Abstract
Trim24 has been implicated in inflammatory processes and lipid metabolism, yet its role in atherosclerosis (AS) remains insufficiently explored. This study aimed to evaluate the diagnostic value of serum Trim24 in AS and its association with disease severity. This prospective case-control study included 137 AS patients and 137 healthy controls. Serum Trim24 and sCD163 levels were measured using ELISA. Clinical characteristics, including lipid profiles, inflammatory markers, and the Gensini score, were collected. Univariate and multivariate logistic regression analyses were performed to identify independent risk factors for AS, severe AS, and severe stenosis. Receiver operating characteristic (ROC) curve analysis was employed to evaluate the diagnostic performance of Trim24. Serum Trim24 levels were significantly higher in AS patients compared to healthy controls. Within the AS group, Trim24 levels were higher in patients with severe AS and severe stenosis compared to their non-severe counterparts. Trim24 exhibited strong diagnostic performance for AS (AUC = 0.928), severe AS (AUC = 0.826), and severe stenosis (AUC = 0.928). Additionally, sCD163 levels were significantly lower in AS patients, especially in those with high Trim24 expression, severe AS, and severe stenosis. ROC analysis showed moderate diagnostic value of sCD163 for AS and its severity (AUC = 0.708–0.778). Curve Estimation revealed a negative correlation between Trim24 and sCD163. Multivariate logistic regression identified Trim24 was an independent risk factors for AS, severe AS, and severe stenosis, respectively. Elevated serum Trim24 levels are associated with increased AS risk and severity, likely through modulation of macrophage polarization. Trim24 demonstrates strong potential as a diagnostic biomarker for AS and its progression, supporting further investigation in larger, more diverse populations.
Similar content being viewed by others
Introduction
Atherosclerosis (AS) is a chronic inflammatory disease characterized by the accumulation of lipid-rich plaques within arterial walls, leading to progressive narrowing and hardening of the arteries1,2. It is a major cause of cardiovascular diseases (CVDs) worldwide, significantly contributing to morbidity and mortality through adverse events including myocardial infarction and stroke3. As of 2019, approximately 330 million people in China were affected by cardiovascular disease, which accounted for 46.74% of all deaths in rural areas and 44.26% in urban areas4. Despite advances in therapeutic strategies, early detection and effective prevention of AS remain challenging due to the lack of sensitive and specific biomarkers for early-stage diagnosis5.
Inflammation and dysregulated lipid metabolism are central to the pathogenesis of AS. The accumulation of lipids in arterial walls triggers an inflammatory response, involving various immune cells, especially macrophages, which are key players in plaque formation and progression6,7. Tripartite Motif Containing 24 (Trim24) is a multifunctional protein implicated in the regulation of both inflammation and lipid metabolism8,9. Our Recent study suggest that it may influence macrophage polarization and contribute to the inflammatory processes in AS10. sCD163 is a soluble scavenger receptor shed by alternatively activated (M2) macrophages and is widely recognized as a biomarker of anti-inflammatory macrophage activity11. Given that Trim24 may regulate macrophage polarization by inhibiting M2 phenotype differentiation, sCD163 is likely to be affected by Trim24-mediated immune modulation. However, no clinical study has yet investigated the association between serum Trim24 and sCD163 in patients with AS. Given its role in these pathways, Trim24 shows promise as a novel biomarker for early diagnosis and disease monitoring in AS, addressing the limitations of traditional markers like cholesterol and C-reactive protein (CRP).
This prospective case-control observational study investigates the clinical diagnostic value of serum Trim24 in patients with AS. By analyzing its levels and correlation with clinical parameters, we aim to evaluate Trim24’s potential as a biomarker for AS diagnosis and predicting the need for interventional therapies such as stenting or angioplasty. Our findings will contribute to the understanding of Trim24’s role in AS and its potential as a novel diagnostic and therapeutic target.
Methods and materials
Patients and study design
This prospective case-control observational study included 137 AS patients who visited our hospital between October 2022 and January 2024, along with 137 healthy age and sex- matched individuals serving as controls. All participants were consecutively enrolled. AS diagnosis was confirmed using ultrasound or coronary CT angiography (CTA) based on the presence of arterial plaques or an intima-media thickness (IMT) ≥ 1.0 mm in any major artery12. Healthy controls were defined as individuals without AS or other severe diseases. The exclusion criteria included: (1) anatomical abnormalities or severe arterial distortion; (2) previous peripheral artery catheterization; (3) inability to complete CTA imaging or poor imaging quality; (4) malignancies, severe liver or renal dysfunction, autoimmune diseases, severe pulmonary diseases, or any other significant medical condition that could interfere with the study results. The study was conducted following the Declaration of Helsinki and approved by the Ethics Committee of The Third Affiliated Hospital of Guangzhou Medical University (Approval No. [2024]099). Written informed consent was obtained from all participants.
Sample size calculation was based on the formula for case-control studies: n = 2*σ2 (Z1−β+Z1−α/2)2/d2, where σ represents the standard deviation of Trim24 in AS patients, Z1−β represents the desired statistical power (0.84 for 80% power), Z1−α/2 represents the critical value for a two-tailed test (1.96 for a 5% significance level), and d represents the expected effect size (0.5 for middle effect size)13. Based on preliminary data and pre-experimental results, σ was estimated to be 1.48 (supplementary file), with a minimum required sample size of 137 per group.
Subgroup analysis
Besides, in the subgroup analysis, the AS patients were further divided into severe/non-severe AS groups and severe/non-severe stenosis groups, respectively. The severity of AS was classified based on the necessity of interventional treatment: patients who underwent percutaneous coronary intervention (PCI), such as stent implantation or balloon angioplasty, were classified as the severe AS group. In contrast, those who did not require PCI and only underwent coronary angiography were classified as the non-severe AS group. The criteria for determining the necessity of PCI included significant coronary artery stenosis (≥ 70% in major epicardial arteries or ≥ 50% in the left main artery), combined with clinical symptoms such as unstable angina or evidence of myocardial ischemia from non-invasive tests (e.g., stress test, myocardial perfusion imaging)12.
For the severity of coronary artery stenosis, patients were categorized into three groups according to their Gensini score, using tertile cutoffs: low (0 ≤ Gensini score ≤ 26), medium (27 ≤ Gensini score ≤ 54.5), and high (Gensini score ≥ 55)14,15. The Gensini score was calculated based on the degree of luminal narrowing and the location of the lesions in the coronary arteries, with higher scores indicating more severe and extensive disease. Patients in the high Gensini score group (≥ 55) were defined as having severe stenosis, indicating a higher burden of atherosclerotic disease and an increased risk of cardiovascular events.
Determination of Trim24 and soluble CD163 (sCD163)
Serum Trim24 (cat. no. EH13196) and soluble sCD163 (cat. no. EH007) levels were measured using two commercial ELISA kits (Wuhan Fine Biotech Co., Ltd., Wuhan, China) according to the manufacturer’s instructions. All samples were tested in duplicate, and results with a coefficient of variation (CV) > 15% between replicates were reanalyzed. All assays were performed using the same reagent lot to ensure consistency. This study focused on early diagnostic assessment; therefore venous blood samples (approximately 5 mL) were collected from all participants in the morning after an overnight fast, within 48 h of enrollment. Samples were centrifuged at 3,000 rpm for 10 min, and the serum was stored at -80 °C until analysis.
Briefly, 100 µL of standard or diluted sample was added to each well of a 96-well plate pre-coated with anti-human Trim24 or CD163 antibodies. After incubation at 37 °C for 90 min and subsequent washing, 100 µL of biotin-labeled detection antibody was added, followed by incubation at 37 °C for 60 min. The wells were washed, and 100 µL of HRP-streptavidin solution was added and incubated for 30 min. The plate was washed again, and 90 µL of TMB substrate was added for color development at 37 °C for 10–20 min. The reaction was stopped with 50 µL of stop solution, and optical density (OD) was measured at 450 nm. Serum Trim24 or sCD163 concentrations were calculated using a standard curve generated from known concentrations of Trim24 or sCD163.
Data collection
Comprehensive clinical data were collected for all participants, including liver and renal function tests, lipid profiles, and complete blood count. Relevant clinical information, such as comorbidities, current alcohol consumer and smoker, was also documented to account for potential confounding factors.
Statistical analysis
Statistical analyses were performed using SPSS 22.0 software. The normality of continuous variables was assessed using the Kolmogorov-Smirnov test. All data were non-normally distributed, presented as median (interquartile range, IQR) and were analyzed using the Mann-Whitney U test. Categorical variables were expressed as frequencies and percentages, and group comparisons were conducted using the chi-square test. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic accuracy of serum Trim24 or sCD163. Post-hoc power analyses based on ROC-derived effect sizes were conducted using G*Power software (version 3.1.9.7) to evaluate statistical power for each subgroup comparison. Spearman’s analysis and Curve Estimation were used for correlation analysis. Binary logistic regression analysis was used to identify independent predictors for the presence and severity of AS. A p-value < 0.05 was considered statistically significant.
Results
Basic clinical characteristics of all participants
The present case-control observational study included 137 AS patients and 137 healthy controls. A total of 94 (68.61%) cases were with severe AS and 109 (79.56%) cases were with Gensini score ≥ 55. As shown in Table 1, AS patients exhibited significantly higher levels of BMI, TC, TG, LDL-C, WBC, hs-CRP, WBC, FIB, HCY, and a higher percentage of current smokers and multi-plaques compared to healthy controls (all p < 0.05). Conversely, HDL-C levels were significantly lower in AS patients than in healthy controls (p < 0.05). No significant differences were observed in other indices between the two groups.
Serum Trim24 is elevated in AS and severe AS patients
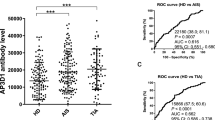
In this study, we evaluated serum Trim24 levels across different patient groups to assess its potential as a biomarker for AS severity. As shown in (Fig. 1), serum Trim24 levels were significantly higher in AS patients than in healthy controls (p < 0.05). Among AS patients, those requiring interventional treatment had significantly higher Trim24 levels compared to the non-severe group (p < 0.05), indicating a correlation between Trim24 expression and disease severity. Furthermore, patients with severe coronary artery stenosis (Gensini score ≥ 55) exhibited significantly higher Trim24 levels than those with non-severe stenosis (p < 0.05), suggesting that Trim24 expression is associated with the extent of coronary artery narrowing.
Correlation of Trim24 with clinical characteristics of AS patients
Next, we divided the AS patients into high and low Trim24 expression groups based on the median Trim24 level (1006.08 pg/ml). Our analysis revealed that age, LDL-C, and HCY levels, along with categorical variables such as current alcohol consumption, the presence of severe AS, and severe stenosis, showed significant differences between the two groups (Table 2). Specifically, the high Trim24 group exhibited significantly higher levels of age, LDL-C, and HCY, and a higher proportion of patients with severe AS and severe stenosis, while the proportion of current alcohol consumers was significantly lower compared to the low Trim24 group (p < 0.05 for all). Furthermore, Spearman’s correlation analysis demonstrated a positive association between serum Trim24 levels and age, LDL-C, and HCY, indicating that elevated Trim24 is linked to increased age, worsened lipid profiles, and elevated homocysteine, which are risk factors for atherosclerosis progression (Table 3).
Diagnostic value of Trim24 for AS and severe AS patients
The diagnostic performance of serum Trim24 for predicting AS, severe AS, and severe stenosis was evaluated using ROC curve analysis. As shown in (Fig. 2), for distinguishing AS patients from healthy controls, the area under the ROC curve (AUC) was 0.928, with a sensitivity of 90.51% and specificity of 87.59% at a cutoff value of > 473.1 pg/mL. For predicting severe AS, the AUC was 0.826, with sensitivity of 91.49% and specificity of 60.47% at a cutoff value of > 688.2 pg/mL. For differentiating severe stenosis (Gensini score ≥ 55) from non-severe stenosis, the AUC was 0.928, with sensitivity of 92.66% and specificity of 89.29% at a cutoff value of > 679.7 pg/mL. Post-hoc power analyses based on the ROC comparisons demonstrated statistical powers of > 99.99% for distinguishing 100% for AS versus healthy controls (AUC = 0.928), severe versus non-severe AS (AUC = 0.826), and > 99.99% for severe versus non-severe stenosis (AUC = 0.928), confirming the adequacy of sample size for these subgroup evaluations. Overall, Trim24 demonstrated good diagnostic value in all three scenarios, supporting its potential role as a biomarker for AS and its severity.
Relationship between Trim24 and sCD163
Then, we compared sCD163 levels among different patient groups, including AS vs. healthy controls, severe AS vs. non-severe AS, severe stenosis vs. non-severe stenosis, and high vs. low Trim24 expression groups. sCD163 levels were significantly lower in AS patients, severe AS, severe stenosis, and high Trim24 expression groups compared to their respective counterparts (p < 0.05 for all, Fig. 3A−C). In addition, ROC curve analyses showed that sCD163 had moderate diagnostic performance for AS, severe AS, and severe stenosis, with corresponding AUC values of 0.708, 0.778, and 0.727, respectively (Fig. 3D−F). Curve Estimation analysis using a cubic model revealed a significant negative correlation between Trim24 and sCD163 levels (p = 0.017) (Fig. 4), suggesting that elevated Trim24 is associated with reduced M2 macrophage polarization, contributing to a pro-inflammatory environment in atherosclerosis.
Serum sCD163 expression and diagnostic performance across different groups. (A–C) sCD163 levels were significantly lower in atherosclerosis (AS) patients compared to healthy controls (A), in severe AS compared to non-severe AS patients (B), and in patients with Gensini score ≥ 55 compared to < 55 (C). (D–F) ROC curve analyses showing the diagnostic value of sCD163 for AS (D), severe AS (E), and severe stenosis based on Gensini score (F). Data were non-normally distributed and analyzed using the Mann–Whitney U test.
Logistic analysis of risk factors for AS and severe AS
To further investigate the potential risk factors for atherosclerosis (AS) and its severity, we performed univariate and multivariate logistic regression analyses on all the parameters discussed above. First, univariate logistic regression was conducted to assess the association of each parameter with AS vs. healthy controls, severe AS vs. non-severe AS, and severe stenosis vs. non-severe stenosis. The detailed results are presented in Supplementary Tables. Since the healthy controls had no comorbidities or IMT measurements, these factors were not included in the model comparing AS patients to healthy controls. Similarly, the Gensini score was excluded from the severe AS vs. non-severe AS, and severe stenosis vs. non-severe stenosis models, as it directly reflects disease severity. The analysis revealed that BMI, TC, TG, HDL-C, LDL-C, WBC, hs-CRP, FIB, HCY, current smoker, sCD163 and Trim24 were significant risk factors for AS, while age, sex, HCY, sCD163 and Trim24 were associated with severe AS, and HCY, sCD163 and Trim24 were linked to severe stenosis (p < 0.05 for all).
Next, significant variables identified in the univariate analysis were included in a multivariate logistic regression model (Table 4). Nagelkerke R2 values were calculated to evaluate the proportion of variance explained by the model. Multicollinearity was checked using the variance inflation factor (VIF), with all variables showing acceptable values (VIF < 10). Additionally, the Box-Tidwell test (with a Bonferroni correction was applied to adjust for multiple testing) was performed to confirm the linearity of the logit for continuous variables. No violations of the linearity assumption were detected in Model 1 and Model 2. In Model 3, TRIM24 showed a potential deviation from linearity (p = 0.002), though it remained a significant predictor and the overall model performance was not affected (see Supplementary Tables). The results showed that BMI, HDL-C, hs-CRP, FIB, HCY, current smoker and Trim24 remained independent risk factors for AS, while age, HCY, sCD163 and Trim24 were independent predictors of severe AS, and sCD163 and Trim24 were independently associated with severe stenosis (p < 0.05 for all).
Discussion
Atherosclerosis (AS) is a leading cause of morbidity and mortality worldwide16, driven by complex interactions between lipid metabolism and chronic inflammation17. Although emerging technologies such as nanomaterial-based biosensors and targeted delivery systems have shown promise in disease detection and treatment18, early-stage blood biomarkers remain essential for convenient, non-invasive diagnosis and risk stratification19. Our study confirms that Trim24 is a valuable biomarker for AS and its severity. We found that higher serum Trim24 levels were significantly associated with increased risk of AS, as well as with more severe cases of AS and greater coronary artery stenosis.
Macrophages are crucial in the development and progression of AS, where their polarization into either pro-inflammatory M1 or anti-inflammatory M2 phenotypes significantly influences disease outcomes20. While M1 macrophages contribute to inflammation and plaque instability, M2 macrophages are associated with anti-inflammatory effects and tissue repair21,22. Ubiquitination is a key post-translational modification involved in regulating protein stability and immune signaling23. Trim24, a known E3 ubiquitin ligase, has been shown to skew macrophage polarization towards the M1 phenotype by promoting Stat6 acetylation, thereby inhibiting M2 macrophage activity and exacerbating the inflammatory environment in AS24,25. Our prior research demonstrated that Trim24 negatively regulates M2 polarization, which may intensify atherosclerotic progression and plaque formation10. In addition, sCD163, an M2 macrophage marker, has been found to correlate with atherosclerosis severity in systemic lupus erythematosus (SLE), where higher sCD163 levels were linked to increased carotid plaque formation26. Another study using ApoE/CD163 double-knockout mice confirmed the protective role of CD163-expressing M2 macrophages against plaque formation by mitigating pro-inflammatory cytokine effects such as TWEAK27. Our data revealed a negative correlation between Trim24 and sCD163 suggesting that Trim24 may inhibit M2 macrophage polarization, leading to a shift toward a more pro-inflammatory M1 macrophage state. Targeting Trim24 to restore macrophage balance offers potential for therapeutic intervention in AS management.
Despite the growing evidence linking Trim24 to various inflammatory diseases, its specific role in atherosclerosis has not been extensively studied. Our previous research revealed that Trim24 contributes to atherosclerotic plaque progression by promoting M1 macrophage polarization and disrupting lipid metabolism, thus worsening inflammation and plaque stability10. Beyond atherosclerosis, Trim24 has also been implicated in cardiovascular remodeling processes, such as cardiomyocyte hypertrophy, where it interacts with dysbindin to influence hypertrophic signaling pathways28. This study builds upon existing knowledge by systematically examining the relationship between Trim24 expression and AS severity. We found that higher Trim24 levels were significantly associated with increased disease severity and more extensive coronary artery stenosis. These results position Trim24 as a critical biomarker for predicting AS progression, and its role in modulating disease severity highlights its therapeutic potential.
Limitations
This study has several limitations. First, it was conducted at a single center with a relatively small sample size, which may limit the generalizability of the findings. Second, only baseline serum levels of Trim24 and sCD163 were assessed, without longitudinal data or outcome follow-up, preventing evaluation of their dynamic changes or prognostic value. Third, although our findings suggest a potential link between Trim24 and macrophage polarization, no mechanistic studies were performed. In addition, clinical information on the duration of comorbidities and medication use (e.g., lipid-lowering or antihypertensive agents) was incomplete and therefore not included in multivariate analyses. Finally, this study did not assess key translational aspects such as assay cost, standardized cutoffs, or comparative cost-effectiveness with conventional biomarkers, which warrant further investigation.
Conclusion
In conclusion, our study underscores the significant role of Trim24 in atherosclerosis, particularly in modulating macrophage polarization and contributing to disease progression. The association between elevated Trim24 levels and reduced sCD163 expression suggests that Trim24 may drive a pro-inflammatory environment, exacerbating AS. Given its diagnostic and potential therapeutic implications, further research is warranted to elucidate the mechanisms underlying Trim24’s role in AS and to validate its clinical application.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Libby, P. The changing landscape of atherosclerosis. Nature 592 (7855), 524–533 (2021).
Björkegren, J. L. M. & Lusis, A. J. Atherosclerosis: recent developments. Cell 185 (10), 1630–1645 (2022).
Bos, D. et al. Atherosclerotic carotid plaque composition and incident stroke and coronary events. J. Am. Coll. Cardiol. 77 (11), 1426–1435 (2021).
In China, T. & Hu, S. S. Report on cardiovascular health and diseases in China 2021: an updated summary. J. Geriatr. Cardiol. 20 (6), 399–430 (2023).
Engelen, S. E., Robinson, A. J. B., Zurke, Y. X. & Monaco, C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed? Nat. Rev. Cardiol. 19 (8), 522–542 (2022).
Kong, P. et al. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal. Transduct. Target. Ther. 7 (1), 131 (2022).
Lin, P., Ji, H. H., Li, Y. J. & Guo, S. D. Macrophage plasticity and atherosclerosis therapy. Front. Mol. Biosci. 8, 679797 (2021).
Xie, W. et al. Tripartite motif containing 24 regulates cell proliferation in colorectal cancer through YAP signaling. Cancer Med. 9 (17), 6367–6376 (2020).
Chen, J., Feng, X., Zhou, X. & Li, Y. Role of the tripartite motif-containing (TRIM) family of proteins in insulin resistance and related disorders. Diabetes Obes. Metab. 26 (1), 3–15 (2024).
Huang, J. H. et al. Bioinspired PROTAC-induced macrophage fate determination alleviates atherosclerosis. Acta Pharmacol. Sin. 44 (10), 1962–1976 (2023).
Nielsen, M. C. et al. Macrophage activation markers, CD163 and CD206, in Acute-on-Chronic liver failure. Cells 9 (5). (2020).
Zhou, A. et al. Carotid contrast-enhanced ultrasonography combined with sirtuin-3 in the diagnosis of plaques in carotid atherosclerosis. Adv. Clin. Exp. Med. 31 (12), 1319–1326 (2022).
Park, S., Kim, Y. H., Bang, H. I. & Park, Y. Sample size calculation in clinical trial using R. J. Minim. Invasive Surg. 26 (1), 9–18 (2023).
Alan, B., Akpolat, V., Aktan, A. & Alan, S. Relationship between osteopenic syndrome and severity of coronary artery disease detected with coronary angiography and Gensini score in men. Clin. Interv Aging. 11, 377–382 (2016).
Xu, Z. et al. Relationship between TyG index and the degree of coronary artery lesions in patients with H-type hypertension. Cardiovasc. Diabetol. 23 (1), 23 (2024).
Agnelli, G., Belch, J. J. F., Baumgartner, I., Giovas, P. & Hoffmann, U. Morbidity and mortality associated with atherosclerotic peripheral artery disease: A systematic review. Atherosclerosis 293, 94–100 (2020).
Gusev, E. & Sarapultsev, A. Atherosclerosis and inflammation: insights from the theory of general pathological processes. Int J. Mol. Sci. 24(9). (2023).
Li, Z. et al. Establishment of a Raman nanosphere based immunochromatographic system for the combined detection of influenza A and B viruses’ antigens on a single T-line. Nanotechnology 35 (50) (2024).
Luo, Z. et al. Cytokine-induced apoptosis inhibitor 1: a comprehensive analysis of potential diagnostic, prognosis, and immune biomarkers in invasive breast cancer. Transl Cancer Res. 12 (7), 1765–1786 (2023).
Li, H. et al. Macrophage subsets and death are responsible for atherosclerotic plaque formation. Front. Immunol. 13, 843712 (2022).
Hou, P. et al. Macrophage polarization and metabolism in atherosclerosis. Cell. Death Dis. 14 (10), 691 (2023).
Barrett, T. J. Macrophages in atherosclerosis regression. Arterioscler. Thromb. Vasc Biol. 40 (1), 20–33 (2020).
Li, Y. et al. Visualization analysis of breast cancer-related ubiquitination modifications over the past two decades. Discov Oncol. 16 (1), 431 (2025).
Yue, L. et al. TRIM24-Mediated acetylation of STAT6 suppresses Th2-Induced allergic rhinitis. Allergy Asthma Immunol. Res. 15 (5), 603–613 (2023).
Hui, Z. et al. Loss of TRIM24 promotes IL-10 expression via CBP/p300-dependent IFNβ1 transcription during macrophage activation. Inflamm. Res. 72 (7), 1441–1452 (2023).
David, C. et al. Soluble CD163 is a biomarker for accelerated atherosclerosis in systemic lupus erythematosus patients at apparent low risk for cardiovascular disease. Scand. J. Rheumatol. 49 (1), 33–37 (2020).
Gutiérrez-Muñoz, C. et al. CD163 deficiency increases foam cell formation and plaque progression in atherosclerotic mice. Faseb J. 34 (11), 14960–14976 (2020).
Chen, X. & Chen, X. The role of TRIM proteins in vascular disease. Curr. Vasc. Pharmacol. 22 (1), 11–18 (2024).
Funding
This work was financially supported by the Tertiary Education Scientific Research Project of Guangzhou Municipal Education Bureau (Grant No. 2024312058), the Guangzhou Science and Technology Project (Grant No. 2024A03J0891 and 2023A03J0373), and the Plan on Enhancing Scientific Research in Guangzhou Medical University (Grant No. 02-410-2302068XM).
Author information
Authors and Affiliations
Contributions
Yuchao Jiang, Jionghua Huang and Yuhui Lin conceived and designed the study. Jin Zhu Huang, Dejin Ou, and Si Huang collected and analyzed the data. Yuchao Jiang and Dejin Ou wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University (Approval No. [2024]099). Informed consent was obtained from all participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiang, Y., Ou, D., Huang, J. et al. Clinical diagnostic value of serum Trim24 in patients with atherosclerosis. Sci Rep 15, 28877 (2025). https://doi.org/10.1038/s41598-025-07545-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07545-x