Abstract
Opioid addiction constitutes a significant global health challenge. Kappa opioid receptor (KOP) modulators have demonstrated efficacy in treating resistant drug abuse cases. However, till date, no selective KOP antagonists have successfully reached the market. In this study, we employed structure-based design to modify JDTic, the most potent KOP antagonist, into a series of novel analogs based on a tetrahydroisoquinoline (THIQ)-valine hybrid scaffold. More than 20 compounds were synthesized and assessed for binding affinities to the three opioid receptor subtypes. Ten derivatives showed a binding Ki of less than 10 µM for KOP. Subsequent functional characterization using [35S]GTP-γ-S and in vivo animal models established that most of these analogs possess mixed agonist and antagonist properties for both kappa and mu opioid receptors. For example, compound (R)-10m exhibited agonist activity for KOP and MOP receptors, with IC50 values of 670 and 94.5 nM, respectively. Subsequent testing indicated that (R)-10m possesses an analgesic profile with a comparable latency to the positive control, Nalbuphine, in the tail-flick animal model, while compound (S)-10h was found to be a sub-micromolar KOP/MOP antagonist. These results suggest that incorporating relatively bulky and hydrophobic appendages to the privileged THIQ-valine scaffold is necessary to retain and fine-tune the affinity and selectivity for KOP.
Similar content being viewed by others
Kappa opioid receptor (KOP) is particularly concentrated in CNS areas implicated in reward, stress response, and cognition such as nucleus accumbens, hippocampus, and locus coeruleus. It is also expressed in the PNS and some non-neuronal tissues. This receptor is activated by the endogenous dynorphin opioid peptide1. KOP agonists possess a strong analgesic effect but, unlike morphine and other Mu opioid receptor (MOP) agonists, this effect is accompanied by a feeling of dysphoria. Interestingly, despite their effectiveness as a pain killer, KOP agonists have been proven to be unable to activate the reward pathway. Therefore, it has been suggested that potent and selective agonists of KOP would have a great potential as non-addictive opioid analgesics2. Till date, all the developed KOP agonists have failed in clinical trials due to their adverse effect on mood. Fortunately, this effect was shown to be mediated by β-arrestin recruitment by the activated KOP that leads to the activation of the p38 MAPK pathway3. Hence, KOP agonists biased towards the Gi signaling pathway with minimal β-arrestin signaling still represent a promising opportunity for the discovery of safe and non-addictive analgesic devoid of the undesirable dysphoric effect.4.
KOP antagonists were proven to block stress-induced depression, anxiety, and drug-seeking behaviors in animal models5,6,7. Scientists at the National Institute on Drug Abuse (NIDA) suggested the effectiveness of KOP antagonists for the treatment of heroin-dependent patients in an open-label study8. Moreover, KOP antagonists were shown to inhibit stress-induced reinstatement of cocaine-associated conditioned place preference (CPP), a model for drug-seeking behavior in mice9. Despite the complicated mechanism of addiction development, a growing evidence refers to the “ĸ overdrive model of opioid addiction”. This hypothesis claims that chronic administration of opiates develops a “ĸ override” to compensate for the excessive stimulation of the µ opioid system. Once the MOP agonist (the illicit drug) is withdrawn, the over-stimulated ĸ system promotes the manifestation of dysphoric mood states, resulting in a desire to reuse MOP agonists in an endeavor to normalize mood8. Taken together, these observations showed that KOP-selective opioid antagonists could be useful for the treatment of opiate addiction as well as for the prevention of relapse.
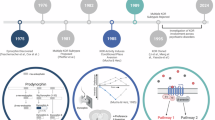
The current strategies to treat addiction are still inefficient in treating some resistant cases and show a high rate of relapse. According to the National Institute of Health (NIH), the usual treatment of opioid addiction includes different combinations of Naltrexone, Methadone, and Buprenorphine (Fig. 1)10. Naltrexone is an opioid antagonist acting on the MOP and used for treating addiction as well as acute opioid toxicity. Methadone is a weak MOP agonist with the ability to relief withdrawal symptoms without causing dependence. However, the majority of patients suffer from relapse to opioid drug abuse perhaps due to dysphoric mood state. The aforementioned “ĸ overdrive model of opioid addiction” links this dysphoric mode to over-activation of ĸ opioid system during sustained opioid administration11. Therefore, scientists have recently turned their attention to the design of KOP antagonists as a more effective treatment of opioid addiction. Unfortunately, the design of a potent and highly selective ligand for KOP has been an elusive task. In 2001, Ivy Carroll and coworkers discovered the non-morphinan compound, JDTic, as the first highly potent and purely selective KOP antagonist (Fig. 1)12. This breakthrough compound is long acting and possesses excellent selectivity for KOP over the other subtypes in the [35S] GTP-γ-S assay (ĸ Ki = 0.006 nM, µ/ĸ ratio = 570, δ/ĸ ratio > 16600). JDTic demonstrated decent activity in animal models of depression, stress-induced cocaine relapse, and opioid withdrawal. These findings encouraged its entry into phase I clinical trials in 2014 to assess safety and pharmacokinetics. However, it was found that the administration of JDTic could lead to some adverse effects including non-sustained ventricular tachycardia (NSVT). Moreover, this compound possesses poor pharmacokinetics demonstrated by low blood–brain barrier penetration and undesirable time course.13 As a result, the clinical trial of this once-promising drug was halted11. Currently, intensive research is focused on obtaining a KOP antagonist with better safety and pharmacokinetic profile. Similarly, Eli Lilly introduced LY-2,456,302, later called CERC-501 and now known as Aticaprant, which is devoid of the NSVT effect, into clinical trials for treatment of depression14. This agent is still being studied in conditions of depression and smoking. Nor-binaltorphimine (nor-BNI), the first selective KOP antagonist, slowed a delayed onset and an exceptionally long duration of action15. Recently, FDA has approved Nalfurafine and Difelikefalin as the first KOP agonists for the treatment of chronic kidney disease-associated pruritus16. To the best of our knowledge, no clinical trials are currently performed on KOP antagonists for treatment of drug abuse17.
The major focus of this study is the design and synthesis of novel KOP ligands that could be useful for treatment of drug dependence, pain, and/or depression. The three opioid receptor subtypes, MOP, KOP, and DOP share 70% sequence identity in the 7 TM domain, with a critical aspartate residue, Asp3.32, and a deep aromatic binding pocket18. The most potent and selective KOP ligand, JDTic, was developed by combining an isoquinoline moiety with the 4-phenylpiperidine pharmacophore which inspired the design of many synthetic opioid ligands19,20,21,22.
Results and discussion
Compounds design
Our approach relied on using the 1,2,3,4-tetrahydroisoquinoline (THIQ) moiety of JDTic, necessary for interaction with the critical Asp3.32, as a “substructure” query to search the ZINC compounds database which currently contains over 230 million compounds in 3D format23. The search retrieved 268,206 hits, which were docked into the orthosteric binding site of the published KOP crystal structure (PDB ID: 4DJH)24. The docking process was performed using the OpenEye software based on similarity of the hits isoquinoline substructure with that of the crystallized JDTic. The binding modes of the top scoring 2700 compounds (ca. 1% of total) were visually examined to eliminate any compounds with reactive functional groups or those showing conformational strain. Priority was given to ligands that fulfill the following requirements: (1) presence of strong salt bridge between the THIQ nitrogen and the critical Asp138; (2) absence of the 4-phenylpiperidine moiety that might contribute to the undesirable pharmacokinetics of JDTic; (3) formation of significant interactions with the selectivity residues, Val108, Val118, Ile294, and Tyr312. These residues differ in other subtypes of opioid receptors and contribute to the subtype selectivity of JDTic and other KOP selective ligands19. Careful investigation of the compounds with the highest docking scores revealed two obvious trends (Figure S1, Supplementary Data). In the first, the THIQ moiety is attached through its nitrogen to the rest of the molecule. The aryl group making contacts with the selectivity residues is connected to the isoquinoline via an amide linkage and a small alkyl moiety is frequently noticed on this linker. In the second chemotype, the THIQ is attached via carbon 3 and connected to the terminal aryl group through a urea linkage. We decided to design a THIQ-valine hybrid scaffold based on the top-scoring compound ZINC-58,154,374 which belongs to the first chemotype (Fig. 2). The presence of the terminal aryl group was necessary to make the required hydrophobic contacts with the aforementioned selectivity residues of KOP. The linker could provide sufficient flexibility of the designed ligands to mimic the V-shaped conformation of JDTic, placing the isopropyl group deeply into the central hydrophobic pocket to make contact with Trp287.
(A) Crystal structure of JDTic in complex with KOP (PDB ID: 4DJH); (B) Proposed binding mode of (R)-phenylacetic acid derivative of the designed scaffold into the binding site of KOP. Ligands are shown as colored sticks, while the protein is displayed as a grey cartoon; (C) Structure of the top-scoring screening hit ZINC-58,154,374 and the (R)-phenylacetic acid derivative of the designed THIQ-valine hybrid.
Chemistry
The synthesis of the THIQ-valine scaffold was achieved by reacting two intermediates, 2 and 4, both prepared from commercially available starting materials. The intermediate 2 was synthesized via the Pictet-Spengler reaction using 2-phenylethylamine (1). Multiple acid catalysts and reaction conditions were tested to optimize the yield and purity of the cyclized product. Conventional methods employing acetic acid (95 °C, 3 h) or hydrochloric acid (90 °C, 9–10 h) yielded the desired product in low amounts, accompanied by side products25,26. Similarly, trifluoroacetic acid at room temperature failed to improve yields. The most efficient condition involved heating 2-phenylethylamine with formic acid and paraformaldehyde at 50 °C overnight, yielding ~ 80% of the cyclized product after flash chromatography purification27. Subsequent functionalization involved amide bond formation at N2 of the tetrahydroisoquinoline scaffold using the amino acids, L- and D- valine (3). To prevent polymerization, the amino group of valine was protected using di-tert-butyl dicarbonate (Boc). Amide coupling between intermediates 2 and 4 required activation of the carboxylic acid using coupling agents such as HBTU or EDCI. Both agents provided comparable yields, though HBTU furnished faster reactions. After the removal of the protecting group, reduction of the amide bond in compound 6 was accomplished using 5 equivalents of LiAlH4 in anhydrous THF to afford the key intermediate 7 in ~ 80% yield. Optimizing reaction temperature and duration was critical to minimizing side products. The final products were synthesized via coupling 7 with various carboxylic acids. While some acids were commercially available, others were prepared via Suzuki coupling of 3-bromobenzoic acid (8) with the appropriate boronic acids. Coupling reactions to synthesize (S)- and (R)-10 final derivatives were performed using EDCI, HOBt, and DIPEA in DMF (Scheme 1).
Reagents and conditions: (i) HCOOH, paraformaldehyde, 50 °C, overnight; (ii) (Boc)2O, 1 M NaOH, THF, H2O, r.t., 12 h; (iii) HBTU, DIPEA, r.t., 16 h; (iv) TFA, DCM, 0 °C then r.t., 3 h., (v) LiAlH4:THF, anhydrous THF, 0 °C then 70 °C, 3 h; (vi) Aryl Boronic acids, Pd(PPh3)4, K2CO3, Toluene, MeOH, 95–105 °C, overnight ; (vii) Different carboxylic acids, EDCI, HOBt, DIPEA, r.t., 16 h.
Biological evaluation
All the prepared compounds were subjected to a radioligand binding assay to determine their affinity to the KOP. The main purpose was to optimize the terminal aryl/aralkyl groups to achieve best complementarity to the aforementioned KOP selectivity pocket. Three structural elements were optimized; size of the aryl moieties, their decoration with H bonding groups, and flexibility of the linker. It was found that all the derivatives possessed variable KOP/MOP selectivity with no affinity to the DOP (Table 1). The receptor binding data shows that relatively small aryl groups, such as pyridyl, thienyl, or phenyl rings in 10a, b, k, and l, failed to provide a high affinity to the KOP, probably due to their inability to fill the large selectivity pocket of this opioid receptor subtype. Increasing the bulkiness of these aromatic groups significantly improved the KOP affinity as noticed with the indole fused ring system in compound 10c and the biphenyl derivatives 10h. H bonding groups could similarly improve the affinity when installed on the meta position (derivatives 10h-j). Substitution on the para position of the biaryl ring system reduced the affinity perhaps due to excessive length and rigidity of this aryl arm (compound 10g). Finally, the effect of the length of the linker to the valine moiety was investigated. Generally speaking, introducing a flexible one-carbon linker could improve the affinity as observed in compounds 10c, d, and m.
Compounds with KOP affinity of less than 10 µM were further studied to determine their selectivity against the other opioid receptor subtypes. All the tested compounds lack the affinity to DOP, while showing variable degrees of selectivity towards MOP and KOP (Table 1). As expected, increasing the bulkiness of the aryl arm slightly enhanced the KOP selectivity, with compound (R)-10d being the most selective. Furthermore, the top six derivatives were tested for acute antinociceptive agonist/antagonist effects in the behavioral tail flick assay (Fig. 3). Interestingly, the relatively bulky compounds with a fused ring or biphenyl systems, (R)-10c, (S)-10h, and (S)-10i, failed to show any significant agonist effect up to 30 mg/kg dose. While the derivative (R)-10d, (S)-10j, and (R)-10m with more flexible linkers and relatively smaller substituents produced a percentage of maximum possible effect (% MPE) comparable to that of the KOP agonist Nalbuphine. These results were consistent with the rat prolactin ELISA test, where only the compounds with agonist activities, (R)-10d, (S)-10j, and (R)-10m, were able to double the normal rat blood prolactin levels. Finally, the top three derivatives in the binding assay, (S)-10h, (S)-10i, and (R)-10m, were tested in the [35S]-GTPγS functional assay to complete their activity profile. As projected by the in vivo data, compounds (S)-10h, and (S)-10i, with the bulky substituted biphenyl arms, failed to produce any agonism in this functional assay. While the more flexible and polar nitrobenzyl derivative (R)-10m was able to activate both KOP and MOP receptor with an EC50 of 666 and 94.5 nM, respectively.
Molecular docking study
To better understand the binding determinants of this series of compounds, the top three ligands with the highest affinities were docked into the orthosteric binding site of KOP. All docked compounds exhibited charge-charge interactions with the critical Asp138 residue, which serves as a key determinant for anchoring the ligand within the binding site via the basic N2 group of the THIQ. Additionally, the isopropyl group contributed to stabilizing the ligands, especially those with bulky substituents on the amide linker, by forming hydrophobic interactions with the aromatic and hydrophobic residues at the base of the active site, such as Trp287, Ile316, and Tyr320. Notably, Trp287 is recognized for its crucial role in the activation mechanism of all GPCRs. Furthermore, the phenyl ring of the THIQ in all compounds interacted with residues at the entrance of the active site, including Val230, Ile294, and Tyr139. Among the most active compounds, (S)-10h and (S)-10i, which feature an amide group attached to a biphenyl moiety with hydroxyl and cyano groups at the 3’ position, respectively, showed a similar orientation in the active site. Their biphenyl systems fit snugly into the selectivity pocket (Figs. 4a and b). Compound (S)-10h formed an additional polar contact between the nitrogen of its amide and Asp138, complementing the ionic bond formed by the THIQ nitrogen with the same residue. Meanwhile, the cyano group in (S)-10i extended towards the top of the binding site, creating an H-bond with the backbone of Cys210, which further stabilized the biaryl group within the hydrophobic selectivity pocket.
Despite its moderate KOP affinity, compound (R)-10m also demonstrated significant interactions within the active site of KOP. This ligand formed bidentate polar bonds with Asp138: an ionic bond with the THIQ N2 and another one with the amide nitrogen (Fig. 4c). These interactions helped the ligand adopt the V-shaped orientation, akin to that of JDTic, enabling contacts with Trp124 in the selectivity pocket. However, due to its relatively smaller aryl substituent, it could not maintain favorable interactions with other hydrophobic residues in the selectivity pocket as effectively as the ligands with bulkier groups.
Proposed binding mode of selected scheme II compounds (a) (S)-10h (left-cyan), (b) (S)-10i (right-pale green) (c) (R)-10m (beige) in the orthosteric binding site of KOP crystal structure (PDB: 4DJH). Protein is shown as a grey cartoon with the important binding site residues displayed as sticks. The yellow dashed indicates polar bonds.
Conclusion
In conclusion, the insights gained from the SAR study and the modeling simulation suggest that the THIQ-valine derivatives with bulky and rigid aryl arms are generally expected to possess a higher selectivity to KOP as well as opioid antagonist effect. While the aryl arms with a small polar substituent and a flexible short linker could produce functionally active opioid ligands with more selectivity to MOP. In this study, two promising leads were introduced; (S)-10h which is a non-selective KOP/MOP antagonist that can be further optimized for the management of drug abuse, and (R)-10m which is a 23-fold selective MOP agonist with an IC50 of 94.5 nM in the [35 S]-GTPγS assay. The latter could also be developed into an analgesic or incorporated into the addiction management plans. Although the activity of the prepared compounds is still sub-optimal compared to the gold-standard JDTic, these THIQ-valine hybrids are characterized by straightforward synthesis, presence of only one stereocenter inherent from the valine amino acid used in their synthesis, absence of reactive or unnecessary functional groups, and no affinity to DOP. Therefore, they represent a viable starting point for further optimization, followed by investigating their analgesic and/or anti-addictive effect in preclinical and clinical studies.
Experimental section
Materials and instrumentation
All solvents and reagents were purchased from Sigma-Aldrich, Acros, and Alfa Aesar, and unless otherwise specified, and used as received from the chemical suppliers. For reactions sensitive to air or moisture, solvents were dried and maintained under an argon atmosphere. Flash column chromatography was performed using Sigma Aldrich silica gel (200–400 mesh) which was used as received. Reaction progress was monitored using analytical thin layer chromatography (TLC) performed on Merck silica gel 60 F254 plates. Visualization was done using UV light (254 nm) or staining with iodine, ninhydrine, phosphomolybdic acid and ferric chloride. Melting points (mp) were determined in open capillaries on an Electrothermal IA9100 melting point apparatus (Drug Design and Discovery lab-Zewail City of Science and Tecnology) and are uncorrected.
Low-resolution mass spectra (MS) were recorded with a Shimadzu QP2010-Plus gas chromatograph/mass spectrometer (GC/MS), electron impact (EI+) 70 eV maintained at 250 °C, equipped with Shimadzu workstation or with electrospray ionization (ESI+) in positive ion me with a Waters Multi-mode ESCi mass detector (The regional center for mycology and biotechnology- Al-Azahar University). The proton and carbon nuclear magnetic resonance1H and13C NMR) were recorded at 400 MHz on a Bruker Ascend Aeon 400 (400 MHz) (Faculty of Pharmacy-Mansoura University) and at 500 MHz on JEOL Delta 500 (500 MHz) spectrometers (Faculty of Science-Mansoura University) at room temperature within the range (19–25 °C). Chemical shifts (δ) are expressed as parts per million (ppm), using tetramethylsilane (TMS) as the internal standard. Proton chemical shifts were internally referenced to the residual proton resonance in CDCl3 (δH 7.26 ppm), d6-DMSO (δH 2.50 ppm) and CD3OD (δH 3.33 ppm and 4.89 ppm). Carbon chemical shifts were internally referenced to the deuterated solvent signals in CDCl3 (δC 77.2 ppm) and d6-DMSO (δC 49.0).
For Signal multiplicities, the following abbreviations are used as follows; s (singlet), d (doublet), dd (doublets of doublet), t (triplet), q (quartet), br s (broad singlet) and m (multiplet). They are defined as multipeak signals when overlap or complex coupling of signals makes definitive descriptions of peaks difficult. Coupling constants (J values) are given in hertz (Hz).
Abbreviations of the solvents are used; DMF:N,N-dimethylformamide, DCM: dichloromethane, DMSO: dimethylsulfoxide, EtOAc: ethyl acetate, EtOH: ethanol, MeOH: methanol, THF: tetrahydrofuran and AcOH: acetic acid.
1,2,3,4-tetrahydroisoquinoline (2)
At 0 ⁰C, 2-phenylethylamine 1 (0.5 gm, 4.13 mmol) was added slowly to formic acid (2 ml) and kept at same temperature (0 ⁰C) for 10 min before the addition of paraformaldehyde (0.125 g, 4.13 mmol). The reaction was left stirring at 50 ⁰C overnight, then it was basified using 1 M solution of NaOH and extracted with DCM. The combined organic layer was dried over anhydrous Na2SO4, filtered and evaporated under reduced pressure. The residue was purified by flash chromatography on silica gel using DCM: MeOH: NH4OH (95:4:1) to yield the required product 2 (0.43 gm, 78%) as a yellow liquid.1H NMR (500 MHz, chloroform-d) δ ppm 2.41 (s, 2 H) 2.95 (t, J = 6.01 Hz, 2 H) 3.29 (t, J = 6.01 Hz, 2 H) 4.16 (s, 2 H) 7.12–7.17 (m, 1 H) 7.21–7.24 (m, 1 H) 7.24–7.29 (m, 2 H). MS (EI+) m/z Calc. for C9H11N: 133.19, found: 133.94 [M+·].
(tert-butoxycarbonyl)-L-valine (S)-4
An aqueous solution of 1 M NaOH (10 ml) was slowly added to a cooled suspension of L-valine (S)-3 (1.2 g, 10.24 mmol) in THF (15 ml) and water (7.5 ml), followed by the dropwise addition of di-tert-butyl dicarbonate (2.46 g, 11.26 mmol). The reaction was stirred at room temperature for 12 h, then acidified with NaHSO4 and extracted with ethyl acetate (3 × 50 ml). The organic layer was cleaned with brine (1 × 50 ml), dried over anhydrous Na2SO4. The solvent was filtered and evaporated under reduced pressure to give a gummy mass (S)-4 (2.18 g, 98%) which was used in the next step without further purification.1H NMR (500 MHz, chloroform-d) δ ppm 0.93 (d, J = 7.21 Hz, 3 H) 1.00 (d, J = 6.41 Hz, 3 H) 1.38–1.49 (m, 9 H), 1.86–1.88 (m, 1 H), 3.76–3.78 (m, 1 H). MS (EI+) m/z Calc. for C10H19NO4: 217.27, found: 217.25 [M+·].
(tert-butoxycarbonyl)-D-valine (R)-4
The protected D isomer was synthesized using the same procedure applied for the L-isomer with D-Valine (R)-20 (1 g, 8.54 mmol), 1 M NaOH (8.3 ml) and di-tert-butyl dicarbonate (2.05 g, 9.39 mmol) to give the desired product (R)-4 as a gummy mass (1.8 g, 97%) which was used in the next step without further purification.1H NMR (500 MHz, chloroform-d) δ ppm 0.93 (d, J = 7.21 Hz, 3 H) 1.00 (d, J = 6.41 Hz, 3 H) 1.43–1.48 (m, 9 H), 1.85–1.88 (m, 1 H), 3.76–3.78 (m, 1 H). MS (EI+) m/z Calc. for C10H19NO4: 217.27, found: 216.94 [M+·].
tert-butyl (S)-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methyl-1-oxobutan-2-yl)carbamate (S)-5
1- Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI) (1.74 g, 9.11 mmol) and 1-hydroxy benzotriazole (HOBt) (1.4 g, 9.11 mmol) were added to a solution of (tert-butoxycarbonyl)-L-valine (S)-4 (1.32 g, 6.08 mmol) in DMF (10 ml) in an ice bath. This mixture was stirred for 15 min before the addition of 1,2,3,4-tetrahydroisoquinoline 2 (0.890 g, 6.68 mmol) and N, N-Diisopropylethylamine (DIPEA) (3.18 ml, 18.23 mmol). The reaction was left stirring at an ambient temperature for 16 h, then extracted with ethyl acetate. The organic phase was sequentially washed with saturated solutions of ammonium chloride (1 × 50 ml), Na2CO3 (1 × 50 ml), water (1 × 50 ml) and brine (1 × 50 ml). The solvent was then dried over anhydrous Na2SO4, filtered and evaporated to dryness on a rotatory evaporator. The resulted viscous oil was purified by flash chromatography and eluted using Hexane: EtOAc (80: 20) to give (S)-5 (1.94 g, 96%) as colorless viscous oil.1H NMR (500 MHz, chloroform-d) δ ppm 0.87–0.94 (m, 3 H) 0.97 (dd, J = 14.61, 6.59 Hz, 3 H) 1.41–1.48 (m, 9 H) 1.93–2.02 (m, 1 H) 2.87(d, J = 5.73 Hz, 1 H) 2.94 (dd, J = 12.60, 6.30 Hz, 1 H) 3.71–3.78 (m, 1 H) 4.56 (dd, J = 8.88, 5.44 Hz, 1 H) 4.67–4.81 (m, 2 H) 5.38 (d, J = 9.74 Hz, 1 H) 7.13–7.18 (m, 2 H) 7.18–7.25 (m, 2 H). MS (EI+) m/z Calc. for C19H28N2O3: 332.44, found: 332.55 [M+·].
tert-butyl (R)-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methyl-1-oxobutan-2-yl)carbamate (R)-5
The R-isomer was synthesized following the protocol described for the S-isomer. From (tert-butoxycarbonyl)-D-valine (R)-4 (0.9 g, 4.14 mmol), EDCI (1.18, 6.2 mmol), HOBt (0.95 g, 6.2 mmol), 1,2,3,4-tetrahydroisoquinoline 19 (0.61 g, 4.55 mmol) and DIPEA (2.17, 12.42 mmol) to give the desired product as a colorless viscous oil (R)-5 (1.31 g, 95%).1H NMR (500 MHz, chloroform-d) δ ppm 0.90 (dd, J = 10.42, 7.21 Hz, 3 H) 0.94–1.01 (m, 3 H) 1.44 (d, J = 4.01 Hz, 9 H) 1.88–2.00 (m, 1 H) 2.83–2.89 (m, 1 H)2.94 (dt, J = 12.02, 6.01 Hz, 1 H) 3.70–3.89 (m, 1 H) 4.52–4.63 (m, 1 H) 4.67–4.81 (m, 2 H) 5.35–5.44 (m, 1 H) 7.09–7.24 (m, 4 H).13C NMR (126 MHz, Chloroform-d) δ ppm 17.44, 19.91, 28.56, 31.84, 43.64, 44.83, 47.73, 55.39, 79.71, 126.36, 126.72, 126.86, 127.24, 128.61, 129.09, 156.10, 171.55. MS (EI+) m/z Calc. for C19H28N2O3: 332.44, found: 332.68 [M+·].
(S)-2-Amino-1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-1-one (S)-6
At 0 °C and under argon, trifluoroacetic acid (5 ml) was added dropwise to a solution of compound (S)-5 (1.0 g, 3.01 mmol) in dichloromethane (10 ml) and the reaction was allowed to warm to room temperature and left on stirrer for 3 h. The reaction mixture was cooled in an ice bath before being basified using saturated solution of NaHCO3 until the pH became 8. This mixture was extracted with DCM (3 × 30 ml), the combined organic layers were washed with brine (1 × 50 ml), dried over anhydrous Na2SO4 and evaporated under reduced pressure to give a pale yellow viscous oil (S)-6 (0.58 g, 83%). This product was used for the following reaction without further purification.1H NMR (500 MHz, chloroform-d) δ ppm 0.87–0.93 (m, 3 H) 0.98 (dd, J = 17.63, 7.21 Hz, 3 H) 1.83–1.97 (m, 1 H) 2.69 (br. s., 2 H) 2.81–2.87 (m, 1 H) 2.87–2.93 (m, 1 H) 3.63–3.75 (m, 2 H) 3.75–3.90 (m, 1 H) 4.61–4.75 (m, 2 H) 7.06–7.20 (m, 4 H). MS (EI+) m/z Calc. for C14H20N2O: 232.33, found: 232.55 [M+·].
(R)-2-amino-1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-1-one (R)-6
The R-isomer was synthesized following the protocol described for the S-isomer from tert-butyl (R)-(1-(3,4-dihydroisoquinolin-2(1 H)-yl)-3-methyl-1-oxobutan-2-yl) carbamate (R)-5 (0.88 g, 2.65 mmol) using trifluoroacetic acid to obtain the deprotected compound (R)-6 ( 0.52 g, 85%) as pale yellow viscous oil.1 H NMR (500 MHz, DMSO-d6) δ ppm 0.81 (dd, J = 12.31, 6.59 Hz, 3 H) 0.83–0.91 (m, 3 H) 1.64–1.78 (m, 1 H) 2.73–2.77 (m, 1 H) 2.78–2.84 (m, 1 H) 2.85–2.92 (m, 1 H) 3.17–3.33 (br., s., 2 H) 3.55 (dd, J = 18.90, 5.73 Hz, 1 H) 3.68–3.73 (m, 1 H) 4.54 (d, J = 17.18 Hz, 1 H) 4.60–4.75 (m, 1 H) 7.15–7.21 (m, 4 H). MS (EI+) m/z Calc. for C14H20N2O: 232.33, found: 232.07 [M+·].
(S)-1-(3,4-dihydroisoquinolin − 2(1 H)-yl)-3-methylbutan-2-amine (S)-7
Under anhydrous conditions, a solution of (S)-6 (0.65 g, 2.8 mmol) in THF (5 ml) was added slowly to 1 M solution of LiAlH4-tetrahydrofuran complex (14 ml) in an ice bath. The reaction was then heated to 70 °C for three hours, then cooled before being cautiously and sequentially quenched by water (2 ml): 30% solution of NaOH (2 ml): water (6 ml). The resulted slurry was left on stirrer for half an hour, filtered on disk funnel, washed with THF and the organic solvent was removed by evaporation on a rotatory evaporator. The aqueous phase was then extracted with ethyl acetate (3 × 0 mL). The organic layers were combined, washed with brine (1 × 30 mL), dried over Na2SO4 and evaporated under vacuum. The residue was purified by flash column chromatography (DCM/Methanol/Ammonia 95:4:1) to give the desired compound (S)-7 as a pale-yellow viscous oil (0.48 g, 78%).1H NMR (500 MHz, chloroform-d) δ ppm 0.97 (t, J = 7.21 Hz, 6 H) 1.62–1.72 (m, 1 H) 2.39–2.46 (m, 1 H) 2.47–2.53 (m, 1 H) 2.60–2.71 (m, 3 H) 2.82–2.94 (m, 4 H) 3.55 (d, J = 14.43 Hz, 1 H) 3.76 (d, J = 14.43 Hz, 1 H) 7.02 (d, J = 6.41 Hz, 1 H) 7.09–7.16 (m, 3 H). MS (EI+) m/z Calc. for C14H22N2: 218.34, found: 218.21 [M+·].
(R)-1-(3,4-dihydroisoquinolin − 2(1H)-yl)-3-methylbutan-2-amine (R)-7
The R-isomer was synthesized following the protocol described for the S-isomer from (R)-2-amino-1-(3,4-dihydroisoquinolin-2(1 H)-yl)-3-methylbutan-1-one (R)-6 (0.95 g, 2.54 mmol) and 1 M solution of LiAlH4-tetrahydrofuran complex (13 ml) to get the reduced form as a pale-yellow viscous oil (R)-7 (0.45 g, 81%). IR (Nujol): 3241 (NH, stretch), 3066 (CH aromatic), 2957 (CH, aliphatic), 1648 (NH, bending).1 H NMR (500 MHz, chloroform-d) δ ppm 0.98 (dd, J = 11.62, 6.81 Hz, 6 H) 1.71 (d, J = 6.41 Hz, 1 H) 2.46 (d, J = 10.43 Hz, 1 H) 2.48–2.54 (m, 1 H) 2.67 (d, J = 5.61 Hz, 1 H) 2.86 (d, J = 5.61 Hz, 2 H) 2.88–2.91 (m, 2 H) 3.18 (t, J = 6.01 Hz, 1 H) 3.56 (d, J = 14.42 Hz, 1 H) 3.76 (d, J = 14.42 Hz, 1 H) 4.06 (s, 1 H) 7.02 (d, J = 5.61 Hz, 1 H) 7.10–7.15 (m, 3 H). MS (EI+) m/z Calc. for C14H22N2: 218.34, found: 218.08 [M+·].
General procedure for the synthesis of [1,1′-biphenyl]-3-carboxylic acid derivatives (Suzuki coupling) (9I-9VI)
A solvent system consisting of toluene, methanol and 2 M solution of K2CO3 (20 ml: 10 ml: 2.5 ml, respectively) was prepared and purged with inert argon for 15 min before the addition of 3-bromobenzoic acid 8 (0.4 g, 1.99 mmol), the appropriate boronic acid (2.39 mmol), and Tetrakis (triphenylphosphine) palladium(0) (0.0199 mmol). The mixture was heated under reflux at 95–105 °C for 24 h. On the next day, the reaction was kept on the hot plate without condenser to reduce the solvent system and ensure the oxidation of residual amount of palladium. The pH was adjusted to 4 with 1 N HCl aqueous solution, then the mixture was extracted with EtOAc (3 × 30 ml). The extractions were combined and washed with brine (3 × 50 ml), dried over Na2SO4, evaporated to dryness and purified by column chromatography on silica gel. Elution was made successively with dichloromethane: methanol (98:2).
[1,1′-biphenyl]-3-carboxylic acid (9I)
Compound 9I was prepared following the general Procedure of the synthesis of [1,1′-biphenyl]-3-carboxylic acid derivatives using phenyl boronic acid, and purified using Hex: EtOAc (70:30) to produce white solid. Yield: 88.7%. Mp:166–169 °C.1H NMR (500 MHz, DMSO-d6) δ ppm 7.37–7.43 (m, 1 H) 7.49 (t, J = 7.61 Hz, 2 H) 7.60 (t, J = 7.61 Hz, 1 H) 7.66–7.72 (m, 2 H) 7.89–7.97 (m, 2 H) 8.18 (t, J = 2.00 Hz, 1 H) 13.15 (br. s., 1 H). MS (EI+) m/z Calc. for C13H10O2: 198.22, found: 198.07 [M+·].
4′-fluoro-[1,1′-biphenyl]-3-carboxylic acid (9II)
Compound 9II was prepared following the general Procedure of the synthesis of [1,1′-biphenyl]-3-carboxylic acid derivatives using 4-fluorophenyl boronic acid, and purified using Hex: EtOAc (70:30) to produce white solid. Yield: 85.6%. Mp: 182–185 °C.1H NMR (500 MHz, chloroform-d) δ ppm 7.08–7.20 (m, 1 H) 7.42–7.49 (m, 2 H) 7.51–7.62 (m, 2 H) 7.62–7.71 (m, 2 H) 8.05–8.20 (m, 1 H). MS (EI+) m/z Calc. for C13H9FO2: 216.21, found: 216.12 [M+·].
3′-hydroxy-[1,1′-biphenyl]-3-carboxylic acid (9III)
Compound 9III was prepared following the general Procedure of the synthesis of [1,1′-biphenyl]-3-carboxylic acid derivatives using 3-hydroxyphenyl boronic acid, and purified using Hex: EtOAc (70:30) to produce white solid. Yield: 77.6%. Mp: 150–152 °C.1H NMR (500 MHz, DMSO-d6) δ ppm 6.80 (d, J = 8.01 Hz, 1 H) 7.04–7.12 (m, 2 H) 7.27 (t, J = 8.01 Hz, 1 H) 7.50–7.57 (m, 1 H) 7.79 (d, J = 8.01 Hz, 1 H) 7.91 (d, J = 7.21 Hz, 1 H) 8.13 (br. s., 1 H). MS (EI+) m/z Calc. for C13H10O3: 214.22, found: 214.28 [M+·].
3′-cyano-[1,1′-biphenyl]-3-carboxylic acid (9IV)
Compound 9I was prepared following the general Procedure of the synthesis of [1,1′-biphenyl]-3-carboxylic acid derivatives using 3-cyanophenyl boronic acid, and purified using Hex: EtOAc (70:30) to produce white solid. Yield: 71.6%. Mp: 165–168 °C.1H NMR (500 MHz, DMSO-d6) δ ppm 7.71 (t, J = 7.61 Hz, 2 H) 7.90 (d, J = 7.21 Hz, 2 H) 8.12 (d, J = 8.01 Hz, 2 H) 8.30 (s, 2 H). MS (EI+) m/z Calc. for C14H9NO2: 223.23, found: 223.26 [M+·].
3-(pyridin-3-yl)benzoic acid (9 V)
Compound 9I was prepared following the general Procedure of the synthesis of [1,1′-biphenyl]-3-carboxylic acid derivatives using 3-pyridinylboronic acid, and purified using Hex: EtOAc (70:30) to produce white solid. Yield: 40%. Mp: 150–152 °C.1H NMR (500 MHz, DMSO-d6) δ ppm 7.52 (td, J = 8.41, 4.81 Hz, 1 H) 7.64 (t, J = 8.01 Hz, 1 H) 7.95–8.02 (m, 2 H) 8.10–8.18 (m, 1 H) 8.21 (s, 1 H) 8.59–8.66 (m, 1 H) 8.89–8.98 (m, 1 H). MS (EI+) m/z Calc. for C12H9NO2: 199.21, found: 199.78 [M+·].
General procedure for the synthesis of amide derivatives (S)-10a-k, (R)-10a-h, l, m
In a two-neck round-bottom flask, HBTU (0.69 mmol) was added to a solution of the carboxylic acid (0.46 mmol) in DCM (5 ml) under cooling. After stirring for 10 min, a solution of compound (S/R)-7 (0.1 g, 0.46 mmol) in DCM (3 ml) was added dropwise followed by triethylamine (1.37 mmol) and the mixture was left for 6 h at room temperature. The mixture was then extracted with DCM and sequentially washed using saturated NaHCO3 aqueous solution (1 × 30 ml), saturated ammonium chloride aqueous solution (1 × 30 ml). The combined organic layer was washed with brine, dried over Na2SO4, filtered and evaporated in vacuo. The crude residue was further purified by flash chromatography using silica gel to afford the required amides.
(S)-3,5-dichloro-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)isonicotinamide (S)-10a
Compound (S)-10a was prepared following the general Procedure for the synthesis of amide derivatives using 3,5-dichloroisonicotinic acid and the amine (S)-7, and purified using Hex: EtOAc (65:35) to produce yellow gummy mass. Yield: 73.4%. IR (Nujol): 3242 (NH, Stretch), 3066 (CH aromatic),2957 (CH, aliphatic), 1645 (C = O, stretch).1H NMR (500 MHz, Chloroform-d) δ ppm 0.99–1.14 (m, 6 H) 2.21–2.34 (m, 1 H) 2.57–2.68 (m, 1 H) 2.70–2.78 (m, 1 H) 2.82–2.92 (m, 3 H) 2.95 (d, J = 9.62 Hz, 1 H) 3.55–3.69 (m, 1 H) 3.81 (d, J = 14.43 Hz, 1 H) 4.22–4.36 (m, 1 H) 6.96–7.07 (m, 1 H) 7.14 (d, J = 4.81 Hz, 2 H) 7.11 (d, J = 5.61 Hz, 1 H) 8.37–8.52 (m, 2 H).13C NMR (126 MHz, chloroform-d) δ ppm 18.31, 18.74, 28.19, 30.42, 36.71, 51.35, 51.99, 56.88, 126.32, 126.58, 126.97, 127.23, 127.32, 128.39, 128.95, 129.14, 142.81, 147.77, 147.86, 162.73. MS (EI+) m/z: 392.15 [M+·]. Anal. Calcd for C20H23Cl2N3O: C, 61.23; H, 5.91; N, 10.71. Found: C, 61.59; H, 6.12; N, 10.98.
(R)-3,5-dichloro-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)isonicotinamide (R)-10a
Compound (R)-10a was prepared following the general Procedure for the synthesis of amide derivatives using 3,5-dichloroisonicotinic acid and the amine (R)-7, and purified using Hex: EtOAc (65:35) to produce yellow gummy mass. Yield: 76.3%.1H NMR (500 MHz, Chloroform-d) δ ppm 1.03 (d, J = 6.41 Hz, 3 H) 1.07 (d, J = 7.21 Hz, 3 H) 2.27 (td, J = 6.81, 4.81 Hz, 1 H) 2.61 (dd, J = 12.42, 5.21 Hz, 1 H) 2.75–2.82 (m, 3 H) 2.83–2.95 (m, 3 H) 3.60 (d, J = 14.43 Hz, 1 H) 3.83 (d, J = 14.43 Hz, 1 H) 4.22–4.36 (m, 1 H) 7.01 (d, J = 6.41 Hz, 1 H) 7.08–7.22 (m, 3 H) 8.42–8.51 (m, 2 H).13C NMR (126 MHz, chloroform-d) δ ppm 18.01, 18.49, 28.43, 30.09, 38.55, 51.06, 51.84, 56.81, 125.91, 126.46, 126.54, 127.05, 128.63, 128.90, 142.63, 147.48, 147.55, 162.37. MS (EI+) m/z: 392.36 [M+·]. Anal. Calcd for C20H23Cl2N3O: C, 61.23; H, 5.91; N, 10.71. Found: C, 61.45; H, 6.13; N, 10.95.
(S)-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-5-nitrothiophene-2-carboxamide (S)-10b
Compound (S)-10b was prepared following the general Procedure for the synthesis of amide derivatives using 5-nitro-thiophene-3-carboxylic acid acid and the amine (S)-7, and purified using Hex: EtOAc (65:35) to produce brown viscous oil. Yield: 71.9%. IR (Nujol): 3288 (NH, Stretch), 3081 (CH aromatic), 2958 (CH, aliphatic), 1623 (C = O, stretch).1H NMR (500 MHz, Chloroform-d) δ ppm 1.00 (d, J = 6.41 Hz, 5 H) 2.17 (d, J = 5.61 Hz, 1 H) 2.68 (dd, J = 12.82, 4.81 Hz, 1 H) 2.79–2.82 (m, 2 H) 2.85–2.89 (m, 2 H) 2.90–2.97 (m, 2 H) 3.71 (d, J = 15.23 Hz, 1 H) 3.79 (d, J = 15.23 Hz, 1 H) 4.18–4.26 (m, 1 H) 6.99–7.04 (m, 1 H) 7.08–7.19 (m, 3 H) 7.39–7.44 (m, 1 H) 7.78 (d, J = 4.01 Hz, 1 H). 13 C NMR (126 MHz, chloroform-d) δ ppm 18.50, 18.74, 28.77, 30.69, 38.84, 50.87, 52.34, 56.20, 57.21, 126.22, 126.28, 126.78, 126.83, 128.44, 128.94, 133.83, 145.52, 154.15, 160.65. MS (EI+) m/z: 373.37 [M+·]. Anal. Calcd for C19H23N3O3S: C, 61.10; H, 6.21; N, 11.25, S 8.58. Found: C, 61.34; H, 6.37; N, 11.42; S, 8.69.
(R )- N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-5-nitrothiophene-2-carboxamide (R)-10b
Compound (R)-10b was prepared following the general Procedure for the synthesis of amide derivatives using 5-nitro-thiophene-3-carboxylic acid acid and the amine (R)-7, and purified using Hex: EtOAc (65:35) to produce brown viscous oil. Yield: 73%.1H NMR (500 MHz, Chloroform-d) δ ppm 1.00 (dd, J = 6.81, 2.00 Hz, 6 H) 2.14–2.25 (m, 1 H) 2.62 (dd, J = 12.82, 5.61 Hz, 1 H) 2.65–2.77(m, 2 H) 2.82–2.92 (m, 3 H) 3.63 (d, J = 14.43 Hz, 1 H) 3.73 (d, J = 14.43 Hz, 1 H) 4.13–4.23 (m, 1 H) 6.61 (d, J = 5.61 Hz, 1 H) 7.01 (d, J = 6.41 Hz,1 H) 7.07–7.19 (m, 3 H) 7.33 (d, J = 4.81 Hz, 1 H) 7.79 (d, J = 4.01 Hz, 1 H).13C NMR (126 MHz, chloroform-d) δ ppm 18.22, 18.39, 29.03, 30.20, 50.76, 52.34, 56.32, 57.22, 125.75, 126.29, 126.48, 128.16, 128.65, 134.01, 134.19, 145.37, 153.84, 160.29. MS (EI+) m/z: 373.07 [M+·]. Anal. Calcd for C19H23N3O3S: C, 61.10; H, 6.21; N, 11.25, S 8.58. Found: C, 60.89; H, 6.42; N, 11.41; S, 8.72.
(S)-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-2-(1H-indol-3-yl)acetamide (S)-10c
Compound (S)-10c was prepared following the general Procedure for the synthesis of amide derivatives using indole-3-carboxylic acid acid and the amine (S)-7, and purified using DCM: MeOH (98:2) to produce yellow viscous oil. Yield: 84.6%.1H NMR (500 MHz, Chloroform-d) δ ppm 0.72 (d, J = 7.21 Hz, 3 H) 0.83 (d, J = 7.21 Hz, 3 H) 1.88 (dd, J = 12.42, 6.81 Hz, 1 H) 2.39–2.52 (m, 2 H) 2.68–2.73 (m, 2 H) 2.89 (s, 1 H) 2.96 (s, 1 H) 3.52 (d, J = 14.43 Hz, 1 H) 3.62 (d, J = 14.43 Hz, 1 H) 3.72 (d, J = 5.61 Hz, 2 H) 3.99–4.09 (m, 1 H) 6.02–6.13 (m, 1 H) 6.94 (d, J = 7.21 Hz, 1 H) 6.99–7.05 (m, 2 H) 7.08 (d, J = 8.01 Hz, 1 H) 7.10–7.19 (m, 3 H) 7.31 (d, J = 8.01 Hz, 1 H) 7.55 (d, J = 8.01 Hz, 1 H) 8.29 (br. s., 1 H).13C NMR (126 MHz, Chloroform-d) δ ppm 17.61, 18.82, 28.27, 30.11, 33.62, 50.62, 51.06, 55.61, 57.70, 109.09, 111.22, 118.77, 119.72, 122.26, 123.81, 125.75, 126.34, 126.58, 126.99, 128.57, 133.87, 136.24, 162.53, 171.83. MS (EI+) m/z: 376.81 [M+·]. Anal. Calcd for C24H29N3O: C, 76.76; H, 7.78; N, 11.19. Found: C, 76.89; H, 7.95; N, 11.37.
(R)-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-2-(1H-indol-3-yl)acetamide (R)-10c
Compound (R)-10c was prepared following the general Procedure for the synthesis of amide derivatives using indole-3-carboxylic acid acid and the amine (R)-7, and purified using DCM: MeOH (98:2) to produce yellow viscous oil. Yield: 81.3%.1H NMR (400 MHz, CHLOROFORM-d) δ ppm 0.74 (d, J = 6.85 Hz, 3 H) 0.81–0.91 (m, 3 H) 1.90 (dd, J = 12.47, 6.60 Hz, 1 H) 2.41–2.53 (m, 2 H) 2.67 (d, J = 6.85 Hz, 1 H) 2.70–2.79 (m, 3 H) 3.49–3.58 (m, 1 H) 3.58–3.81 (m, 3 H) 4.07 (t, J = 5.62 Hz, 1 H) 6.05 (d, J = 6.85 Hz, 1 H) 6.96 (d, J = 7.09 Hz, 1 H) 7.00–7.07 (m, 2 H) 7.10 (d, J = 7.34 Hz, 1 H) 7.12–7.23 (m, 3 H) 7.33 (d, J = 8.07 Hz, 1 H) 7.57 (d, J = 8.07 Hz, 1 H) 8.28 (br. s., 1H).13C NMR (101 MHz, CHLOROFORM-d) δ ppm 17.67, 18.90, 28.36, 30.19, 33.69, 50.73, 51.16, 55.71, 57.85, 109.09, 111.32, 118.81, 119.77, 122.31, 123.92, 125.82, 126.42, 126.63, 127.06, 128.64, 133.89, 136.34, 171.95. MS (EI+) m/z: 375.23 [M+·]. Anal. Calcd for C24H29N3O: C, 76.76; H, 7.78; N, 11.19. Found: C, 76.85; H, 7.73; N, 11.10.
(S)-1N-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-2-(4-isobutylphenyl)propanamide (S)-10d
Compound (S)-10d was prepared following the general Procedure for the synthesis of amide derivatives using 4-isobutyl-alpha-methylphenylacetic acid and the amine (S)-7, and purified using Hex: EtOAc (70:30) to produce colorless viscous oil. Yield: 61%. IR (Nujol): 3300 (NH, Stretch), 3063 (CH aromatic), 2954 (CH, aliphatic), 1640 (C = O, stretch).1H NMR (500 MHz, chloroform-d) δ ppm 0.72 (d, J = 6.41 Hz, 2 H) 0.75–0.90 (m, 10 H) 1.46–1.53 (m, 3 H) 1.75–1.86 (m, 2 H) 1.90–2.00 (m, 1 H) 2.36–2.45 (m, 4 H) 2.60–2.67 (m, 1 H) 2.69–2.76 (m, 1 H) 2.78–2.82 (m, 1 H) 3.49–3.64 (m, 3 H) 3.93–4.06 (m, 1 H) 6.89–6.98 (m,1 H) 6.98–7.03 (m, 2 H) 7.04–7.18 (m, 5 H).13C NMR (126 MHz, chloroform-d) δ ppm 17.24, 17.64, 18.17, 18.77, 22.26, 22.31, 29.12, 29.81, 29.90, 30.13, 44.95, 46.87, 46.99, 50.92, 51.19, 51.45, 56.26, 58.36, 125.56, 126.05, 126.51, 127.31, 128.58, 129.45, 138.73, 140.50, 174.57. MS (EI+) m/z: 406.40 [M+·]. Anal. Calcd for C27H38N2O: C, 79.76; H, 9.42; N, 6.89. Found: C, 80.02; H, 9.58; N, 7.02.
(R)-1N-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-2-(4-isobutylphenyl)propanamide (R)-10d
Compound (R)-10d was prepared following the general Procedure for the synthesis of amide derivatives using 4-isobutyl-alpha-methylphenylacetic acid and the amine (R)-7, and purified using Hex: EtOAc (70:30) to produce colorless viscous oil. Yield: 65.5%. IR (Nujol): 3307 (NH, Stretch), 3021 (CH aromatic), 2954 (CH, aliphatic), 1644 (C = O, stretch).1H NMR (500 MHz, chloroform-d) δ ppm 0.86 (d, J = 7.21 Hz, 3 H) 0.90–0.94 (m, 3 H) 1.04 (d, J = 1.60 Hz, 3 H) 1.05 (d, J = 1.60 Hz, 3 H)1.62 (d, J = 7.21 Hz, 3 H) 1.94–2.06 (m, 2 H) 2.59 (d, J = 8.01 Hz, 2 H) 2.66 (dd, J = 12.82, 5.61 Hz, 1 H) 2.83 (dd, J = 12.82, 9.62 Hz, 1 H) 2.91–2.97(m, 1 H) 2.99–3.05 (m, 3 H) 3.61 (q, J = 7.21 Hz, 1 H) 3.79–3.90 (m, 2 H) 4.19–4.27 (m, 1 H) 6.21 (d, J = 8.82 Hz, 1 H) 7.19 (d, J = 8.01 Hz, 3 H)7.23 (dd, J = 7.61, 3.61 Hz, 1 H) 7.29 (d, J = 2.40 Hz, 1 H) 7.32–7.34 (m, 2 H) 7.43–7.46 (m, 1 H).13C NMR (126 MHz, chloroform-d) δ ppm 17.32, 18.06, 18.81, 22.26, 27.85, 30.15, 30.30, 44.93, 46.73, 50.03, 50.52, 55.35, 57.19, 125.87, 126.43, 126.64, 127.21, 128.59, 128.77, 129.11, 129.35, 133.25, 133.60, 138.83, 140.33, 174.72. MS (EI+) m/z: 406.14 [M+·]. Anal. Calcd for C27H38N2O: C, 79.76; H, 9.42; N, 6.89. Found: C, 79.98; H, 9.59; N, 7.17.
(S)-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)cinnamamide (S)-10e
Compound (S)-10e was prepared following the general Procedure for the synthesis of amide derivatives using cinnamic acid and the amine (S)-7, and purified using Hex: EtOAc (70:30) to produce yellow viscous oil. Yield: 89% IR (Nujol): 3272 (NH, Stretch), 3062 (CH aromatic), 2956 (CH, aliphatic), 1653 (C = O, stretch), 1615 (C = C).1H NMR (500 MHz, chloroform-d) δ ppm 1.03 (t, J = 6.01 Hz, 5 H) 2.19 (d, J = 7.21 Hz, 1 H) 2.69 (dd, J = 12.82, 5.61 Hz, 1 H) 2.77–2.98 (m, 4 H) 3.68–3.77 (m, 1 H) 3.77–3.85 (m, 1 H) 4.28–4.38 (m, 1 H) 6.66–6.75 (m, 1 H) 7.03 (d, J = 7.21 Hz, 1 H) 7.08–7.18 (m, 3 H) 7.37 (d, J = 7.21 Hz, 1 H) 7.40–7.44 (m, 2 H) 7.48 (t, J = 7.61 Hz, 1 H) 7.55 (d, J = 8.01 Hz, 2 H) 7.69 (d, J = 7.21 Hz, 1 H) 7.77 (d, J = 8.01 Hz, 1 H) 8.02 (s, 1 H).13C NMR (126 MHz, chloroform-d) δ ppm 18.34, 18.97, 28.95, 30.68, 50.93, 51.83, 56.27, 57.78, 125.95, 126.06, 126.10, 126.62, 126.82, 127.38, 127.85, 128.88, 129.07, 129.21, 130.20, 134.11, 135.77, 140.49, 168.08. MS (EI+) m/z: 320.23 [M+·]. Anal. Calcd for C23H28N2O: C, 79.27; H, 8.10; N, 8.04. Found: C, 79.44; H, 8.34; N, 8.23.
(R)-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)cinnamamide (R)-10e
Compound (R)-10e was prepared following the general Procedure for the synthesis of amide derivatives using cinnamic acid and the amine (R)-7, and purified using Hex: EtOAc (70:30) to produce yellow viscous oil. Yield: 87.8%.1H NMR (500 MHz, chloroform-d) δ ppm 0.98 (t, J = 6.81 Hz, 6 H) 2.07–2.17 (m, 1 H) 2.59 (dd, J = 12.82, 5.61 Hz, 1 H) 2.67–2.74 (m, 2 H) 2.83–2.92 (m, 3 H) 3.64–3.76 (m, 2 H) 4.21–4.29 (m, 1 H) 6.05 (br. s., 1 H) 6.42 (d, J = 15.23 Hz, 1 H) 7.02–7.06 (m, 1 H) 7.07–7.11 (m, 1 H) 7.11–7.15 (m, 2 H) 7.31–7.35 (m, 3 H) 7.46–7.50 (m, 2 H) 7.61 (d, J = 15.23 Hz, 1 H).13C NMR (126 MHz, chloroform-d) δ ppm 17.89, 18.72, 28.77, 30.31, 50.48, 51.23, 56.27, 57.79, 120.99, 125.67, 126.22, 126.57, 127.73, 128.62, 128.69, 129.46, 134.11, 134.27, 134.85, 140.72, 166.07. MS (EI+) m/z: 320.28 [M+·]. Anal. Calcd for C23H28N2O: C, 79.27; H, 8.10; N, 8.04. Found: C, 79.49; H, 8.34; N, 8.25.
(S)-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-[1,1′-biphenyl]-3-carboxamide (S)-10f
Compound (S)-10f was prepared following the general Procedure for the synthesis of amide derivatives using 3-biphenylcarboxylic acid and the amine (S)-7, and purified using Hex: EtOAc (75:25) to produce yellow viscous oil. Yield: 80.5%.1H NMR (500 MHz, chloroform-d) δ ppm 0.99–1.06 (m, 6 H) 2.15–2.25 (m, 1 H) 2.67 (dd, J = 12.82, 5.61 Hz, 1 H) 2.73–2.84 (m, 2 H) 2.85–2.98 (m, 3 H) 3.66–3.75 (m, 1 H) 3.76–3.83 (m, 1 H) 4.27–4.36 (m, 1 H) 6.65 (br. s., 1 H) 7.03 (d, J = 6.41 Hz, 1 H) 7.07–7.17 (m, 3 H) 7.33–7.39 (m, 1 H) 7.42 (t, J = 7.61 Hz, 2 H) 7.47 (t, J = 7.61 Hz, 1 H) 7.54 (d, J = 8.01 Hz, 2 H) 7.66–7.71 (m, 1 H) 7.72–7.78 (m, 1 H) 8.00 (s, 1 H).13C NMR (126 MHz, chloroform-d) δ ppm 18.08, 18.71, 28.85, 30.38, 50.71, 51.65, 56.15, 57.67, 125.66, 125.73, 125.81, 126.28, 126.56, 127.12, 127.60, 128.62, 128.82, 128.96, 129.91, 130.87, 134.01, 135.63, 140.24, 141.53, 167.83. MS (EI+) m/z: 398.80 [M+·]. Anal. Calcd for C27H30N2O: C, 81.37; H, 7.59; N, 7.03. Found: C, 81.26; H, 7.76; N,7.32.
(R)-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-[1,1′-biphenyl]-3-carboxamide (R)-10f
Compound (R)-10f was prepared following the general Procedure for the synthesis of amide derivatives using 3-biphenylcarboxylic acid and the amine (R)-7, and purified using Hex: EtOAc (75:25) to produce yellow viscous oil. Yield: 77.1%. IR (Nujol): 3296 (NH, Stretch), 3061 (CH aromatic), 2956 (CH, aliphatic), 1632 (C = O, stretch).1H NMR (500 MHz, chloroform-d) δ ppm 0.98–1.09 (m, 6 H) 2.15–2.26 (m, 1 H) 2.60–2.68 (m, 1 H) 2.70–2.80 (m, 2 H) 2.83–2.94 (m, 3 H) 3.63–3.71 (m, 1 H) 3.71–3.80 (m, 1 H) 4.23–4.35 (m, 1 H) 6.51 (d, J = 8.01 Hz, 1 H) 7.03 (d, J = 6.41 Hz, 1 H) 7.08–7.18 (m, 3 H) 7.33–7.50 (m, 4 H) 7.54 (d, J = 7.21 Hz, 2 H) 7.72 (d, J = 8.01 Hz, 1 H) 7.69 (d, J = 8.01 Hz, 1 H) 7.98 (s, 1 H).13C NMR (126 MHz, chloroform-d) δ ppm 18.08, 18.75, 29.17, 30.35, 50.88, 51.87, 56.43, 57.98, 125.64, 125.67, 125.79, 126.17, 126.56, 127.16, 127.63, 128.64, 128.85, 128.99, 129.90, 134.28, 134.63, 135.84, 140.32, 141.63, 167.84. MS (EI+) m/z: 375 [M+·]. Anal. Calcd for C27H30N2O: C, 81.37; H, 7.59; N, 7.03. Found: C, 81.54; H, 7.75; N, 7.29.
(S)-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-4′-fluoro-[1,1′-biphenyl]-3-carboxamide (S)-10g
Compound (S)-10g was prepared following the general Procedure for the synthesis of amide derivatives using 4′-fluoro-3-biphenylcarboxylic acid and the amine (S)-7, and purified using Hex: EtOAc (70:30) to produce colorless viscous oil. Yield: 76.8%.1H NMR (500 MHz, chloroform-d) δ ppm 0.97–1.08 (m, 6 H) 2.19 (dd, J = 12.42, 6.81 Hz, 1 H) 2.68 (dd, J = 12.82, 5.61 Hz, 1 H) 2.75–2.98 (m, 5 H) 3.72 (d, J = 15.23 Hz, 1 H) 3.81 (d, J = 15.23 Hz, 1 H) 4.28–4.38 (m, 1 H) 7.03 (d, J = 7.21 Hz, 1 H) 7.07–7.18 (m, 5 H) 7.42–7.52 (m, 3 H) 7.64 (d, J = 8.01 Hz, 1 H) 7.70–7.78 (m, 1 H) 7.97 (s, 1 H).13C NMR (126 MHz, chloroform-d) δ ppm 18.10, 18.64, 29.16, 30.31, 50.82, 51.80, 56.36, 57.81, 115.63, 115.80, 125.51, 125.68, 126.18, 126.54, 128.61, 128.67, 128.74, 129.03, 129.71, 130.88, 134.22, 134.56, 135.84, 136.34, 140.58, 161.60, 167.76. MS (EI+) m/z: 418.90 [M+·]. Anal. Calcd for C27H29FN2O: C, 77.85; H, 7.02; N, 6.73. Found: C, 77.61; H, 7.26; N, 6.98.
(R)-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-4′-fluoro-[1,1′-biphenyl]-3-carboxamide (R)-10g
Compound (R)-10g was prepared following the general Procedure for the synthesis of amide derivatives using 4′-fluoro-3-biphenylcarboxylic acid and the amine (R)-7, and purified using Hex: EtOAc (70:30) to produce colorless viscous oil. Yield: 73%.1H NMR (500 MHz, chloroform-d) δ ppm 0.97–1.03 (m, 6 H) 2.10–2.20 (m, 1 H) 2.72 (dd, J = 12.42, 5.21 Hz, 1 H) 2.82–3.02 (m, 5 H) 3.74–3.81 (m, 1 H) 3.81–3.89 (m, 1 H) 4.20 (dd, J = 9.22, 6.01 Hz, 1 H) 4.26–4.36 (m, 1 H) 7.02 (d, J = 6.41 Hz, 1 H) 7.05–7.11 (m, 3 H) 7.12–7.17 (m, 2 H) 7.42–7.54 (m, 3 H) 7.62 (d, J = 7.21 Hz, 1 H) 7.77 (d, J = 8.01 Hz, 1 H) 8.00 (s, 1 H).13C NMR (126 MHz, Chloroform-d) δ ppm 18.11, 18.64, 29.13, 30.33, 50.81, 51.78, 56.34, 57.80, 115.63, 115.80, 125.51, 125.69, 126.18, 126.54, 128.61, 128.67, 128.74, 129.02, 129.71, 130.88, 134.21, 134.54, 135.82, 136.34, 140.58, 161.61, 167.76. MS (EI+) m/z: 417.97 [M+·]. Anal. Calcd for C27H29FN2O: C, 77.85; H, 7.02; N, 6.73. Found: C, 78.07; H, 7.16; N, 6.91.
(S)-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-3′-hydroxy-[1,1′-biphenyl]-3-carboxamide (S)-10h
Compound (S)-10h was prepared following the general Procedure for the synthesis of amide derivatives using 3′-hydroxy-3-biphenylcarboxylic acid and the amine (S)-7, and purified using DCM: MeOH (97:3) to produce creamy gummy solid. Yield: 68.6%. M.p.: 45-48oC.1H NMR (500 MHz, chloroform-d) δ ppm 1.01 (d, J = 6.41 Hz, 6 H) 1.97–2.11 (m, 1 H) 2.77 (dd, J = 12.82, 4.01 Hz, 1 H) 2.89 (d, J = 4.81 Hz, 1 H) 2.93–3.05 (m, 3 H) 3.07–3.14 (m, 1 H) 3.84 (d, J = 15.23 Hz, 1 H) 3.99 (d, J = 15.23 Hz, 1 H) 4.47 (br. s., 1 H) 6.66 (d, J = 8.01 Hz, 1 H) 6.90 (s, 1 H) 6.95 (d, J = 8.01 Hz, 1 H) 7.05 (d, J = 7.21 Hz, 1 H) 7.02 (d, J = 7.21 Hz, 1 H) 7.08–7.15 (m, 3 H) 7.30–7.35 (m, 1 H) 7.48 (d, J = 7.21 Hz, 1 H) 7.72 (d, J = 8.01 Hz, 1 H) 7.92 (s, 1 H).13C NMR (126 MHz, chloroform-d) δ ppm 18.13, 19.04, 26.99, 31.37, 50.14, 50.87, 54.84, 56.92, 114.03, 114.72, 118.49, 125.47, 126.03, 126.32, 126.81, 126.98, 128.66, 128.83, 129.83, 129.93, 131.60, 132.71, 134.58, 140.96, 141.23, 156.74, 168.42. MS (EI+) m/z: 414.11 [M+·]. Anal. Calcd for C27H30N2O2: C, 78.23; H, 7.29; N, 6.76. Found: C, 78.51; H, 7.46; N, 6.95.
(R)-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-3′-hydroxy-[1,1′-biphenyl]-3-carboxamide (R)-10h
Compound (R)-10h was prepared following the general Procedure for the synthesis of amide derivatives using 3′-hydroxy-3-biphenylcarboxylic acid and the amine (R)-7, and purified using DCM: MeOH (97:3) to produce creamy gummy solid. Yield: 64%. M.p.: 45-48oC. IR (Nujol): 3295 (OH and NH, broad), 3063 (CH aromatic), 2921 (CH, aliphatic), 1633 (C = O, stretch).1H NMR (500 MHz, chloroform-d) δ ppm 1.01 (d, J = 6.41 Hz, 6 H) 2.00–2.08 (m, 1 H) 2.77 (dd, J = 12.82, 4.01 Hz, 1 H) 2.89 (d, J = 4.81 Hz, 1 H) 2.94–3.05 (m, 3 H) 3.08–3.17 (m, 1 H) 3.84 (d, J = 15.23 Hz, 1 H) 3.99 (d, J = 15.23 Hz, 1 H) 4.47 (br. s., 1 H) 6.66 (d, J = 8.01 Hz, 1 H) 6.90 (s, 1 H) 6.95 (d, J = 8.01 Hz, 1 H) 7.05 (d, J = 7.21 Hz, 1 H) 7.02 (d, J = 7.21 Hz, 1 H) 7.08–7.17 (m, 3 H) 7.29–7.34 (m, 1 H) 7.48 (d, J = 7.21 Hz, 1 H) 7.72 (d, J = 8.01 Hz, 1 H) 7.92 (s, 1 H).13C NMR (126 MHz, chloroform-d) δ ppm 18.39, 19.29, 27.25, 31.63, 50.40, 51.12, 55.10 (s, 1 C) 57.18, 114.29, 114.98, 118.75, 125.73, 126.28, 126.58, 127.07, 127.24, 128.92, 129.09, 130.09, 130.19, 132.97, 134.84, 141.22, 141.49, 157.00, 168.67.MS (EI+) m/z: 414.61 [M+·]. Anal. Calcd for C27H30N2O2: C, 78.23; H, 7.29; N, 6.76. Found: C, 78.50; H, 7.43; N, 6.95.
(S)-3′-cyano-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-[1,1′-biphenyl]-3-carboxamide (S)-10i
Compound (S)-10i was prepared following the general Procedure for the synthesis of amide derivatives using 3′-cyano-3-biphenylcarboxylic acid and the amine (S)-7, and purified using Hex: EtOAc (60:40) to produce off-white powder. Yield: 78.8%. M.p.: 108-110oC. IR (Nujol): 3316 (NH, stretch), 3063 (CH aromatic), 2957 (CH, aliphatic), 2357 (C ≡ N), 1643 (C = O, stretch).1H NMR (500 MHz, chloroform-d) δ ppm 1.03 (d, J = 7.21 Hz, 6 H) 2.14 (br. s., 1 H) 2.65 (d, J = 9.62 Hz, 1 H) 2.77–2.99 (m, 5 H) 3.70 (br. s., 1 H) 3.79 (br. s., 1 H) 4.36 (br. s., 1 H) 6.99–7.03 (m, 1 H) 7.05–7.15 (m, 3 H) 7.35–7.45 (m, 2 H) 7.58–7.63 (m, 2 H) 7.65 (d, J = 7.21 Hz, 1 H) 7.74 (d, J = 7.21 Hz, 1 H) 7.95 (br. s., 1 H) 8.02 (br. s., 1 H).13C NMR (126 MHz, Chloroform-d) δ ppm 18.22, 18.90, 28.73, 30.79, 50.68, 51.64, 56.15, 57.96, 125.80, 126.10, 126.24, 126.33, 126.48, 126.59, 128.62, 129.01, 129.78, 130.41, 133.79, 133.91, 135.69, 140.31, 140.53, 167.75, 169.25. MS (EI+) m/z: 423.56 [M+·]. Anal. Calcd for C28H29N3O: C, 79.40; H, 6.90; N, 9.92. Found: C, 79.18; H, 6.75; N, 10.16.
(S)-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-3-(pyridin-3-yl)benzamide (S)-10j
Compound (S)-10j was prepared following the general Procedure for the synthesis of amide derivatives using 3-(pyridine-3-yl)benzoic acid and the amine (S)-7, and purified using DCM: MeOH (98:2) to produce colorless viscus oil. Yield: 59%. IR (Nujol): 3271 (NH, stretch), 3061 (CH aromatic), 2958 (CH, aliphatic), 1634 (C = O, stretch).1H NMR (500 MHz, chloroform-d) δ ppm 1.03 (d, J = 7.21 Hz, 6 H) 2.10 (d, J = 5.61 Hz, 1 H) 2.72 (dd, J = 12.82, 4.81 Hz, 1 H) 2.86–2.99 (m, 3 H) 2.99–3.09 (m, 2 H) 3.85 (d, J = 12.82 Hz, 2 H) 4.41 (br. s., 1 H) 7.05 (d, J = 7.21 Hz, 1 H) 7.08–7.12 (m, 1 H) 7.13–7.18 (m, 2 H) 7.32–7.38 (m, 2 H) 7.51 (t, J = 7.61 Hz, 1 H) 7.68 (d, J = 7.21 Hz, 1 H) 7.82 (d, J = 8.01 Hz, 1 H) 7.87 (d, J = 8.01 Hz, 1 H) 8.03 (s, 1 H) 8.60 (d, J = 4.81 Hz, 1 H) 8.85 (d, J = 2.40 Hz, 1 H).13C NMR (126 MHz, Chloroform-d) δ ppm 18.13, 18.91, 27.26, 31.05, 49.72, 50.96, 55.00, 56.41, 123.67, 126.17, 126.60, 126.72, 126.78, 128.67, 129.19, 129.80, 132.20, 133.03, 134.63, 135.69, 135.92, 138.02, 148.04, 148.56, 167.64. MS (EI+) m/z: 400.65 [M+·]. Anal. Calcd for C26H29N3O: C, 78.16; H, 7.32; N, 10.52. Found: C, 78.40; H, 7.51; N, 10.64.
(R)-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-2-(2-methyl-3-nitrophenyl)acetamide (R)-10m
Compound (R)-10m was prepared following the general procedure for the synthesis of amide derivatives using 2-methyl-3-nitro-phenylacetic acid and the amine (R)-7, and purified using DCM: MeOH (98:2) to produce golden viscous oil. Yield: 87.2%. IR (Nujol): 3295(NH, stretch), 3066 (CH aromatic), 2958 (CH, aliphatic), 1643 (C = O, stretch), 1538(N-O, stretch), 1348 (N-O, stretch).1H NMR (500 MHz, Chloroform-d) δ ppm 0.83 (d, J = 6.85 Hz, 3 H) 0.80 (d, J = 6.85 Hz, 3 H) 1.90 (dd, J = 12.23, 6.60 Hz, 1 H) 2.25 (s, 3 H) 2.43–2.50 (m, 1 H) 2.51–2.60 (m, 1 H) 2.65–2.70 (m, 1 H) 2.72–2.82 (m, 3 H) 3.48 (d, J = 14.67 Hz, 1 H) 3.57–3.69 (m, 3 H) 3.94–4.05 (m, 1 H) 5.89 (br. s., 1 H) 6.90 (d, J = 6.85 Hz, 1 H) 6.97 (t, J = 7.95 Hz, 1 H) 7.03 (d, J = 7.58 Hz, 1 H) 7.05–7.15 (m, 2 H) 7.33 (d, J = 7.58 Hz, 1 H) 7.51 (d, J = 8.07 Hz, 1 H).13C NMR (126 MHz, Chloroform-d) δ 15.23, 18.02, 18.73, 27.45, 30.68, 41.68, 50.66, 54.85, 56.83, 123.07, 126.42, 126.52, 126.67, 127.19, 128.77, 131.45, 134.53, 136.80, 151.33, 170.20. MS (EI+) m/z: 396.22 [M+·]. Anal. Calcd for C23H29N3O3: C, 69.85; H, 7.39; N, 10.62. Found: C, 69.16; H, 7.71; N, 9.90.
General procedure of the synthesis of amide derivatives (S)-10k, l / (R)-10L
Under an inert argon atmosphere and at 0 °C, acid chloride (0.55 mmol) was added to a solution of compound (S/R)-7 (0.1 g, 0.46 mmol) in chloroform (5 ml) and diisopropylethylamine (DIPEA) (0.69 mmol) over a period of 15 min. After stirring at room temperature for 2 h, the reaction mixture was extracted using chloroform and saturated solution of Na2CO3 (1 × 30 mL), water (1 × 30 mL), and brine (1 × 30 mL). The organic phase was dried over Na2SO4, concentrated in vacuo and purified by flash column chromatography on silica gel.
(S)-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-4-(trifluoromethyl)benzamide (S)-10k
Compound (S)-10k was prepared following the latter general procedure for the synthesis of amide derivatives using 3-trifluoromethybenzoic acid and the amine (S)-7, and purified using DCM: MeOH (98:2) to produce golden viscous oil. Yield: 79.6%.1H NMR (500 MHz, Chloroform-d) δ ppm 1.03 (d, J = 1.60 Hz, 3 H) 1.04 (br. s., 3 H) 2.11 (dt, J = 12.82, 6.41 Hz, 1 H) 2.80 (dd, J = 12.82, 4.81 Hz, 1 H) 2.87–2.98 (m, 2 H) 2.99–3.06 (m, 2 H) 3.10–3.16 (m, 1 H) 3.87–3.98 (m, 2 H) 4.37–4.46 (m, 1 H) 7.06 (d, J = 6.41 Hz, 1 H) 7.11–7.21 (m, 3 H) 7.37–7.40 (m, 1 H) 7.60 (d, J = 8.01 Hz, 1 H) 7.93–7.97 (m, 1 H) 8.06 (s, 1 H).13C NMR (126 MHz, chloroform-d) δ ppm 18.23, 18.91, 26.68, 31.18, 49.64, 50.89, 54.54, 55.92, 122.56, 124.41, 126.50, 126.76, 127.21, 127.84, 128.10, 128.74, 128.89, 130.24, 130.97, 132.48, 135.16, 166.69. MS (EI+) m/z: 390.74 [M+·]. Anal. Calcd for C22H25F3N2O: C, 67.68; H, 6.45; N, 7.17. Found: C, 67.90; H, 6.73; N, 7.41.
(S)-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-4-fluorobenzamide (S)-10L
Compound (S)-10L was prepared following the general Procedure for the synthesis of amide derivatives using 4-fluorobenzoic acid and the amine (S)-7, and purified using DCM: MeOH (98:2) to produce golden viscous oil. Yield: 71.9%. IR (Nujol): 3235 (NH, stretch), 3056 (CH aromatic), 2966 (CH, aliphatic), 1630 (C = O, stretch).1H NMR (500 MHz, Chloroform-d) δ ppm 1.00 (d, J = 3.21 Hz, 3 H) 1.01 (d, J = 2.40 Hz, 3 H) 2.15–2.23 (m, 1 H) 2.61–2.67 (m, 1 H) 2.69–2.79 (m, 2 H) 2.82–2.93 (m, 3 H) 3.66–3.78 (m, 2 H) 4.22–4.30 (m, 1 H) 6.48 (d, J = 6.41 Hz, 1 H) 7.00–7.03 (m, 1 H) 7.07 (s, 1 H) 7.08–7.11 (m, 2 H) 7.12–7.15 (m, 2 H) 7.77–7.81 (m, 2 H).13C NMR (126 MHz, chloroform-d) δ ppm 18.05, 18.63, 28.69, 30.31, 50.63, 51.56, 56.07, 57.46, 115.37, 115.54, 125.81, 126.37, 126.52, 128.63, 129.23, 129.31, 131.01, 133.86, 163.59, 165.58, 166.72. MS (EI+) m/z: 341.41 [M+·]. Anal. Calcd for C21H25FN2O: C, 74.09; H, 7.40; N, 8.23. Found: C, 73.87; H, 7.57; N, 8.51.
(R)-N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)-3-methylbutan-2-yl)-4-fluorobenzamide (R)-10L
Compound (R)-10L was prepared following the general Procedure for the synthesis of amide derivatives using 4-fluorobenzoic acid and the amine (R)-7, and purified using DCM: MeOH (98:2) to produce golden viscous oil. Yield: 67.1%.1H NMR (500 MHz, Chloroform-d) δ ppm 0.78 (dd, J = 6.85, 1.96 Hz, 6 H) 1.94 (dd, J = 12.23, 6.85 Hz, 1 H) 2.44 (dd, J = 12.72, 5.62 Hz, 1 H) 2.52–2.74 (m, 5 H) 3.46–3.60 (m, 2 H) 4.00–4.12 (m, 1 H) 6.42 (d, J = 7.09 Hz, 1 H) 6.77–6.84 (m, 2 H) 6.85–6.97 (m, 4 H) 7.58 (dd, J = 8.68, 5.26 Hz, 2 H).13C NMR (126 MHz, chloroform-d) δ ppm 18.16, 18.73, 28.12, 30.62, 50.51, 51.36, 55.64, 57.05, 115.40, 115.62, 126.12, 126.64, 126.75, 128.73, 129.43, 129.52, 130.77, 133.38, 163.46, 165.96, 166.83. MS (EI+) m/z: 341.20 [M+·]. Anal. Calcd for C21H25FN2O: C, 74.09; H, 7.40; N, 8.23. Found: C, 73.91; H, 7.69; N, 8.01.
Biological evaluation
Radioligand competitive binding assay
The competitive radioligand binding testing was performed on the Chinese hamster ovarian cell lines expressing the mouse monocloned opioid receptors as being previously reported [doi: https://doi.org/10.1021/jm801272c; doi: https://doi.org/10.1021/cn2000348; doi: https://doi.org/10.1021/jm301247n]. In brief, different opioid receptors; KOP, MOP, and DOP, were respectively labelled using [3 H]nor-BNI, [3 H]NLX, and [3 H]NTI. Compounds under investigations were incubated with the aliquots of the membrane protein (30 µg) in presence of corresponding radioligand all within Tris/Borate/EDTA buffer (50mM Tris, 3mM magnesium chloride, 0.2mM EDTA; pH = 7.7) for 2 h at room temperature. Filtration using Brandel M24 harvester (Biomedical Research aDevelopment Laboratories®, Gaithersburg, MD, USA) was used to separate the bounded radioactive ligands from the free radioligands. Drug potency in relation to radioligand displacement was estimated in duplicates from the specific binding using the Hill plots′ linear regression analysis. The IC50 values of selected compounds were then determined using the Cheng-Prusoff equation following correction to the Ki values.
[35S]-GTPγS stimulated binding functional assay
Functional assay was conducted on the same cell membranes being adopted within the above described competitive radioligand binding testing. Tested synthesized compounds (5µM) were incubated with the membrane protein (10 µg), Guanosine diphosphate (10 µg), and 0.1nM [35S]-GTPγS within the Tris/Borate/EDTA analysis buffer (50mM Tris, 3mM magnesium chloride, 0.2mM EDTA; pH = 7.7) for 2 h at room temperature [doi:https://doi.org/10.1021/jm4012214]. Non-labelled GTPγS (20µM) was used to determine the non-specific bindings. DAMGO (5µM) and Salvinorin A (5µM) were included within the assay as full agonists for MOP and KOP, respectively.
Tail-flick test
All experimental protocols were approved by the Research Ethics Committee and Institutional Animal Care and Use Committee, Faculty of Pharmacy, Suez Canal (approval number SCU-Pharm202501RA1), and all methods were carried out according to the European Union guidelines and in accordance with ARRIVE guidelines (https://arriveguidelines.org) [https://doi.org/10.1186/s12917-020-02451-y]. In brief, male Wistar mice of approximately the same weight (170 ± 20 g) were purchased from Laboratory Animal House, Faculty of Veterinary Medicine, Suez Canal University, Egypt, and acclimatized following routine experimental conditions. Mice were kept at room temperature, 12 h dark/light cycles, and both food and water were available ad libitum. The warm-water tail-flick experiment was conducted as per the reported procedure by Rollman and Coderre [doi: https://doi.org/10.1016/0024-3205(83)90103-0]. In brief, the baseline latency of the tested mice was recorded in response to a prewarmed water bath with a temperature being maintained at (50 ± 0.1 °C). Mice were held with care and ½ of their tails were dipped within the warm water bath and average baseline latency was recorded at 3 ± 0.3 s. Both the test synthesized compounds and control agent (nalbuphine) were then administered subcutaneously at 0.3 mg/Kg to the respective mice groups (n = 4–5/group). Latency to a rapid tail-flick following drug administration was recorded by an investigator being blind to treatment groups. Cut-off time was set at 15 s to avoid the damage of the mice tail tissues. Anti-nociception effects were quantified as the percentage of maximum possible effect (% MPE) being estimated through the following equation; % MPE [(test latency–control latency)/(10–control latency)]×100 [PMID: 14163985].
Prolactin assay
Following drug administration, blood samples were collected via retro-orbital sinus bleeds from Isoflurane anesthetized mice and centrifuged at 1000 g/4 °C for 10 min. Plasma was removed, and prolactin (25,496 Dalton) was measured within 1:20 plasma dilutions [doi: https://doi.org/10.1242/bio.060088] using the commercially available Mice Prolactin Sandwich ELISA Kit (#ab100736, Abcam®, MA, USA) as per the manufacturer′s instructive protocol. Results are represented in ng/mL and One-way ANOVA and Dunnett post-hoc test were done for assessing statistical significances (p < 0.05) between the test group in relation to control.
Molecular docking
Molecular docking was performed in the OpenEye software 2023.2.3 using the FRED module ( Fast Rigid Exhaustive Docking).28 First, a file of the prepared receptor grids was generated using the make-receptor command as implemented in FRED. Then, a multi-conformer algorithm was used to produce a library of the ligand(s) conformers. The crystal structure of the human kappa opioid receptor in complex with JDTic (PDB ID: 4DJH) has been used for the docking simulation. The conformer library of the docked ligands was generated using the OpenEye software and the default settings. The docking box was centered on the sidechain carboxylate carbon of Asp138, with a volume of 25,160 Å2. The shape complementarity scoring function of the FRED module, Chemgauss4, was used to rank the docked poses. The score consists of the following components: steric, acceptor, donors, coordinating groups, metals, lone pairs, polar hydrogens and chelator coordinating groups. Visual examination was used to insure the presence of the critical interactions and the top scoring poses were visualized using the Pymol 2.5.5 software module.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files]. The biological activity dose-response curves as well as the docking files are available from the corresponding author on reasonable request.
References
Kitchen, I., Slowe, S. J., Matthes, H. W. & Kieffer, B. Quantitative autoradiographic mapping of mu-, delta- and kappa-opioid receptors in knockout mice lacking the mu-opioid receptor gene. Brain Res. 778(1), 73–88 (1997).
Albert-Vartanian, A. et al. Will peripherally restricted kappa-opioid receptor agonists (pKORAs) relieve pain with less opioid adverse effects and abuse potential? J. Clin. Pharm. Ther. 41(4), 371–382. https://doi.org/10.1111/jcpt.12404 (2016).
Manglik, A. et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature 537 (7619), 185–190. https://doi.org/10.1038/nature19112 (2016).
Liu, H. et al. Heterodimerization of the kappa opioid receptor and neurotensin receptor 1 contributes to a novel beta-arrestin-2-biased pathway. Biochim. Biophys. Acta. 1863 (11), 2719–2738. https://doi.org/10.1016/j.bbamcr.2016.07.009 (2016).
Lutz, P. E. & Kieffer, B. L. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 36(3), 195–206. https://doi.org/10.1016/j.tins.2012.11.002 (2013).
Robinson, S. E. Buprenorphine-containing treatments: Place in the management of opioid addiction. CNS Drugs 20(9), 697–712 (2006).
Lobe, M. M. M., Verma, S., Patil, V. M. & Iyer, M. R. A review of kappa opioid receptor antagonists and their clinical trial landscape. Eur. J. Med. Chem. 287, 117205. https://doi.org/10.1016/j.ejmech.2024.117205 (2025).
Rothman, R. B. et al. An open-label study of a functional opioid kappa antagonist in the treatment of opioid dependence. J. Subst. Abuse Treat. 18 (3), 277–281 (2000).
Schindler, A. G., Li, S. & Chavkin, C. Behavioral stress may increase the rewarding Valence of cocaine-associated cues through a dynorphin/kappa-opioid receptor-mediated mechanism without affecting associative learning or memory retrieval mechanisms. Neuropsychopharmacology: Official Publication Am. Coll. Neuropsychopharmacol. 35(9), 1932–1942. https://doi.org/10.1038/npp.2010.67 (2010).
del Narciso, B., Orts Castro, B. B. & Calvo Vecino, A. Anesthetic considerations in a patient taking Disulfiram and Naltrexone for chronic alcoholism. Rev. Esp. Anestesiol. Reanim. 58(2), 128–129 (2011).
Carroll, F. I., Harris, L. S. & Aceto, M. D. Effects of jdtic, a selective kappa-opioid receptor antagonist, on the development and expression of physical dependence on morphine using a rat continuous-infusion model. Eur. J. Pharmacol. 524 (1–3), 89–94. https://doi.org/10.1016/j.ejphar.2005.09.013 (2005).
Thomas, J. B. et al. Identification of the first trans-(3R,4R)- dimethyl-4-(3-hydroxyphenyl)piperidine derivative to possess highly potent and selective opioid kappa receptor antagonist activity. J. Med. Chem. 44(17), 2687–2690 (2001).
Melief, E. J. et al. Duration of action of a broad range of selective κ-opioid receptor antagonists is positively correlated with c-Jun N-terminal kinase-1 activation. Mol. Pharmacol. 80(5), 920–929. https://doi.org/10.1124/mol.111.074195 (2011).
Domi, E. et al. Preclinical evaluation of the kappa-opioid receptor antagonist CERC-501 as a candidate therapeutic for alcohol use disorders. Neuropsychopharmacology 43 (9), 1805–1812. https://doi.org/10.1038/s41386-018-0015-y (2018).
Banks, M. L. & Rice, K. C. Effects of the kappa-opioid receptor antagonist nor-binaltorphimine on methamphetamine-vs-food choice in male rhesus monkeys. Drug Alcohol Depend. 266, 112518. https://doi.org/10.1016/j.drugalcdep.2024.112518 (2025).
Wala, K. & Szepietowski, J. C. Difelikefalin in the treatment of chronic kidney Disease-Associated pruritus: A systematic review. Pharmaceuticals (Basel) 15(8). https://doi.org/10.3390/ph15080934 (2022).
Li, W. et al. Major depressive disorder and kappa opioid receptor antagonists. Translational Perioperative Pain Med. 1(2), 4–16 (2016).
Zhao, M., Wang, J. Y., Jia, H. & Tang, J. S. Roles of different subtypes of opioid receptors in mediating the ventrolateral orbital cortex opioid-induced Inhibition of mirror-neuropathic pain in the rat. Neuroscience 144(4), 1486–1494. https://doi.org/10.1016/j.neuroscience.2006.11.009 (2007).
Wu, H. et al. Structure of the human kappa-opioid receptor in complex with JDTic. Nature 485(7398), 327–332. https://doi.org/10.1038/nature10939 (2012).
Kormos, C. M. et al. Simple tetrahydroisoquinolines are potent and selective kappa opioid receptor antagonists. ACS Med. Chem. Lett. 8(7), 742–745. https://doi.org/10.1021/acsmedchemlett.7b00115 (2017).
Kormos, C. M. et al. Potent and selective tetrahydroisoquinoline kappa opioid receptor antagonists of lead compound (3 R)- N-[1 R)-1-(Cyclohexylmethyl)-2-methylpropyl]-7-hydroxy-1,2,3,4-tetrahydroisoquinoline-3-carboxamide (CDTic). J. Med. Chem. 61(17), 7546–7559. https://doi.org/10.1021/acs.jmedchem.8b00674 (2018).
Ondachi, P. W. et al. Potent and selective tetrahydroisoquinoline kappa opioid receptor antagonists of lead compound (3 R)-7-Hydroxy- N-[(1 S)-2-methyl-1-(piperidin-1-ylmethyl)propyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxamide (PDTic). J. Med. Chem. 61(17), 7525–7545. https://doi.org/10.1021/acs.jmedchem.8b00673 (2018).
Irwin, J. J. & Shoichet, B. K. ZINC–a free database of commercially available compounds for virtual screening. J. Chem. Inf. Model. 45(1), 177–182. https://doi.org/10.1021/ci049714+ (2005).
Wu, H. et al. Structure of the human κ-opioid receptor in complex with JDTic. Nature 485(7398), 327–332. https://doi.org/10.1038/nature10939 (2012).
Liu, F. et al. Discovery of tetrahydro-beta-carbolines as inhibitors of the mitotic Kinesin KSP. Bioorg. Med. Chem. 18(12), 4167–4177. https://doi.org/10.1016/j.bmc.2010.05.024 (2010).
Zhang, X. et al. 2,3-Diamino acid modifying 3S-tetrahydroisoquinoline-3-carboxylic acids: Leading to a class of novel agents with highly unfolded conformation, selective in vitro anti-platelet aggregation and potent in vivo anti-thrombotic activity. Bioorg. Med. Chem. 18 (4), 1536–1554. https://doi.org/10.1016/j.bmc.2010.01.009 (2010).
Zhao, M. et al. Synthesis of new class dipeptide analogues with improved permeability and antithrombotic activity. Bioorg. Med. Chem. 14(14), 4761–4774. https://doi.org/10.1016/j.bmc.2006.03.026 (2006).
McGann, M. FRED pose prediction and virtual screening accuracy. J. Chem. Inf. Model. 51(3), 578–596. https://doi.org/10.1021/ci100436p (2011).
Acknowledgements
The work has been entirely funded by The U.S. - Egypt Science and Technology Joint Fund, Grant #1086, Cycle 18, from the National academy of Sciences (USA) and Science, Technology, and Innovation Funding Authority (STDF, Egypt).
Author information
Authors and Affiliations
Contributions
A. A. and H. S. performed all the chemical synthesis and chromatographic purification. S. C. and A.C. performed the spectroscopic analysis and structure elucidation. M. E. performed the biological evaluation and data analysis. M. H. and K. D. Conceived the idea and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Abdelwaly, A., Safwan, H., Chatterjee, S. et al. Novel dual kappa/mu opioid ligands based on a tetrahydroisoquinoline-valine hybrid nucleus. Sci Rep 15, 36138 (2025). https://doi.org/10.1038/s41598-025-08398-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08398-0