Abstract
This study compared the efficacy of nonvitrectomizing vitreous surgery (NVS) and pars plana vitrectomy (PPV) for idiopathic epiretinal membrane by evaluating both the central macular thickness (CMT) and disorganization of retinal inner layers (DRIL), an indicator first used for structural assessment. Medical records of 117 patients (117 eyes; NVS: 54 eyes; PPV: 63 eyes) were retrospectively analyzed. Baseline best-corrected visual acuity (BCVA), CMT, and DRIL points showed no significant intergroup differences. Postoperatively, BCVA improved, whereas CMT and DRIL points decreased greatly at 1 and 6 months in both groups. While postoperative CMT and DRIL points did not differ significantly between groups, BCVA improvement was comparable at 1 month but obviously greater in the NVS group at 6 months. Eyes with no/mild DRIL had better visual prognoses than those with severe DRIL, though marked visual improvement occurred even in cases with severe DRIL. No ERM recurrence was observed. In conclusion, NVS, which simplifies the surgical procedure and preserves the vitreous integrity, can achieve non-inferior visual functional and retinal structural outcomes compared to PPV in the treatment of iERM.
Similar content being viewed by others
Introduction
Idiopathic epiretinal membrane (iERM), a common cause of visual impairment, can lead to distortion and disorganization of the retinal structure. Vitrectomy has been widely used for the treatment of this disorder1,2, while postoperative cataract remains inevitable3,4. As a matter of fact, the vitreous body in patients with ERM always remains intact and has little impact on vision compared with the proliferative membrane itself. Nonvitrectomizing vitreous surgery (NVS), aiming to remove ERMs without excising the vitreous, has been proposed as an alternative procedure to ERMs for its minimally invasive nature and reduced risk of postoperative cataract5,6.
However, there have been no reports about retinal inner layer changes and their relationship with vision prognosis after NVS. In this study, we attempted to observe the retinal anatomical alteration by optical coherence tomography (OCT) and elaborate whether NVS could affect the anatomical and functional prognoses as compared with the traditional vitrectomy.
Materials and methods
Study participants
In this retrospective case series, we enrolled 117 patients (117 eyes) who underwent surgical treatment for iERM at the Qingdao Eye Hospital between May 2020 and May 2023, including 63 patients treated by pars plana vitrectomy (PPV) and 54 patients treated by NVS. The study was conducted in accordance with the tenets of Declaration of Helsinki and approved by the Institutional Review Board of Qingdao Eye Hospital (2020-01). Written informed consent was obtained from all the studied subjects for sample collection and subsequent analyses.
All included patients were aged ≥ 18 years, completed at least 6 months of postoperative follow-up, and experienced no severe or symptomatic vitreous opacity. Patients who had secondary ERM with coexisting or preceding ocular diseases, previous vitrectomy surgery, ocular trauma, high myopia, or any other eye diseases requiring further treatment were excluded from the study. Patient demographics, preoperative and postoperative best-corrected visual acuity (BCVA), central macular thickness (CMT), and disorganization of retinal inner layers (DRIL) were evaluated.
Retinal structure assessment
Spectral domain OCT (SD-OCT) (Spectralis; Heidelberg Engineering, Heidelberg, Germany) was performed to measure the CMT and DRIL. The CMT was defined as the average retinal thickness of the circular area 1 mm from the foveal center. The DRIL within the central 2000-µm area was scored based on distinguishability (0 for distinguishable, 1 for indistinguishable) and boundary regularity (0 for regular, 1 for irregular) between the ganglion cell inner plexiform layer complex and inner nuclear layer (INL) and between the INL and outer plexiform layer, resulting in a total score ranging from 0 to 4 points. The severity of DRIL was then classified into 3 grades: no DRIL, grade 0 (0 points); presence of mild DRIL, grade 1 (1–3 points); presence of severe DRIL, grade 2 (4 points)7. All OCT images were qualitatively and quantitatively reviewed by two independent and masked observers (S.X., W.C.), and possible disagreements were resolved by a third observer (M.Z.).
Surgical techniques
In the PPV group, 25-gauge PPV was performed using the Constellation Vision System (Alcon Laboratories, Fort Worth, TX, USA). The entire vitreous was removed, and the ERM was completely peeled. The internal limiting membrane (ILM) was also peeled if there was any residual ILM at the macular surface extending to the arcades. Then the peripheral retina was inspected, and air/fluid exchange was performed.
In the NVS group, two sclerotomies were made, and self-sealed 25-gauge trocars (Synergetics, O’Fallon, MO, USA) were placed. Both the ERM and ILM were peeled and removed completely under an intraocular illumination. Immediately after the ILM removal, the retinal surface presented a transient gray appearance, sometimes accompanied by subtle hemorrhage, which serves as the indicator of successful ILM peeling. The excised ILM was clearly visible as a glistening transparent sheet, while the removed ERM became a strip. The distinctive appearance of these membranes allowed dye-free peeling. After the peripheral retina was inspected, the incarcerated vitreous was excised, and the sclerotomy site was sutured if there was any leakage. Balanced salt solution was finally injected to maintain the normal intraocular pressure.
Phacoemulsification combined with intraocular lens implantation was performed after the PPV/NVS procedure for all the patients.
Statistical analysis
The SPSS 26.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Snellen BCVA values were converted to the logarithm of the minimum angle of resolution (logMAR) values. In each group, the BCVA and CMT were compared using the repeated analysis of variance (ANOVA), and the DRIL points were compared using the Friedman rank sum test before and after surgery. Between groups, the BCVA and CMT at each time point were compared using the t-test, and the DRIL grades were compared using the Mann–Whitney rank sum test. A P value < 0.05 was considered statistically significant.
Results
The mean patient age was 65.79 ± 7.28 years old in the PPV group and 65.62 ± 6.74 years old in the NVS group (P = 0.893). Females accounted for 65.1% and 66.7% in the two groups, respectively.
BCVA
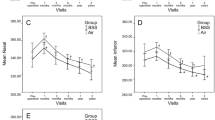
The mean baseline BCVA was 0.68 ± 0.32 logMAR in the PPV group and 0.59 ± 0.27 logMAR in the NVS group (P = 0.118). Substantial visual enhancement was achieved in both groups at postoperative 1 and 6 months (both P < 0.001). The postoperative BCVA was not significantly different at 1 month (P = 0.460) but had statistical difference at 6 months (P = 0.028) between groups (Table 1, Fig. 1a).
CMT
No significant difference in CMT was revealed at baseline between the PPV group (463.00 ± 108.63 μm) and the NVS group (460.00 ± 95.89 μm) (P = 0.875). Postoperatively, both groups exhibited markedly reduced CMT at 1 and 6 months (all P < 0.001). There was still no significant difference between the two groups at either follow-up time point (P = 0.249, 0.265) (Table 1, Fig. 1b).
DRIL
The DRIL measurements were 2.74 ± 1.26 points and 2.73 ± 1.12 points at baseline in the NVS and PPV groups, respectively, with no significant difference (P = 0.802), and decreased markedly at postoperative 1 and 6 months in both groups (all P < 0.001). Postoperative measurements still showed no significant difference between groups at either time point (P = 0.596, 0.998) (Table 1, Fig. 1c).
There existed a strong correlation between BCVA and DRIL severity. In the NVS group, the baseline BCVA differed remarkably between eyes with no/mild DRIL (33 eyes, 61.1%; 0.54 ± 0.30 logMAR) and those with severe DRIL (21 eyes, 38.9%; 0.68 ± 0.19 logMAR) (P = 0.041). Although BCVA improved greatly at postoperative 1 and 6 months in all eyes, the difference between eyes with no/mild and severe DRIL remained significant (P = 0.018, 0.017). Similarly, in the PPV group, BCVA in eyes presenting no/mild DRIL (39 eyes, 61.9%; 0.56 ± 0.24 logMAR) was significantly different from that in eyes with severe DRIL (24 eyes, 38.1%; 0.88 ± 0.34) at baseline (P < 0.001), and improved markedly at the two postoperative time points in all eyes, with marked difference between eyes with varied severities of DRIL (P = 0.041, 0.017) (Table 2).
Surgical complications
Surgical complications such as postoperative vitreous hemorrhage, retinal detachment, and endophthalmitis were observed in neither group during the follow-up period. There was no sign of ERM recurrence on the retinal appearance and OCT examination at postoperative 6 months. The CMT gradually decreased, and the DRIL ameliorated in both groups (Figs. 2 and 3).
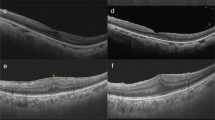
Imaging examinations reveal anatomical changes in patients treated by nonvitrectomizing vitreous surgery. A 68-year-old female patient. (1a) Fundus photography shows macular pucker with posterior vitreous detachment. (1b) Preoperative optical coherence tomography (OCT) reveals epiretinal membrane (ERM) with a central retinal thickness value of 624 μm and the tortured retina with DRIL at 4 points, grade 2. (1c) OCT at 3 months after surgery demonstrates a decrease of central retinal thickness to 393 μm and reorganized retinal layers (0 points, grade 0). The vision is improved from preoperative Snellen 0.15 to Snellen 0.3. A 60-year-old female patient. The paramacular ERM on fundus photography (2a) and rough retinal surface with DRIL at 4 points, grade 2 on OCT (2b) are presented. The rough surface becomes smooth postoperatively with distinct retinal layers and DRIL at 1 point, grade 1 (2c). The vision is improved from preoperative Snellen 0.15 to Snellen 0.4. A 68-year-old male patient. (3a) Fundus photography shows the opaque ERM and high reflection in the posterior pole. (3b) The preoperative tight adhesion, elevated foveal pit, and disorganized inner layers are demonstrated on the OCT image with DRIL at 1 point, grade 1. (3c) Postoperatively, the foveal pit is present, and retinal layers are well-defined with DRIL at 0 points, grade 0. The vision is improved from preoperative Snellen 0.4 to Snellen 1.0
Imaging examinations reveal anatomical changes in patients treated by pars plana vitrectomy. A 65-year-old female patient. (4a) Fundus photography shows ERM with twisted vessels. (4b) Preoperative OCT reveals the elevated and disorganized retina and intra-layer schisis with DRIL at 4 points, grade 2. (4c) OCT at 3 months after surgery demonstrates the retinal structure was reformed with DRIL at 2 points, grade 1. The vision is improved from preoperative Snellen 0.3 to Snellen 0.4. A 70-year-old female patient. (5a) Fundus photography shows ERM presenting as a translucent membrane on the macular surface. (5b) Preoperative OCT reveals the nerve fiber layer is stretched and the inner layers are disorganized with DRIL at 3 points, grade 2. (5c) OCT at 3 months after surgery demonstrates the traction is relieved and the retinal layers are distinct with DRIL at 0 points, grade 0. The vision is improved from preoperative Snellen 0.4 to Snellen 0.8. A 72-year-old male patient. (6a) Fundus photography shows ERM presenting as a white strip with DRIL at 4 points, grade 2 (6b). (6c) OCT at 3 months after surgery demonstrates DRIL at 0 points, grade 0. The vision is improved form preoperative Snellen 0.5 to Snellen 1.0.
Discussion
The efficacy of PPV as the conventional treatment for symptomatic ERM has been documented well1,2. However, postoperative nuclear sclerosis seems unavoidable4. Lens removal surgery is not suitable for patients without cataract when vitrectomy is performed. Moreover, the vitreous is essential for normal intraocular metabolism and pharmacokinetics, especially when intraocular medication is needed.
NVS can treat retinal diseases without removing the vitreous, with the advantage of preventing postoperative nuclear sclerosis5. Although iERM typically presents as a partial membrane with an intact vitreous, the application of NVS for this benign condition has been limited in clinical practice5,6. Sawa et al.5 evaluated the therapeutic effects of NVS on iERM from the perspective of nuclear sclerosis stabilization. Reibaldi et al.6 compared the visual improvement by NVS and PPV in 79 eyes with iERM. In the current study, the surgical outcomes after PPV and NVS were compared, and the OCT parameters of DRIL and CMT were employed as the indicators for retinal rehabilitation. The structural changes were found to align closely with the vision enhancement. The CMT was reduced by 27% after PPV and 31% after NVS, similar to the results (both 33%) disclosed by Reibaldi et al6. However, the visual improvement was more remarkable in our study, 42.6% after PPV and 49.1% after NVS, than the previously reported rates of 26.8% and 33.3%6. This discrepancy might be attributed to the combined cataract surgery in our cohort. To eliminate the impact of post-vitrectomy cataract progression on visual outcomes, all enrolled senile patients underwent concurrent cataract extraction during the PPV/NVS procedure. Notably, postoperative visual recovery reflects the functional contribution of both anterior and posterior segment interventions. To more accurately evaluate the influence of ERM removal on visual function, a comparative analysis of preoperative and postoperative visual acuity in eyes undergoing NVS without combined cataract surgery would be preferable, although this falls beyond the scope of this study. The better postoperative visual acuity at 6 months after NVS compared to PPV may result from the shorter operation time, reduced phototoxicity, avoidance of dye application, and preservation of intraocular metabolism with the vitreous. Overall, the NVS procedure was not inferior to conventional PPV in the functional and anatomical improvement for patients with iERM.
As for the OCT parameters for ERM, various prognostic micro-structural factors such as CMT, ellipsoid zone disruption, and the inner-retinal layer irregularity index, ERM stages, and DRIL have been employed, among which DRIL and CMT are strongly correlated with unfavorable postoperative visual outcomes7,8,9. DRIL was first described by Sun et al.10 as the horizontal extent in microns for which boundaries between the ganglion cell inner plexiform layer, INL, and outer plexiform layer could not be identified, and later was disclosed to be a predictor for the resolution of diabetic macular edema11,12. To our knowledge, the current study represents the first application of DRIL as a quantitative indicator of retinal structural changes after NVS for the treatment of iERM.
In our series, cases with varying degrees of DRIL showed comparable distribution between the two groups. The postoperative reduction in DRIL points indicated a rehabilitation trend that paralleled visual improvement. It should be noted that even patients with severe baseline DRIL achieved greatly improved postoperative vision, which was superior to the outcomes reported by Karasavvidou et al.9 These findings suggest that surgical interventions are worthwhile for ERM even at the advanced stage. Furthermore, the NVS procedure is proved as effective as conventional vitrectomy for severe cases.
In terms of postoperative complications, the potential recurrence of ERM remains a primary concern, despite the lack of a standardized definition. The recurrence incidence is associated with the definition, examination approach, follow-up period, and technique of membrane peeling (combined with ILM peeling or not). It was reported that ERM recurred in 0% to 58% of cases after PPV, but only 4.3% of cases had clinically significant recurrence requiring re-operation13. In the previous two reports on NVS for ERM, the rate of postoperative ERM recurrence was demonstrated to be 33%5 and 7.5%6. The comparatively high percentage reported by Sawa et al.5 could be attributed to the surgical procedure, in which 20- or 23-gauge instruments were used, and the residual membrane in the vitreous might be the cellular source of postoperative proliferation. In their series, a second surgical intervention was performed in 30% of cases. It is notable that neither study employed ILM peeling during the operation.
It was disclosed that the neighboring remnant membrane and hyperreflective dots on the retinal surface and postoperative inner retinal wrinkling persisting for over 1 month were predisposing OCT findings for ERM recurrence13. In the current study, both the DRIL severity and CRT were observed dynamically over the 6-month follow-up period. The retina appeared flat with no sign of recurrence on OCT at postoperative 6 months, a time point exceeding the typical high-risk period for reproliferation. Moreover, ILM peeling, which was reported as a helpful procedure14,15, might contribute to the low rate of recurrence. Reichel et al.16 identified postoperative persistence of foveal ERM with varying severity degrees in 84.4% of eyes, for which insufficient peeling seemed to be the major predisposing factor. Similarly, de Novelli et al.17 discovered that patients who did not have the ILM removed may have a higher recurrence rate. In this study, the extensive ERM and ILM peeling decreased the risk for disease recurrence.
Regarding the surgical timing for ERM, prolonged non-surgical monitoring is often recommended for patients with BCVA better than 20/50. The risks of PPV are also assessed to avoid unnecessary complications18. The outcomes in our series demonstrated that both PPV and NVS could improve the anatomic appearance and vision regardless of the ERM stage, suggesting that symptomatic patients at early ERM stage deserve early surgical interventions.
Practically, surgeons need to weigh the costs, risks, and benefits of a surgical intervention. This study disclosed that the postoperative retinal restoration process after NVS was parallel to that after PPV. As long as the traction force was relieved, the preserved vitreous would not interfere in the retinal rehabilitation. On OCT images, the reconstruction of the disorganized retinal inner layers was independent of vitreous removal. In addition, vitreous removal in PPV would not accelerate or improve the rehabilitation with greater benefits compared with NVS. Moreover, there was no remarkable complication or ERM recurrence after NVS. The value of NVS was highlighted with its advantages of cost saving, prevention of after-cataract, simplified surgical procedure, intraocular metabolism protection, and patient rehabilitation comfort, which might encourage the early treatment for iERM.
As a retrospective study, there were limitations in patient decision bias, surgeon preference, and non-randomized assignment in our series. The pros and cons of either surgery were fully explained to all patients. Surgeons were experienced and competent to perform the sophisticated procedure. The large sample size and well-matched baseline data in both groups ensured the high reliability of the results. Although the follow-up period was relatively short, which was due to the incomplete OCT data and loss to follow-up in some patients beyond 6 months, the promising 6-month surgical outcomes support the need for prospective investigations with longer-term follow-up.
Data availability
Data are available from the corresponding author on reasonable request.
References
Margherio, R. R. et al. Removal of epimacular membranes. Ophthalmology 92, 1075–1083 (1985).
de Bustros, S. et al. Vitrectomy for idiopathic epiretinal membranes causing macular pucker. Br. J. Ophthalmol. 72, 692–695 (1988).
Reibaldi, M. et al. Iatrogenic retinal breaks in 25-gauge vitrectomy under air compared with the standard 25-gauge system for macular diseases. Retina 34(8), 1617–1622 (2014).
Almony, A. et al. Small-gauge vitrectomy does not protect against nuclear sclerotic cataract. Retina 32(3), 499–505 (2012).
Sawa, M. et al. Nonvitrectomizing vitreous surgery for epiretinal membrane long-term follow-up. Ophthalmology 112(8), 1402–1408 (2005).
Reibaldi, M. et al. Transconjunctival nonvitrectomizing vitreous surgery versus 25-gauge vitrectomy in patients with epiretinal membrane: A prospective randomized study. Retina 35(5), 873–879 (2015).
Zur, D. et al. Disorganization of retinal inner layers as a biomarker for idiopathic epiretinal membrane after macular surgery-The DREAM Study. Am. J. Ophthalmol. 196, 129–135 (2018).
Kunavisarut, P. et al. Idiopathic epiretinal membranes: Visual outcomes and prognostic factors. Turk. J. Ophthalmol. 52(2), 109–118 (2022).
Karasavvidou, E.-M., Panos, G. D. & Koronis, S. Optical coherence tomography biomarkers for visual acuity in patients with idiopathic epiretinal membrane. Eur. J. Ophthalmol. 31(6), 3203–3213 (2021).
Sun, J. K. et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA. Ophthalmol. 132, 1309–1316 (2014).
Sun, J. K. et al. Neural retinal disorganization as a robust marker of visual acuity in current and resolved diabetic macular edema. Diabetes 64, 2560–2570 (2015).
Radwan, S. H. et al. Association of disorganization of retinal inner layers with vision after resolution of center-involved diabetic macular edema. JAMA Ophthalmol. 133, 820–825 (2015).
Ahn, S. J., Woo, S. J. & Park, K. H. Recurrence of idiopathic epiretinal membrane and its predisposing factors: An optical coherence tomography study. Retina 41(3), 516–524 (2021).
Azuma, K. et al. Effects of internal limiting membrane peeling combined with removal of idiopathic epiretinal membrane: A systematic Review of literature and meta-analysis. Retina 37, 1813–1819 (2017).
Schechet, S. A., DeVience, E. & Thompson, J. T. The effect of internal limiting membrane peeling on idiopathic epiretinal membrane surgery, with a review of the literature. Retina 37, 873–880 (2017).
Reichel, F. F. et al. Persistence and recurrence after removal of idiopathic epiretinal membrane. Eye (Lond). 39(2), 314–319 (2025).
De Novelli, F. J. et al. Surgical removal of epiretinal membrane with and without removal of internal limiting membrane: Comparative study of visual acuity, features of optical coherence tomography, and recurrence rate. Retina 39(3), 601–607 (2019).
Chen, X. et al. Progression to surgery for patients with idiopathic epiretinal membranes and good vision. Ophthalmic. Surg. Lasers. Imaging. Retina. 49, S18–S22 (2018).
Acknowledgements
The authors would like to thank Ping Lin for her linguistic and editorial assistance.
Author information
Authors and Affiliations
Contributions
N.C. designed the study and revised the manuscript. N.C., S.X., and D.C. analyzed the data. S.X. and D.C. drafted the manuscript. N.C. and B.Y. performed the surgery. M.Z. and W.C. performed OCT analysis. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xue, S., Zhou, M., Chen, D. et al. Nonvitrectomizing vitreous surgery for idiopathic epiretinal membrane. Sci Rep 15, 26265 (2025). https://doi.org/10.1038/s41598-025-08410-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08410-7