDA approvals for JAKis as treatment options for AA in the near future also increases. Future research directions concerning JAKis in AA are expected to concentrate on several key areas: (1) long-term efficacy and safety evaluations; (2) combination therapies with other treatment modalities; (3) biomarker investigations to predict treatment responses; and (4) strategies for maintenance therapy following discontinuation.
Platelet concentrates, including PRP, PRF, and CGF, have garnered significant attention in the field of AA due to their autologous blood origin, non-immunogenicity, and straightforward preparation methods45. PRP offers a rich source of exogenous growth factors that can regulate hair growth cycle-related proteins and signaling pathways46. By promoting cell proliferation, preventing apoptosis, facilitating vascularization, PRP effectively regulates the hair growth cycle. A randomized controlled clinical trial comprising 11 trials demonstrated that compared to placebo interventions, PRP injections significantly increased the number of hair follicles as well as hair thickness and density47. In addition, patients’ overall satisfaction with PRP treatment was also high, and no significant adverse reactions were observed. However, the clinical application of PRP in AA treatment does have certain limitations: despite numerous studies demonstrating its positive effect on hair growth, there is still a lack of long-term follow-up to determine its efficacy over time. The exact mechanism of action for PRP remains incompletely understood, and the absence of standardized protocols for preparation schemes, injection levels and distances, optimal injection concentrations and frequencies, as well as injection volumes etc., along with the lack of standardized methods for evaluating hair regeneration may impact research outcomes and restrict comprehensive evaluation of clinical efficacy and widespread adoption of PRP48. Overall, PRP is presently utilized as a promising clinical treatment option for hair regeneration therapy, characterized by its high safety profile and low incidence of adverse reactions. However, it is not considered an absolutely effective or specific treatment. These issues necessitate further scholarly attention in order to expand the application of self-derived products within an evidence-based model49.
While this study systematically elucidates the regional heterogeneity of alopecia areata (AA) disease burden and its association patterns with the Sociodemographic Index (SDI), it should be specifically noted that the current analysis could not comprehensively address regional disparities in therapeutic accessibility due to the absence of critical parameters in existing global public health databases (e.g., Global Burden of Disease [GBD], Institute for Health Metrics and Evaluation [IHME]), including national healthcare resource allocation, insurance coverage, and clinical adoption rates of innovative therapies. This data gap may compromise the holistic interpretation of disease burden distribution mechanisms, particularly hindering quantitative assessment of healthcare accessibility disparities as potential modifiers when deciphering the underlying drivers behind declining incidence in high-SDI nations versus escalating burden in low-SDI regions. We propose that establishing multidimensional surveillance systems integrating therapeutic modalities, pharmaceutical accessibility, and health policy interventions would substantially enhance the precision of global AA burden estimation models, thereby informing equitable resource allocation strategies.
Limitation
This study has several limitations. First, regional heterogeneity in data sources may compromise result accuracy: the GBD 2019 database relies on markedly divergent regional datasets (e.g., fragmented hospital records in sub-Saharan Africa versus comprehensive healthcare data in Western Europe), which may systematically underestimate mild cases and true prevalence rates; diagnostic inconsistencies—such as misclassifying alopecia areata (AA) as “unspecified alopecia” without standardized tools like dermoscopy—further exacerbate reporting inaccuracies, particularly in regions with low dermatologist-to-population ratios. Second, the model inadequately addresses complex sociocultural factors: GBD modeling insufficiently incorporates region-specific subtype distributions, environmental confounders, and cultural perceptions (e.g., stigma-driven underreporting or appearance-related anxiety-induced overreporting). Third, analytical granularity constraints exist: current burden estimates apply only to national-level interpretations and cannot be directly extrapolated to individuals, while the bibliometric analysis exclusively included English-language publications, potentially introducing language bias. Although AA significantly impacts quality of life, its lack of mortality association may lead to underdetection in epidemiological surveillance, and cross-disciplinary variables such as economic status and psychological impacts remain unsystematized in existing databases. These limitations underscore the need for future assessments integrating multi-source heterogeneous data and AI-assisted technologies to optimize evaluation frameworks; nevertheless, our findings retain critical relevance for regional health policy formulation.
Conclusion
The DALYs, incidence and prevalence number of patients with AA increased significantly from 1990 to 2019, despite a decrease in standardized rate of DALYs, incidence, and prevalence due to the expanding global population base. It is worth mentioning that countries with high SDI have observed a decrease in the incidence of AA; nevertheless, they continue to bear one of the highest disease burdens worldwide. Conversely, low SDI countries have experienced an escalation in the disease burden related to AA. These findings underscore that AA remains a significant public health concern globally and its disease burden persists at alarming levels. Furthermore, young individuals and women appear to be disproportionately affected by this condition necessitating targeted health policies tailored towards addressing regional disparities and specific populations. In terms of research hotspots in the field of AA, the treatment program represented by JAKis and autologous derivatives undoubtedly stands out as one of the most promising avenues. However, there is a need for further strengthening the level of evidence in related studies, as well as conducting long-term efficacy observations and assessing potential adverse reactions of these drugs.
Data availability
The data utilized in this study are accessible through the links to public data resources outlined in the Methods section or can be obtained from the corresponding author on reasonable request.
Abbreviations
- AA:
-
Alopecia areata
- DALYs:
-
Disability adjusted life years
- GBD:
-
Global burden of disease
- SDI socio:
-
demographic index
- JAK:
-
Janus kinases
- QoL:
-
Quality of life
- ASR:
-
Age-standardized rate
- UI:
-
Uncertainty interval
- YLD:
-
Years of life lived with disability
- YLL:
-
Years of life lost
- EAPC:
-
Estimated annual percentage change
- CI:
-
Confidence interval
- PRP:
-
Platelet rich plasma
- PRF:
-
Platelet rich fibrin
- CGF:
-
Concentrated growth factor
References
Meah, N. et al. The alopecia areata consensus of experts (ACE) study: Results of an international expert opinion on treatments for alopecia areata. J. Am. Acad. Dermatol. 83(1), 123–130. https://doi.org/10.1016/j.jaad.2020.03.004 (2020).
Meah, N. et al. The alopecia areata consensus of experts (ACE) study part II: Results of an international expert opinion on diagnosis and laboratory evaluation for alopecia areata. J. Am. Acad. Dermatol. 84(6), 1594–1601. https://doi.org/10.1016/j.jaad.2020.09.028 (2021).
Passeron, T. et al. Inhibition of T-cell activity in alopecia areata: Recent developments and new directions. Front. Immunol. 14, 1243556. https://doi.org/10.3389/fimmu.2023.1243556 (2023).
Rencz, F. et al. Alopecia areata and health-related quality of life: A systematic review and meta-analysis. Br. J. Dermatol. 175(3), 561–571. https://doi.org/10.1111/bjd.14497 (2016).
Titeca, G. et al. The psychosocial burden of alopecia areata and androgenetica’: A cross-sectional multicentre study among dermatological out-patients in 13 European countries. J. Eur. Acad. Dermatol. Venereol. 34(2), 406–411. https://doi.org/10.1111/jdv.15927 (2020).
Zhou, C. et al. Alopecia areata: An update on etiopathogenesis, diagnosis, and Management. Clin. Rev. Allergy Immunol. 61(3), 403–423. https://doi.org/10.1007/s12016-021-08883-0 (2021).
Pratt, C. H. et al. Alopecia areata. Nat. Rev. Dis. Primers. 3, 17011. https://doi.org/10.1038/nrdp.2017.11 (2017).
Freitas, E., Guttman-Yassky, E. & Torres, T. Baricitinib for the treatment of alopecia Areata. Drugs 83(9), 761–770. https://doi.org/10.1007/s40265-023-01873-w (2023).
Shimizu, Y. et al. Regenerative medicine strategies for hair growth and regeneration: A narrative review of literature. Regen Ther. 21, 527–539. https://doi.org/10.1016/j.reth.2022.10.005 (2022).
Barbulescu, C. C. et al. Harnessing the power of regenerative therapy for vitiligo and alopecia Areata. J. Invest. Dermatol. 140(1), 29–37. https://doi.org/10.1016/j.jid.2019.03.1142 (2020).
Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 396(10258), 1204–1222 (2020). https://doi.org/10.1016/S0140-6736(20)30925-9
Quantifying risks and interventions that have affected the burden Of diarrhoea among children younger than 5 years: an analysis of the global burden of disease study 2017. Lancet Infect. Dis. 20(1), 37–59. https://doi.org/10.1016/S1473-3099(19)30401-3 (2020).
Zhang, S. et al. The global burden and associated factors of ovarian cancer in 1990–2019: Findings from the global burden of disease study 2019. BMC Public. Health. 22(1), 1455. https://doi.org/10.1186/s12889-022-13861-y (2022).
Global age-sex. -specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: A comprehensive demographic analysis for the global burden of disease study 2019. Lancet 396(10258), 1160–1203. https://doi.org/10.1016/S0140-6736(20)30977-6 (2020).
Zhang, W. et al. Global burden of prostate Cancer and association with socioeconomic status, 1990–2019: A systematic analysis from the global burden of disease Study. J. Epidemiol. Glob. Health. 13(3), 407–421. https://doi.org/10.1007/s44197-023-00103-6 (2023).
Li, S. J. et al. Association of Out-of-Pocket health care costs and financial burden for patients with alopecia Areata. JAMA Dermatol. 155(4), 493–494. https://doi.org/10.1001/jamadermatol.2018.5218 (2019).
Mesinkovska, N. et al. Burden of illness in alopecia areata: A cross-sectional online survey study. J. Investig Dermatol. Symp. Proc. 20(1), S62–S68. https://doi.org/10.1016/j.jisp.2020.05.007 (2020).
Mostaghimi, A. et al. All-cause health care resource utilization and costs among adults with alopecia areata: A retrospective claims database study in the united States. J. Manag. Care Spec. Pharm. 28(4), 426–434. https://doi.org/10.18553/jmcp.2022.28.4.426 (2022).
Anderson, P. et al. Alopecia areata treatment patterns and satisfaction: Results of a real-world cross-sectional survey in Europe. Dermatol. Ther. (Heidelb). 14(12), 3243–3258. https://doi.org/10.1007/s13555-024-01280-3 (2024).
Al-Dhubaibi, M. S. et al. Trichoscopy pattern in alopecia areata: A systematic review and meta-analysis. Skin. Res. Technol. 29(6), e13378. https://doi.org/10.1111/srt.13378 (2023).
Joshi, T. P. et al. Burden of atopic disease in black and Hispanic patients with alopecia areata: A case-control study in the all of Us research program. Int. J. Dermatol. 62(7), e393–e394. https://doi.org/10.1111/ijd.16528 (2023).
Lee, H. et al. Racial characteristics of alopecia areata in the united States. J. Am. Acad. Dermatol. 83(4), 1064–1070. https://doi.org/10.1016/j.jaad.2019.06.1300 (2020).
Hayran, Y. et al. Evaluation of HLA class I and HLA class II allele profile and its relationship with clinical features in patients with alopecia areata: A case-control study. J. Dermatolog. Treat. 33(4), 2175–2181. https://doi.org/10.1080/09546634.2021.1937478 (2022).
Pulopulos, M. M., Baeken, C. & De Raedt, R. Cortisol response to stress: The role of expectancy and anticipatory stress regulation. Horm. Behav. 117 https://doi.org/10.1016/j.yhbeh.2019.104587 (2020).
Thom, E. Stress and the hair growth cycle: Cortisol-Induced hair growth Disruption. J. Drugs Dermatol. 15(8), 1001–1004 (2016).
Singam, V. et al. Association of alopecia areata with hospitalization for mental health disorders in US adults. J. Am. Acad. Dermatol. 80(3), 792–794. https://doi.org/10.1016/j.jaad.2018.07.044 (2019).
Lephart, E. D. Human scalp hair: Modulation by various factors and hormones do estrogens inhibit or stimulate-A perplexing perspective. J. Cosmet. Dermatol. 18(6), 1860–1865. https://doi.org/10.1111/jocd.12888 (2019).
Oh, H. S. & Smart, R. C. An Estrogen receptor pathway regulates the telogen-anagen hair follicle transition and influences epidermal cell proliferation. Proc. Natl. Acad. Sci. U S A. 93(22), 12525–12530. https://doi.org/10.1073/pnas.93.22.12525 (1996).
Desai, M. K. & Brinton, R. D. Autoimmune disease in women: Endocrine transition and risk across the Lifespan. Front. Endocrinol. (Lausanne). https://doi.org/10.3389/fendo.2019.00265 (2019).
Natri, H. et al. The pregnancy pickle: Evolved immune compensation due to pregnancy underlies sex differences in human Diseases. Trends Genet. 35(7), 478–488. https://doi.org/10.1016/j.tig.2019.04.008 (2019).
Rosenberg, A. M. et al. Quantitative mapping of human hair greying and reversal in relation to life stress. Elife https://doi.org/10.7554/eLife.67437 (2021).
Tan, I. J. & Jafferany, M. Psychosocial impact of alopecia areata in paediatric and adolescent populations: A systematic review. J. Paediatr. Child. Health. 60(12), 778–782. https://doi.org/10.1111/jpc.16678 (2024).
Ismail, F. F. & Sinclair, R. JAK Inhibition in the treatment of alopecia areata—a promising new dawn?. Expert Rev. Clin. Pharmacol. 13(1), 43–51. https://doi.org/10.1080/17512433.2020.1702878 (2020).
O’Shea, J. J., Holland, S. M. & Staudt, L. M. JAKs and stats in immunity, immunodeficiency, and cancer. N. Engl. J. Med. 368(2), 161–170. https://doi.org/10.1056/NEJMra1202117 (2013).
Xing, L. et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 20(9), 1043–1049. https://doi.org/10.1038/nm.3645 (2014).
King, B. et al. Two phase 3 trials of baricitinib for alopecia Areata. N. Engl. J. Med. 386(18), 1687–1699. https://doi.org/10.1056/NEJMoa2110343 (2022).
Kwon, O. et al. Efficacy and safety of baricitinib in patients with severe alopecia areata over 52 weeks of continuous therapy in two phase III trials (BRAVE-AA1 and BRAVE-AA2). Am. J. Clin. Dermatol. 24(3), 443–451. https://doi.org/10.1007/s40257-023-00764-w (2023).
King, B. A. & Craiglow, B. G. Janus kinase inhibitors for alopecia areata. J. Am. Acad. Dermatol. 89(2S), S29–S32. https://doi.org/10.1016/j.jaad.2023.05.049 (2023).
Guttman-Yassky, E. et al. Ritlecitinib and brepocitinib demonstrate significant improvement in scalp alopecia areata biomarkers. J. Allergy Clin. Immunol. 149(4), 1318–1328. https://doi.org/10.1016/j.jaci.2021.10.036 (2022).
King, B. et al. Efficacy and safety of Ritlecitinib in adults and adolescents with alopecia areata: A randomised, double-blind, multicentre, phase 2b-3 trial. Lancet 401(10387), 1518–1529. https://doi.org/10.1016/S0140-6736(23)00222-2 (2023).
Lensing, M. & Jabbari, A. An overview of JAK/STAT pathways and JAK Inhibition in alopecia areata. Front. Immunol. 13, 955035. https://doi.org/10.3389/fimmu.2022.955035 (2022).
Yan, D. et al. The efficacy and safety of JAK inhibitors for alopecia areata: A systematic review and meta-analysis of prospective studies. Front. Pharmacol. 13, 950450. https://doi.org/10.3389/fphar.2022.950450 (2022).
Gupta, A. K. et al. Systematic review of newer agents for the management of alopecia areata in adults: Janus kinase inhibitors, biologics and phosphodiesterase-4 inhibitors. J. Eur. Acad. Dermatol. Venereol. 37(4), 666–679. https://doi.org/10.1111/jdv.18810 (2023).
King, B. et al. Integrated safety analysis of baricitinib in adults with severe alopecia areata from two randomized clinical trials. Br. J. Dermatol. 188(2), 218–227. https://doi.org/10.1093/bjd/ljac059 (2023).
Qu, Q. et al. Efficacy of Platelet-Rich plasma plus basic fibroblast growth factor on the treatment of androgenic Alopecia. Plast. Reconstr. Surg. 151(4), 630e–640e. https://doi.org/10.1097/PRS.0000000000010000 (2023).
Li, Z. J. et al. Autologous platelet-rich plasma: A potential therapeutic tool for promoting hair growth. Dermatol. Surg. 38(7 Pt 1), 1040–1046. https://doi.org/10.1111/j.1524-4725.2012.02394.x (2012).
Papakonstantinou, M. et al. Autologous Platelet-Rich plasma treatment for androgenic alopecia: A systematic review and meta-analysis of clinical Trials. Plast. Reconstr. Surg. 151(5), 739e–747e. https://doi.org/10.1097/PRS.0000000000010076 (2023).
Atiyeh, B., Oneisi, A. & Ghieh, F. Platelet-Rich plasma facial rejuvenation: Myth or Reality?. Aesthetic Plast. Surg. 45(6), 2928–2938. https://doi.org/10.1007/s00266-021-02300-9 (2021).
Gentile, P. & Garcovich, S. Systematic review: Platelet-rich plasma use in facial rejuvenation. Plast. Reconstr. Surg. 152(1), 72e–82e. https://doi.org/10.1097/PRS.0000000000010150 (2023).
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
DHF and YZH were the principal architects of the study design and paper composition; YHW and XX conducted the data analysis; LYX and CML critically reviewed the article for its intellectual content. All authors contributed to the critical review and editing of the manuscript. All authors had full access to the data in the study and accepted the responsibility for submitting this study for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ding, H., Yu, Z., Yao, H. et al. Global burden of alopecia areata from 1990 to 2019 and emerging treatment trends analyzed through GBD 2019 and bibliometric data. Sci Rep 15, 25869 (2025). https://doi.org/10.1038/s41598-025-07224-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07224-x