Abstract
The pathogenesis of pulmonary fibrosis involves structural remodeling and functional impairment of lung tissue, accompanied by increased secretion of pro-inflammatory mediators and abnormal synthesis of the extracellular matrix (ECM). Thrombospondin-2 (THBS2), an ECM glycoprotein encoding gene, has been extensively studied in liver and heart fibrosis. However, its role in idiopathic pulmonary fibrosis (IPF) in humans remains incompletely understood. Lung fibroblasts were obtained from normal individuals and IPF patients, and THBS2 expression was detected. Then, THBS2 overexpression and knockdown cell models as well as exogenous human THBS2 active protein administration cell models were established to explore the role of THBS2 in cell aggressive phenotype, collagen synthesis and proinflammatory mediator secretion. Furthermore, TGF-β1 inhibitor was used to investigate the underlying mechanism of THBS2 affecting collagen synthesis. Finally, in the bleomycin (BLM) -induced pulmonary fibrosis model, the severity of pulmonary fibrosis in mice was evaluated by administering exogenous mouse THBS2 active protein. THBS2 expression was significantly up-regulated in lung tissues of IPF patients and in IPF lung fibroblasts. THBS2 Overexpression and exogenous human THBS2 active protein markedly enhanced the proliferation and migration of fibroblasts and increased the levels of COL1A1, COL1A2, COL3A1, LOX and LOXL2. These effects were attenuated after knockdown of THBS2 in IPF fibroblasts. Animal models also confirmed that exogenous mouse THBS2 protein could aggravate bleomycin-induced pathological changes and collagen deposition in lung tissues of mice. Using TGF-β1 inhibitor SB525334 reduced the protein expression of downstream molecules (TGFBR1, TGFBR2, P-Smad2/3) and collagen synthesis but did not inhibit the upregulation of post-translational modification enzymes LOX and LOXL2 involved in collagen synthesis. Meanwhile, we observed that THBS2 overexpression significantly promoted inflammatory secretome (IL-1β, IL-6 and IL-8). THBS2 is overexpressed in IPF. Functionally, THBS2 promotes the invasive phenotype (proliferation and migration), collagen synthesis and inflammation secretome in fibroblasts. Mechanistically, THBS2 promotes collagen synthesis through the TGF-β1/Smad2/3 signaling pathway.
Similar content being viewed by others
Introduction
Idiopathic pulmonary fibrosis (IPF) is a long-term, progressive condition affecting the interstitium of the lungs, marked by elevated mortality rates and a scarcity of effective treatment choices. The annual incidence of IPF is 2–30 cases per 100,000 people worldwide1, and the median survival of diagnosed patients is only 2–4 years2. At present, although the IPF patients with drug therapy can improve lung function, but not significantly increase the survival rate3,4. Most patients eventually die of respiratory failure.
In the process of IPF, the increase of myofibroblasts and the deposition of ECM are considered to be the key factors in its development. Collagen is a major component of the ECM and maintains the structural integrity of the lung5,6. Collagen synthesis begins with the transcription of DNA, and the associated collagen genes (like COL1A1 and COL3A1) are transcribed into messenger RNA (mRNA) in the nucleus, subsequently translated into collagen precursors (procollagen) in the rough endoplasmic reticulum7. Collagen precursors undergo post-translational modifications like hydroxylation, glycosylation, and cross-linking8,9. Lysine oxidase (LOX) catalyzes the conversion of amino groups of lysine and hydroxylysine to aldehyde groups, forming cross-links between collagen molecules and improving the mechanical properties and durability of collagen9. Collagen synthesis also involves multiple signaling pathways and cytokines. TGF-β1 has been identified as a key promoter in pulmonary fibrosis, activating the Smad signaling pathway through its receptor to induce fibroblast proliferation and collagen synthesis10,11. Moreover, the release of proinflammatory mediators such as TNF-α and IL-1β can trigger fibroblast activation and enhance collagen production. This phenomenon may also be associated with the senescence of fibroblasts12. Overall, despite extensive research on fibrosis, our understanding remains incomplete and managing IPF prognosis continues to be challenging. This emphasizes the importance of gaining a more thorough understanding of its underlying mechanisms.
Thrombospondin-2 is the second member of the thrombospondin family and is encoded by the THBS2 gene. It is a disulfide-linked trimeric glycoprotein that is released by a variety of types of cells, including mesenchymal fibroblasts, endothelial cells and immune cells13,14. Previous studies have found THBS2 can induce damage and promote inflammation and fibrosis in multiple organs. For instance, THBS2 exacerbates LPS-induced inflammation and apoptosis in mouse renal tubular epithelial cells15. THBS2 has been demonstrated to activate hepatic stellate cells and facilitate liver fibrosis while its levels can serve as indicators for assessing the severity of liver fibrosis and disease activity16,17. In addition, THBS2 deficiency has been shown to reduce cardiac fibrosis18,19. However, there are currently no supporting data on the therapeutic role of THBS2 in IPF or pulmonary fibrosis.
In this study, we mainly elucidated the role of THBS2 in IPF fibroblasts and found THBS2 promoted lung fibroblasts aggressive phenotype and collagen synthesis. THBS2 can also regulate the pro-inflammatory secretory state of fibroblasts to promote aging in adjacent normal fibroblasts. This study provides ideas for exploring the pathogenic mechanism of pulmonary fibrosis, and provides a solid theoretical basis for the subsequent development of therapeutic regimens targeting THBS2 gene and its downstream factors.
Materials and methods
RNA-seq analysis
Publicly available data from the Gene Expression Omnibus Database (GEO) were used. Three IPF-derived fibroblasts and three normal lung fibroblasts were selected from the reference number GSE119007 data set. Normal lung tissues (n = 11) and IPF tissues (n = 22) were selected from the reference number GSE110147 data set. Data were imported in R 4.4.1 and analyzed using the DESeq2 v1.44.0 R package. The false discovery rate (FDR) was determined using the approach established by Benjamini and Hochberg. The correlations were calculated by Spearman’s correlation analysis. Data visualization was achieved by using ggplot2.
Primary pulmonary fibroblasts
All subjects provided written informed consent prior to study onset, and the ethics committee of Zhongnan Hospital, Wuhan University (Wuhan, China), approved the study (approval No.2024022K). The study was performed in accordance with the Declaration of Helsinki. All subjects provided written informed consent prior to study onset. Lung fibroblasts were obtained from nine patients diagnosed with IPF (IPF-FBS), all of whom had not received steroid or immunosuppressive treatment at the time of biopsy. As a control, primary normal lung fibroblasts (N-FBs) were derived from patients undergoing thoracic surgery for non-fibrotic lung conditions.
Primary human lung fibroblasts were isolated as described previously20. Lung tissue was finely cut into small pieces (approximately 1 mm3) and subjected to enzymatic digestion in Hank’s balanced salt solution containing collagenase I, papain, protease, and calcium chloride for 1 h at 37 °C. Following enzymatic dissociation, a glass pipettor was used for mechanical disruption, and the resulting cell suspension was centrifuged at 800 × g for 5 min to collect the cell pellet. The pellet was then resuspended in fibroblast culture medium (MEM Alpha with 10% fetal bovine serum (FBS) and antibiotics). The cell suspension was seeded onto culture plates, allowing fibroblasts to preferentially adhere. Adherent fibroblasts were cultured in a 37 °C incubator with 5% CO₂. For experimentation, cells between passages 4 and 7 were used.
Cell line culture
We used MRC5 cells for in vitro experiments21,22. Fibroblasts cell line MRC5 cells were obtained from American Type Culture Collection. All cells were grown in MEM Alpha enriched with 10% FBS and antibiotics, maintained in a humidified environment at 37 °C with 5% CO2.
Plasmids and transfection
Fibroblasts were transfected with a fusion efficiency of 70–80% using THBS2 plasmid vector (2 μg) or the corresponding empty vector (2 μg) from Sino Biological (Beijing, China). Transient transfection was performed using Lipofectamine 3000 (Invitrogen, USA), followed by incubation in MEM Alpha supplemented with 10% FBS for 6–8 h. Following a 48-h period, the cells were rinsed twice with PBS and then collected in cell lysate. The efficiency of transfection was evaluated using RT-qPCR and Western blot techniques.
Small interfering RNA
The siRNAs (On-Target and Non-specific Control) were purchased from RiboBio (Guangzhou, Guangdong, China). Cells were transfected with THBS2 targeted siRNA (THBS2 siRNA) or Non-specific control siRNA (siNC) using Lipofectamine 2000 reagent (11668-019, Invitrogen, USA). The mRNA knockdown efficiency was confirmed by RT-qPCR and western blot.
Recombinant THBS2 protein was administered in vitro and in vivo
Cells were starved for 24 h after reaching 70% confluence, and then treated with Recombinant Human Thrombospondin-2 Protein (rhTHBS2, 50 ng/ml, 100 ng/ml and 200 ng/ml) (R&D Systems, 1635-T2-050, USA) in serum-free medium for 48 h. Recombinant mouse Thrombospondin-2 Protein (rmTHBS2, 25 μg/kg and 50 μg/kg) was specially synthesized and supplied from Sino Biological (Beijing, China). Mice in each group were intratracheal injected with saline or bleomycin. After the establishment of bleomycin fibrosis model, THBS2 protein was instilled by nasal drip every 3 days, and the animals were sacrificed after 21 days.
Real time quantitative PCR (RT-qPCR)
Using the TRIzol reagent (15596018, Invitrogen, USA), total RNA was isolated from cells. The RNA concentration was measured using a NanoDrop One spectrophotometer (Thermo Fisher Scientific), and subsequently, reverse transcription was performed to synthesize cDNA with the FastKing cDNA RT reagent kit (KR116-02, TIANGEN, Beijing, China). Then, RT-qPCR was performed using the UltraSYBR Mixture (CW0956M, Jiangsu, China).
THBS2-F, 5′- GACACGCTGGATCTCACCTAC-3′
THBS2-R, 5′- GAAGCTGTCTATGAGGTCGCA-3′;
COL1A1-F, 5′- TGAAGGGACACAGAGGTTTCAG-3′
COL1A1-R, 5′- GTAGCACCATCATTTCCACGA-3′;
COL1A2-F, 5′- GGCCCTCAAGGTTTCCAAGG-3′
COL1A2-R, 5′- CACCCTGTGGTCCAACAACTC-3′;
COL3A1-F, 5′- TGGTCTGCAAGGAATGCCTGGA-3′
COL3A1-R, 5′- TCTTTCCCTGGGACACCATCAG-3′;
P16-F, 5′- CTCGTGCTGATGCTACTGAGGA-3′
P16-R, 5′- GGTCGGCGCAGTTGGGCTCC-3′;
P21-F, 5′- TGTCCGTCAGAACCCATGC-3′
P21-R, 5′- AAAGTCGAAGTTCCATCGCTC-3′;
P53-F, 5′- CCTCAGCATCTTATCCGAGTGG-3′
P53-R, 5′- TGGATGGTGGTACAGTCAGAGC-3′;
Actin-F, 5′- TGCTATCCAGGCTGTGCTAT-3′
Actin-R, 5′- AGTCCATCACGATGCCAGT-3′;
β-actin served as the housekeeping gene for calculating relative gene expression using the 2−ΔΔCT method.
Western blot assays
Total proteins of cells were lysed in ice-cold RIPA buffer with PMSF, a protease inhibitor. The samples were then separated by SDS-PAGE gel electrophoresis and transferred to PVDF membranes. The membranes were blocked with 5% milk for one hour and subsequently incubated with primary antibodies at 4 °C overnight: β-actin (AC026, 1:50,000, ABclonal), THBS2 (A8561, 1:1000, ABclonal), Collagen 1A1 (A16891, 1:1000, ABclonal), Collagen 1A2 (A5786, 1:1000, ABclonal), Collagen 3A1 (A0817, 1:1000, ABclonal), LOX (A11504, 1:1000, ABclonal), LOXL2 (A14638, 1:500, ABclonal), P21 (381102, 1:500, ZENBIO), P-P53 (AP0083, 1:400, ABclonal), P53 (A0263, 1:1000, ABclonal), TGF-β1 (A2124, 1:2000, ABclonal), TGFBR1 (A16983, 1:1000, ABclonal), TGFBR2 (A1415, 1:1000, ABclonal), P-Smad2/3 (AP1343, 1:2000, ABclonal), Smad2/3 (A18674, 1:1000, ABclonal). Following a wash with TBST, the samples were incubated for one hour at room temperature with horseradish peroxidase-conjugated secondary antibodies. Chemiluminescence detection was employed to visualize protein bands, and quantification was carried out using ImageJ. All Western blot experiments were conducted a minimum of three times.
Cell migration assay
Cell migration assays were conducted using 24-well Transwell chambers (8.0 μm; Corning, USA). Following cell digestion, the culture medium was removed via centrifugation, and the cells were rinsed 1–2 times with PBS. The cell density was then adjusted to 1 × 10^4. A volume of 200 µl of the cell suspension was introduced into the Transwell chamber, while 600 µl of medium supplemented with 15% FBS was added to the lower chamber of the 24-well plate. After washing the cells twice with sterile PBS, the non-migrated cells on the upper surface were carefully removed using a cotton swab. The chambers were fixed with 4% formaldehyde (Solarbio, China) for 30 min and allowed to air dry. Subsequently, the cells were stained with 0.1% crystal violet for 30 to 60 min and washed three times with PBS. Any excess moisture in the upper chamber was gently wiped away with a cotton swab. The migrated cells to the bottom surface were captured using an inverted microscope.
EdU cell proliferation
Cell proliferation was measured using an EdU kit (RiboBio, Guangzhou, Guangdong, China) according to the manufacturer’s instructions. Fibroblasts and MRC5 cells were seeded and cultured in 96-well plates. When the cells reached confluence, appropriate amount of EdU reagent was added to the cells, and the cells were incubated for 2 h. After incubation, the cells were fixed and permeabilized. The pre-prepared Apollo staining solution was added for staining and microphotography was performed under a fluorescence microscope.
Senescence-associated β-galactosidase (SA-β-Gal) staining
SA-β-gal activity staining was conducted according to the manufacturer’s instructions (C0602, Beyotime, Shanghai, China). In brief, cells were washed with PBS and then fixed using the fixative solution provided in the kit. They were subsequently stained with a 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (X-gal) solution for 48 h at 37 °C. Imaging was carried out using bright-field microscopy, where senescent cells appeared blue.
Histological analysis and Immunofluorescence staining
The left lung of the mouse was excised and fixed in 4% formaldehyde. After dehydration with ethanol, they were embedded in paraffin and sectioned at 4 μm. These sections were stained with hematoxylin and eosin (H&E) or Masson’s trichrome. Ashcroft scoring was used to evaluate the degree of fibrosis. Digital images of the slides were obtained by light microscopy (Nikon, Japan). The whole lung area and fibrosis area were manually delineated. The percentage of fibrosis was calculated by the formula: fibrosis (%) = (fibrosis area/total lung area) × 100%. For immunohistochemistry, the following primary antibodies were used: Collagen I (Abcam, ab270993, 1:500) and Collagen III (Proteintech, 22734-1-AP, 1:1000). The slides were sealed with a neutral adhesive and examined under a light microscope (Nikon, Japan).
ABplex human cytokine 12-Plex assay kit
Prepare the standard and reagent, add 50μL of each gradient standard or test sample per well, add 5μL of microsphere suspension per well, and incubate at 37 °C for 1 h. Then magnetic wash once, add 50μL antibody detection solution, incubate at 37 °C for 0.5 h, wash once. 50μL fluorescein solution was added and incubated at 37 °C for 15 min without light. Add 70μL wash buffer and test using ABplex-100.
Statistical analysis
All data were analyzed using SPSS 22.0 or GraphPad Prism 8.0, and results are presented as mean ± SEM. One-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test was used for comparisons between multiple groups, while Student’s t-test was employed for comparisons between two groups. A p-value of less than 0.05 was considered statistically significant.
Results
THBS2 mRNA and protein expression are increased in IPF
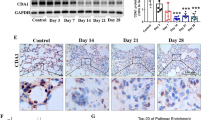
First, we queried the publicly available data from the Gene Expression Omnibus (GEO) Database (GSE119007) for genes differentially expressed at least twofold over control fibroblasts (Fig. 1A and Table S1). THBS2 was significantly up-regulated in the IPF-derived lung fibroblasts [false discovery rate (FDR) = 2.86 × 10−3] (Fig. 1B). Furthermore, THBS2 mRNA was positively correlated with collagen associated marker mRNA like COL1A1 and COL1A2 in lung fibroblasts (Fig. 1C). In contrast to these findings, other THBS family members were not significantly expressed in IPF fibroblasts and not associated with collagen gene expression (Fig. 1C). We then re-examined the publicly available data in the GEO database (GSE110147) and similarly found that THBS2 expression was significantly upregulated in lung tissue from IPF patients compared to normal lung tissue (FDR = 6.69 × 10⁻⁸) (Fig. 1D).
THBS2 mRNA and protein expression are increased in IPF. (A) Volcano map of DEGs from differential analysis of the GSE119007 dataset: Volcano map of differentially expressed genes (DEGs) between idiopathic pulmonary fibrosis (IPF) and the control groups (CTRL); red dots represent up-regulated DEGs. The gray dots represent genes not differentiated. The blue dots represent downregulated DEGs. (B) Log-transformed normalized gene expression values in IPF-derived fibroblasts (n = 3) and normal lung fibroblasts (n = 3), with differential expression FDR-adjusted P value. (C) Partial Spearman correlation of THBS family (THBS1, THBS3, THBS4 and COMP) and THBS2 with fibrosis-associated genes. Correlation P values were adjusted for FDR for each cytokine separately. Colors (from blue to red) indicate Spearman’s correlation coefficient, from negative to positive values. Dot sizes are proportional to − log10 (adjusted P value): the larger the dot, the lower the FDR value. X denotes nonsignificant values (FDR > 0.05). (D) log-transformed normalized THBS2 gene expression and FDR-adjusted p-values for differential expression in normal lung tissues (n = 11) and IPF tissues (n = 22) obtained from the GSE110147 dataset. (E) Western blot analysis of THBS2 protein in human IPF lung tissues (n = 9) and healthy control lung tissues (n = 7). (F) Quantitative statistical mapping of protein expression levels. n = 7 for Control group and n = 9 for IPF group. Data are shown as the mean ± SEM. ****P < 0.0001. (G) THBS2 mRNA levels were significantly increased in IPF and healthy control lung fibroblasts. n = 9 for each group. Data are shown as the mean ± SEM. ****P < 0.0001. (H) Western blot analysis of THBS2 protein and COL1A1, COL1A2, and COL3A1 proteins in human IPF-derived and healthy control lung fibroblasts. (I) Quantitative statistical mapping of protein expression levels. n = 9 per group. Data are shown as the mean ± SEM. ***P < 0.001, ****P < 0.0001.
To further verify the results of the dataset, we collected lung tissues from 7 healthy individuals and 9 patients with IPF (Table S2). Compared with the control lung tissues, the level of THBS2 protein in the lung tissues of IPF patients was significantly increased (Fig. 1E–F). We then examined THBS2 expression in primary human lung fibroblast isolated from fresh lung tissues of nine IPF patients (IPF-FBs) and nine normal lung tissues (N-FBs) (Table S2). THBS2 mRNA and protein, collagen associated marker protein levels were visibly increased in primary IPF fibroblast compared with those in primary normal fibroblast (Fig. 1G–I). These data show considerable elevation of THBS2 in IPF.
THBS2 promotes an aggressive phenotype in human lung fibroblasts
In view of the higher expression of THBS2 in IPF fibroblasts, we investigated the effects of THBS2 overexpressed on the phenotype of fibroblasts, specifically their proliferative capacity and migratory capacity. We transfected N-FBs with a plasmid containing a cDNA encoding human THBS2 and detected a significant increase in THBS2 protein expression 48 h post-transfection (Fig. 2A). We then measured cell proliferation using EdU assays in THBS2 overexpressed N-FBs and Vector controls. As shown in Fig. 2C, THBS2 overexpression significantly increased fibroblasts proliferation. In addition, THBS2 overexpression also caused more fibroblasts to enter the trans-pore compartment (Fig. 2B), indicating that THBS2 promotes the migratory ability of normal lung fibroblasts. We reached the same conclusion after overexpressing THBS2 in the normal lung fibroblast cell line MRC5 (Fig. S1A-C). Our data support the conclusion that THBS2 can promote the proliferative and migratory capacity of normal lung fibroblasts.
THBS2 promotes an aggressive phenotype in human lung fibroblasts. (A) N-FBs were transfected with THBS2 plasmid and the expression level of THBS2 protein was detected by Western blot. n = 3 for each group. Data are shown as the mean ± SEM. **P < 0.01. (B) Transwell chamber was used to detect the effect of THBS2 transfection on the migration ability of N-FBs cells. Scale bar, 200μm. n = 3 for each group. Data are shown as the mean ± SEM. ***P < 0.001. (C) EdU assay was used to detect the proliferation ability of N-FBs stimulated by THBS2 plasmid and negative control vector. Scale bar, 100 μm. n = 3 for each group. Data are shown as the mean ± SEM. **P < 0.01. (D) IPF-FBs with THBS2 siRNA (si-THBS2#2) was transfected and the expression level of THBS2 protein was detected by Western blot. n = 6 for each group. Data are shown as the mean ± SEM. **P < 0.01. (E) Forty-eight hours after transfection of THBS2 siRNA (si-THBS2#2) and NC siRNA (si-NC), equal numbers of fibroblasts were loaded into transwell chambers. Images of migrating fibroblasts from the crystal violet staining assay are shown. Scale bar, 200 μm. n = 6 for each group. Data are shown as the mean ± SEM. ****P < 0.0001. (F) Proliferation of THBS2-deficient cells. After transfection of THBS2 siRNA (si-THBS2#2) or NC siRNA (si-NC) for 48 h, EdU method was used to detect the proliferation of IPF-FBs cells. Scale bar, 100 μm. n = 6 for each group. Data are shown as the mean ± SEM. ****P < 0.0001.
To test whether knocking down expression of THBS2 can attenuates the migration and proliferation ability of IPF fibroblasts, we employed siRNA to knock down THBS2 in IPF-FBs. As the results show, three THBS2 siRNA (si-THBS2#1, si-THBS2#2 and si-THBS2#3, 100 nM) could down-regulate the expression of THBS2 mRNA and protein at 48 h after transfection. However, compared with si-THBS2#1 and si-THBS2#3, the knockdown efficiency of si-THBS2#2 was the highest (Figs. 2D and S1D–F). Therefore, we selected si-THBS2#2 for the follow-up experiment. we observed the si-THBS2 group exhibited markedly decreased cell proliferation and migration compared with the control group among fibroblasts derived from IPF patients (Fig. 2E–F).
THBS2 promotes collagen synthesis accompanied by abnormal collagen cross-linking
Considering the positive correlation between THBS2 and collagen markers, we subsequently sought to determine whether THBS2 regulates collagen expression in fibroblasts. Collagen type I and collagen type III are the most abundant collagen types during the development of IPF23. THBS2 overexpression significantly increased COL1A1, COL1A2 and COL3A1 mRNA and protein levels (Fig. 3A–C). Under normal conditions, moderate collagen cross-linking is essential to maintain the function of lung tissue24. Their hyperactivity may lead to excessive cross-linking of collagen and aggravation of fibrosis25. Our findings demonstrated that the protein levels of LOX and LOXL2 were markedly elevated following the overexpression of THBS2 (Fig. 3B–C). These results suggest THBS2 may be involved in the regulation of abnormal cross-linking of collagen. In addition, THBS2 overexpression in the normal fibroblast cell line MRC5 also significantly increased the protein levels of collagen-related markers and LOX, LOXL2 (Fig. S2A), which further verified the above conclusion.
THBS2 promotes collagen synthesis accompanied by abnormal collagen cross-linking. (A) The expression levels of COL1A1, COL1A2, and COL3A1mRNA in N-FBs transfected with THBS2 plasmid and control empty vector were detected by RT-qPCR. n = 3 for each group. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01. (B) The protein expression levels of collagen markers COL1A1, COL1A2, COL3A1, LOX and LOXL2 in THBS2 transfected N-FBs were detected by Western blot. n = 3 for each group. (C) The protein expression levels of each collagen marker were quantitatively analyzed. n = 3 for each group. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01. (D) RT-qPCR was used to detect the mRNA expression levels of COL1A1, COL1A2, and COL3A1in IPF-FBs transfected with si-THBS2#2 (si-THBS2#2) or si-NC (si-NC). n = 6 for each group. Data are shown as the mean ± SEM. ***P < 0.001, ****P < 0.0001. (E) Western blot was used to detect the protein expression levels of collagen markers COL1A1, COL1A2, COL3A1, LOX and LOXL2 in IPF-FBs transfected with si-THBS2#2 (si-THBS2#2). n = 6 for each group. (F) Quantitative analysis of protein expression levels of each collagen marker. n = 6 for each group. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To test if the effects are true, we used siRNA (si-THBS2#2) interference to knock down THBS2 in IPF-FBs. THBS2-deficent IPF-FBs showed decreased COL1A1, COL1A2 and COL3A1 mRNA and protein levels, with concomitant decreases in LOX and LOXL2 (Fig. 3D–F). Furthermore, in order to eliminate off-target interference, we examined whether si-THBS2#1 and si-THBS2#3 could affect the expression of collagen markers. The results showed that compared with siNC, the protein levels of COL1A1 and COL3A1 in the si-THBS2#1 and si-THBS2#3 groups did not decrease. There was no statistically significant difference (Fig. S1G).
In vitro rhTHBS2 administration directly promoted the collagen synthesis and invasive phenotype of fibroblasts
To investigate whether increased THBS2 expression in normal lung fibroblasts directly alters their fibrotic response, primary normal lung fibroblasts (N-FBs) were isolated and treated with varying concentrations of recombinant human THBS2 protein to assess its effect on collagen synthesis. The results showed that mRNA expression levels of COL1A1 and COL3A1 increased with escalating THBS2 concentrations (50 ng/ml, 100 ng/ml and 200 ng/ml), with the most significant increase observed at 200 ng/ml (Fig. 4A). Protein levels of COL1A1, COL1A2, COL3A1, LOX and LOXL2 were also significantly higher in the 200 ng/ml group compared to the 0 ng/ml group, with statistically significant differences (Fig. 4B–C). Cells were then treated with 200 ng/ml rhTHBS2 at different time points (0H, 12H, 24H, 48H) to assess fibrosis marker expression, with the most pronounced increase at 48 h (Fig. 4D–E). Based on these results, 200 ng/ml rhTHBS2 for 48 h was selected to further examine its effect on fibroblast invasiveness. The Transwell assay demonstrated a significant increase in the number of cells migrating to the lower chamber in the 200 ng/ml group (Fig. 4F). EdU proliferation assays showed that rhTHBS2 treatment significantly promoted cell proliferation, as evidenced by a marked increase in the proportion of proliferating (EdU-positive) cells relative to the total number of cells in the visible field (Fig. 4G).
In vitro recombinant human THBS2 protein promotes the collagen synthesis and invasive phenotype. (A) The mRNA expression levels of COL1A1 and COL3A1 in N-FBs stimulated with different concentrations of rhTHBS2 were detected by RT-qPCR. n = 6 for each group. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, nsP > 0.05. (B) Western blot analysis of COL1A1, COL1A2, COL3A1, LOX and LOXL2 protein expression in N-FBs stimulated with different concentrations of rhTHBS2 for 48H, with the most significant increase at 200 ng/ml. n = 6 for each group. (C) Quantitative analysis of protein levels of COL1A1, COL1A2, COL3A1, LOX and LOXL2. n = 6 for each group. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, nsP > 0.05. (D) Western blot analysis of COL1A1, COL1A2, COL3A1, LOX and LOXL2 protein expression at different time points after N-FBs stimulation with 200 ng/ml rhTHBS2, with the most significant increase at 48H. n = 6 for each group. (E) Quantitative analysis of protein levels of COL1A1, COL1A2, COL3A1, LOX and LOXL2. n = 6 for each group. Data are shown as the mean ± SEM. **P < 0.01, ****P < 0.0001, nsP > 0.05. (F) After stimulation of N-FBs with 200 ng/ml rhTHBS2 for 48 h, equal amounts of fibroblasts were loaded into transwell chambers. Images of migrating fibroblasts from the crystal violet staining assay are shown. Scale bar, 200 μm. n = 6 for each group. Data are shown as the mean ± SEM. **P < 0.01. (G) After stimulation of N-FBs with 200 ng/ml rhTHBS2 for 48 h, the proliferation of N-FBS was detected by EdU assay. Scale bar, 100 μm. n = 6 for each group. Data are shown as the mean ± SEM. ****P < 0.0001.
THBS2 promotes collagen synthesis via TGF-β1 signaling pathway
TGF-β1 signaling is one of the most important pathways in the process of pulmonary fibrosis26. Next, we investigated whether THBS2 promoted collagen synthesis via TGF-β1 signaling. Western blot analysis was performed on THBS2-overexpressing N-FBs (Fig. 5A) and MRC5 cell lines (Fig. S2B), revealed a significant increase in TGF-β1 levels after THBS2 treatment. In addition, the expression of TGFBR1 and TGFBR2 was up-regulated, accompanied by a significant increase in phosphorylated Smad2/3 (P-Smad2/3), a downstream signal molecule of TGF-β1, but the total protein level of Smad2/3 was unaffected. However, IPF-FBS treated with si-THBS2#2 showed reduced levels of TGF-β1 and downstream signaling molecules (Fig. 5B). For further testing, N-FBs and MRC5 overexpressing THBS2 were treated with SB525334, a known TGF-β1 inhibitor27. SB525334 down-regulated the expression levels of related molecules downstream of TGF-β1 (Figs. 5C and S2C). The TGF-β1 inhibitor also inhibited the expressions of COL1A1, COL1A2 and COL3A1 in overexpressed THBS2 N-FBs and MRC5 (Figs. 5D–F and S2D). Interestingly, SB525334 did not inhibit the protein expression of LOX and LOXL2 in N-FBs and MRC5 overexpressing THBS2 (Figs. 5E–F and S2D), suggesting that THBS2 may affect the collagen cross-linking abnormality by directly or indirectly affecting the expression of LOX/LOXL2, but not entirely directly dependent on TGF-β1. Taken together, these findings suggest THBS2 enhances collagen synthesis and promotes ECM synthesis and secretion by mediating TGF-β1/Smad2/3 signaling. However, SB525334 reversed the pro-fibrotic effect of THBS2 overexpression.
THBS2 promotes collagen synthesis via TGF-β1 signaling pathway. (A) N-FBs was transfected with THBS2 or control plasmid for 48 h, Western blot was used to detect the expression levels of TGF-β1, TGFBR1, TGFBR2, P-Smad2/3 and Smad2/3. n = 3 for each group. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, nsP > 0.05. (B) IPF-FBs were transfected with si-THBS2#2 or si-NC, and the expression levels of TGF-β1 pathway-related molecules were detected by Western blot. n = 6 for each group. Data are shown as the mean ± SEM. ***P < 0.001, ****P < 0.0001, nsP > 0.05. (C) THBS2-oeverexpressed fibroblasts were treated with TGF-β receptor inhibitor (SB525334, Selleck, USA) in the presence of 10 μM, and the expression levels of TGF-β1 pathway-related molecules were detected by Western blot. n = 3 for each group. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, nsP > 0.05. (D) THBS2-oeverexpressed fibroblasts were treated with SB525334 and DMSO, respectively, and the mRNA expression levels of COL1A1, COL1A2, and COL3A1 were detected by RT-qPCR. n = 3 for each group. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01. (E) THBS2 over-expressed transfected N-FBs were treated with SB525334, and the protein expression levels of collagen markers COL1A1, COL1A2, COL3A1, LOX and LOXL2 were detected by Western blot. n = 3 for each group. (F) Quantitative statistical analysis of protein expression of TGF-β1 pathway-related molecules. n = 3 for each group. Data are shown as the mean ± SEM. *P < 0.05, nsP > 0.05.
THBS2 regulates fibroblasts a pro-inflammation secretory state
In addition to producing a substantial amount of collagen-rich extracellular matrix, fibroblasts derived from IPF also release a variety of pro-inflammatory and pro-fibrotic factors that encourage adjacent normal fibroblasts to undergo senescence and fibrosis28. Specifically, when these factors are released by fibroblasts, they affect adjacent normal fibroblasts and cause normal fibroblasts to enter a senescent state, which is usually accompanied by decreased cell proliferation and sustained secretion of inflammatory factors (cellular senescence-associated secretory phenotype, SASP)29,30. To test whether THBS2 regulates the secretion of fibrosis/inflammatory mediators in fibroblasts, supernatants from Vector and THBS2 overexpressed N-FBs were collected and analyzed using a human cytokine 12-Plex assay kit. The expression of several cytokines, including IL-1β, IL-6 and IL-8, was increased in the supernatant of THBS2-overexpressing N-FBs (Fig. 6A). The mRNA levels of these cytokines were also increased in THBS2-overexpressing N-FBs and MRC5 cells (Figs. 6B and S3A), suggesting that THBS2 promotes a secretory phenotype in fibroblasts. Previous study suggested these cytokines can stimulate cells to undergo senescence, thus we evaluated the combined effects of these secreted products on naïve cells by utilizing conditioned media (CM) (Figs. 6C and S3B). THBS2 overexpression promoted the ratio of SA-β-gal positive cell numbers (Figs. 6D and S3C) and the expression of senescence marker (p16, p21 and p53) in naïve fibroblasts exposed to conditioned media (Figs. 6E–G and S3D–F). These data suggested THBS2 can promote the pro-inflammatory secretory state of fibroblasts, thereby promoting the aging of adjacent normal fibroblasts.
THBS2 regulates fibroblasts a pro-inflammation secretory state. (A) The supernatant (CM) of N-FBs cells overexpressing THBS2 was collected, and the expression level of SASP was detected. n = 3 for each group. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, nsP > 0.05. (B) RT-qPCR analysis of SASP mRNA levels in N-FBs cells overexpressing THBS2. n = 3 for each group. Data are shown as the mean ± SEM. *P < 0.05, ***P < 0.001. (C) Schematic representation of fibroblasts transfected before CM collection and transfer. Forty-eight hours after transfection, CM of empty vector or THBS2 transfected cells were collected and transferred into recipient normal healthy donor fibroblasts for another 48 h. n = 3 for each group. (D) Representative images of SA-β-gal staining and quantitative positive percentages in CM with N-FBs overexpressing THBS2. Scale bar, 100 μm. n = 3 for each group. Data are shown as the mean ± SEM. ****P < 0.0001. (E) RT-qPCR analysis of P16, P21 and P53 mRNA levels in CM with N-FBs overexpressing THBS2. n = 3 for each group. Data are shown as the mean ± SEM. **P < 0.01, ***P < 0.001. (F) Analysis of P16, P21 and P53 protein levels by Western blotting. n = 3 for each group. (G) Quantitative analysis of protein levels of P16, P21 and P53. n = 3 for each group. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01.
THBS2 can aggravate the pathological changes of lung tissue in mice induced by bleomycin
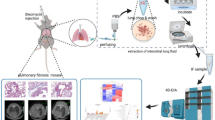
To further verify the role of THBS2 in the progression of pulmonary fibrosis in vivo. Pulmonary fibrosis was induced in mice by bleomycin exposure, followed by intranasal delivery of recombinant THBS2 protein (25 or 50 μg/kg) every 3 days, and lung tissue harvesting after 21 days (Fig. 7A). Body weight, which was assessed every 3 days, was significantly reduced in the bleomycin injury group compared with the Saline group and further worsened in the THBS2 plus bleomycin group of mice (Fig. 7B). In parallel, we found a dose-dependent acceleration in mortality in the THBS2-exposed group (Fig. 7C). We next examined changes in collagen levels, and hydroxyproline quantification showed that hydroxyproline levels were also significantly enhanced after THBS2 administration, suggesting increased collagen deposition in lung tissue of mice (Fig. 7D). Western immunoblotting showed that rmTHBS2 promoted the expression of fibrosis markers COL1A1, COL1A2 and COL3A1 in a dose-dependent manner (Fig. 7E-F). Further mouse lung histological staining showed enhanced inflammatory cells and collagen deposition in the lungs of THBS2-treated mice after bleomycin injury relative to control mice (Fig. 7G), and immunohistochemistry also suggested that COLI and COLIII deposition in the affected tissues also increased gradually in a rmTHBS2 dose-dependent manner (Fig. 7H). These common observations confirm THBS2 as a promoter of collagen deposition in fibrotic lungs.
THBS2 enhances bleomycin-triggered pulmonary fibrosis. (A) Schematic representation of the experimental approach. WT mice were intratracheal injected with sterile saline or bleomycin. They were treated with or without recombinant THBS2 protein (25 μg/kg and 50 μg/kg) and euthanized after 21 days. (B) The body weights of THBS2 treated and control mice were measured and represented by bars. n = 15 for each group. (C) Survival curves of mice within 21 days are shown. n = 15 for each group. (D) Total lung hydroxyproline content was quantified after 21 days. n = 6 for each group. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, ****P < 0.0001. (E) Protein levels of collagen markers measured by Western blot at 21 days. (F) The protein expression levels of each collagen marker were quantitatively analyzed. n = 6 for each group. Data are shown as the mean ± SEM.*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, nsP > 0.05. (G) Representative whole-lung images of hematoxylin–eosin (H&E) (top) from 21-day lung sections; Representative images of 21-day lung tissues stained with H&E (middle) and Masson trichrome (bottom). H&E staining and Ashcroft score were used to measure lung section morphology. The area of lung fibrosis was examined and quantified by Masson`s trichrome stain; Blue color, collagen fiber; red color, cytoplasm or muscle fiber. Scale bars, 2.5 μm (top) and 100 μm (middle and bottom). n = 6 for each group. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (H) Immunohistochemical images showed CoLI and CoLIII positive areas and staining was quantified. Scale bar, 100 μm. n = 6 for each group. Data are shown as the mean ± SEM. **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
IPF is a fatal lung disease associated with aging, which can lead to scarring and destruction of lung structures, respiratory failure, and eventually death31. Our study reveals THBS2 is involved in the main therapeutic mechanism of pulmonary fibrosis by mediating TGF-β1/Smad2/3 to regulate collagen synthesis, leading to accelerated collagen deposition.
THBS2, an ECM protein-coding gene, encodes a secreted glycoprotein that is part of the extracellular matrix and can serve as a marker for the severity of tissue fibrosis32,33. Our work also supports this conclusion by showing that THBS2 is significantly enhanced in primary cultured human lung fibroblasts and MRC5, and functional assays further demonstrate that THBS2 directly enhances the proliferation and migration ability of fibroblasts. Type I and III collagen are located in the interstitial matrix, these collagen deposits initially play a protective role, but excessive and persistent collagen deposits lead to remodeling of the lung architecture34. Experiments showed THBS2 promotes the production of type I collagen and type III collagen in fibroblasts, whereas knockdown of THBS2 inhibits the deposition of these collagens. Further validating these findings in a gain-of-function model, our study showed that intranasal instillation of rmTHBS2 in bleomycin-induced pulmonary fibrosis in mice aggravated BLM-induced IPF with a gradual increase in collagen deposition. LOX and LOXL2 are involved in the cross-linking process of essential collagen for maintaining the structure of lung tissue. In pulmonary fibrosis, abnormal collagen cross-linking leads to excessive collagen accumulation, stiffening the lung tissue and impairing normal gas exchange function35. Our experiments confirmed THBS2 could promote the expression levels of LOX and LOXL2, suggesting that the process of THBS2 promoting collagen synthesis was also accompanied by the enhancement of collagen cross-linking.
TGF-β1 plays a key role by inducing the synthesis and release of a variety of cytokines including PDGF, TNF-α, IL-6 and IL-1336, and it is widely regarded as a major pro-fibrotic cytokine. In the present study, THBS2 overexpression in fibroblasts resulted in significantly increased levels of TGF-β1 and various other cytokines in CM. THBS2 overexpression induced the activation of molecules related to the TGF-β1 signaling pathway recruitment, while inhibition of TGF-β1 attenuated this recruitment and decreased the expression levels of COL1A1, COL1A2 and COL3A1. Interestingly, the upregulation of LOX and LOXL2 was uninhibited. These results suggest that THBS2 may promote collagen expression by mediating TGF-β1-induced collagen mRNA expression and that the effect of THBS2 on collagen cross-linking may be directly formed or not directly dependent on TGF-β signaling. Through literature review, some scholars have proved there is a related signaling pathway between LOX and TGF-β37, and LOX may act through TGF-β signaling pathway38,39. However, some studies suggest that the activation of LOX/LOXL2 is influenced by multiple factors and is not solely dependent on the drive of TGF-β1. AZM reduces LOX/LOXL2 production by inhibiting JNK/c-Jun pathway40. Activation of IKKβ/NFκB pathway increases LOX production41. The domain SRCR1 + 2 of LOXL2 may contribute to promoting fibrosis independently of its enzyme activity by accelerating the fibrosis process through ECM deposition, primarily collagen and elastin42. Regulation of LOX activity by IGF-II also affects pulmonary fibrosis progression43. Therefore, we may hope that further experiments in the future can clarify the specific molecular mechanism of THBS2 regulating abnormal collagen cross-linking in collagen synthesis, and provide new ideas for the treatment of fibrosis and other diseases.
Studies have shown that pulmonary fibroblasts with metabolic activity, hypersecretion and anti-apoptotic senescence phenotypes are abundant in the lungs of IPF patients, and they promote the release of various fibrotic growth factors like TGF-β1, IL-6, IL-1β and MMP-12. Results in the secretion and deposition of ECM proteins30,44,45,46. THBS2 has been reported to play a pivotal role in disease pathogenesis through paracrine signaling pathways31,47. Our study showed THBS2 overexpression significantly increased the secretion of many soluble mediators belonging to SASP released by fibroblasts. Furthermore, our findings provide strong evidence that CM significantly affects the activation and aging state of normal lung fibroblasts. This suggests that upregulation of THBS2 in fibroblasts promotes paracrine activation of the fibrotic process and accelerates the progression of cells to a senescent state. Senescent fibroblasts typically exhibit reduced proliferation and impaired function; however, they also release numerous inflammatory mediators and matrix remodeling enzymes, which promote the proliferation of neighboring cells. This contributes to local tissue fibrosis and creates a complex negative feedback loop. Moving forward, it will be crucial to investigate the pathway through which SASP, induced by THBS2-mediated secretion of inflammatory factors, influences the senescence of the fibroblast niche and further drives the progression of pulmonary fibrosis.
Limitations of the study
Our study has certain limitations. MRC5 cells, originating from fetal tissue, exhibit distinct biological properties compared to adult lung fibroblasts, and they are in a quiescent state, lacking an active fibrotic response. Furthermore, the availability of primary fibroblast samples is restricted. Lung tissue obtained from patients with benign tumors may present differences in cell phenotype and function when compared to healthy individuals, potentially influencing the fibroblasts’ biological characteristics. To address these limitations, future investigations will focus on utilizing immortalized cell lines for model development, alongside efforts to expand the sample size for more comprehensive studies. In addition, THBS2 overexpression in CM resulted in increased SASP levels, including IL-6, IL-8, IL-1β, etc. However, further studies are needed to understand the specific contribution of individual secreted factors to these paracrine effects.
Conclusions
THBS2 is overexpressed in IPF. Functionally, THBS2 promoted the invasive phenotype, collagen synthesis and inflammation secretome in fibroblasts. Mechanistically, THBS2 promotes collagen synthesis through the TGF-β1/Smad2/3 signaling pathway. THBS2 can also regulate the pro-inflammatory secretory state of fibroblasts to promote aging in adjacent normal fibroblasts.
Data availability
The raw RNA-seq data are available in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) under the dataset identifiers GSE119007 and GSE110147. For further inquiries, please contact the corresponding author.
References
Martinez, F. J. et al. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 3, 17074. https://doi.org/10.1038/nrdp.2017.74 (2017).
RicheldI, L., Collard, H. R. & Jones, M. G. Idiopathic pulmonary fibrosis. Lancet 389(10082), 1941–1952. https://doi.org/10.1016/s0140-6736(17)30866-8 (2017).
Knüppel, L. et al. A novel antifibrotic mechanism of nintedanib and pirfenidone. Inhibition of collagen fibril assembly. Am. J. Respir. Cell Mol. Biol. 57(1), 77–90. https://doi.org/10.1165/rcmb.2016-0217OC (2017).
Myllärniemi, M. & Kaarteenaho, R. Pharmacological treatment of idiopathic pulmonary fibrosis—preclinical and clinical studies of pirfenidone, nintedanib, and N-acetylcysteine. Eur. Clin. Respir. J. https://doi.org/10.3402/ecrj.v2.26385 (2015).
Snijder, J. et al. Pulmonary fibrosis: A disease of alveolar collapse and collagen deposition. Expert Rev. Respir. Med. 13(7), 615–619. https://doi.org/10.1080/17476348.2019.1623028 (2019).
Mei, Q. et al. Idiopathic pulmonary fibrosis: An update on pathogenesis. Front. Pharmacol. 12, 797292. https://doi.org/10.3389/fphar.2021.797292 (2021).
Kaarteenaho-Wiik, R. et al. Type I and III collagen protein precursors and mRNA in the developing human lung. J. Pathol. 203(1), 567–574. https://doi.org/10.1002/path.1547 (2004).
Stegen, S. et al. HIF-1α metabolically controls collagen synthesis and modification in chondrocytes. Nature 565(7740), 511–515. https://doi.org/10.1038/s41586-019-0874-3 (2019).
Devos, H. et al. Reviewing the regulators of COL1A1. Int. J. Mol. Sci. https://doi.org/10.3390/ijms241210004 (2023).
Margadant, C. & Sonnenberg, A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 11(2), 97–105. https://doi.org/10.1038/embor.2009.276 (2010).
Kim, K. K., Sheppard, D. & Chapman, H. A. TGF-β1 signaling and tissue fibrosis. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a022293 (2018).
Lin, Y. & Xu, Z. Fibroblast senescence in idiopathic pulmonary fibrosis. Front. Cell Dev. Biol. 8, 593283. https://doi.org/10.3389/fcell.2020.593283 (2020).
Carlson, C. B., Lawler, J. & Mosher, D. F. Structures of thrombospondins. Cell. Mol. Life Sci. CMLS 65(5), 672–686. https://doi.org/10.1007/s00018-007-7484-1 (2008).
Kozumi, K. et al. Transcriptomics identify thrombospondin-2 as a biomarker for NASH and advanced liver fibrosis. Hepatology 74(5), 2452–2466. https://doi.org/10.1002/hep.31995 (2021).
Shen, Y., Yu, J., Jing, Y. & Zhang, J. MiR-106a aggravates sepsis-induced acute kidney injury by targeting THBS2 in mice model. Acta Cir. Bras. 34(6), e201900602. https://doi.org/10.1590/s0102-865020190060000002 (2019).
Chen, W. et al. Multitranscriptome analyses reveal prioritized genes specifically associated with liver fibrosis progression independent of etiology. Am. J. Physiol. Gastrointest. Liver Physiol. 316(6), G744-g754. https://doi.org/10.1152/ajpgi.00339.2018 (2019).
Iwadare, T. et al. Circulating thrombospondin 2 levels reflect fibrosis severity and disease activity in HCV-infected patients. Sci. Rep. 12(1), 18900. https://doi.org/10.1038/s41598-022-23357-9 (2022).
Hsu, C. H. et al. miR-29a-3p/THBS2 axis regulates PAH-induced cardiac fibrosis. Int. J. Mol. Sci. https://doi.org/10.3390/ijms221910574 (2021).
Reinecke, H. et al. Lack of thrombospondin-2 reduces fibrosis and increases vascularity around cardiac cell grafts. Cardiovasc. Pathol. 22(1), 91–95. https://doi.org/10.1016/j.carpath.2012.03.005 (2013).
Ghavami, S. et al. Autophagy and the unfolded protein response promote profibrotic effects of TGF-β(1) in human lung fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 314(3), L493-l504. https://doi.org/10.1152/ajplung.00372.2017 (2018).
Han, D. et al. Hesperidin inhibits lung fibroblast senescence via IL-6/STAT3 signaling pathway to suppress pulmonary fibrosis. Phytomedicine 112, 154680. https://doi.org/10.1016/j.phymed.2023.154680 (2023).
Sun, J. et al. LncRNA DACH1 protects against pulmonary fibrosis by binding to SRSF1 to suppress CTNNB1 accumulation. Acta Pharm. Sin. B 12(9), 3602–3617. https://doi.org/10.1016/j.apsb.2022.04.006 (2022).
Burgess, J. K. & Weckmann, M. Matrikines and the lungs. Pharmacol. Ther. 134(3), 317–337. https://doi.org/10.1016/j.pharmthera.2012.02.002 (2012).
Di Mauro, P. et al. LOX, but not LOXL2, promotes bone metastasis formation and bone destruction in triple-negative breast cancer. J. Bone Oncol. 44, 100522. https://doi.org/10.1016/j.jbo.2024.100522 (2024).
Andugulapati, S. B. et al. Biochanin-A ameliorates pulmonary fibrosis by suppressing the TGF-β mediated EMT, myofibroblasts differentiation and collagen deposition in in vitro and in vivo systems. Phytomedicine 78, 153298. https://doi.org/10.1016/j.phymed.2020.153298 (2020).
Yang, W. et al. Nintedanib alleviates pulmonary fibrosis in vitro and in vivo by inhibiting the FAK/ERK/S100A4 signalling pathway. Int. Immunopharmacol. 113(Pt A), 109409. https://doi.org/10.1016/j.intimp.2022.109409 (2022).
Heo, J. Y. et al. TGF-β1 receptor inhibitor SB525334 attenuates the epithelial to mesenchymal transition of peritoneal mesothelial cells via the TGF-β1 signaling pathway. Biomedicines https://doi.org/10.3390/biomedicines9070839 (2021).
Blokland, K. E. C. et al. Regulation of cellular senescence is independent from profibrotic fibroblast-deposited ECM. Cells https://doi.org/10.3390/cells10071628 (2021).
Coppé, J. P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6(12), 2853–2868. https://doi.org/10.1371/journal.pbio.0060301 (2008).
Yamada, Z. et al. Senescence of alveolar epithelial cells impacts initiation and chronic phases of murine fibrosing interstitial lung disease. Front. Immunol. 13, 935114. https://doi.org/10.3389/fimmu.2022.935114 (2022).
Caporarello, N. et al. PGC1α repression in IPF fibroblasts drives a pathologic metabolic, secretory and fibrogenic state. Thorax 74(8), 749–760. https://doi.org/10.1136/thoraxjnl-2019-213064 (2019).
Pantano, L. et al. Molecular characterization and cell type composition deconvolution of fibrosis in NAFLD. Sci. Rep. 11(1), 18045. https://doi.org/10.1038/s41598-021-96966-5 (2021).
Kimura, T. et al. Serum thrombospondin 2 is a novel predictor for the severity in the patients with NAFLD. Liver Int. 41(3), 505–514. https://doi.org/10.1111/liv.14776 (2021).
Shao, S. et al. Lysyl hydroxylase 3 increases collagen deposition and promotes pulmonary fibrosis by activating TGFβ1/Smad3 and Wnt/β-catenin pathways. Arch. Med. Sci. AMS 16(2), 436–445. https://doi.org/10.5114/aoms.2018.81357 (2020).
Ma, H. Y. et al. LOXL4, but not LOXL2, is the critical determinant of pathological collagen cross-linking and fibrosis in the lung. Sci. Adv. 9(21), eadf0133. https://doi.org/10.1126/sciadv.adf0133 (2023).
Jolly, M. K. et al. Epithelial-mesenchymal transition, a spectrum of states: Role in lung development, homeostasis, and disease. Dev. Dyn. 247(3), 346–358. https://doi.org/10.1002/dvdy.24541 (2018).
Wen, X. et al. LOXL2, a copper-dependent monoamine oxidase, activates lung fibroblasts through the TGF-β/Smad pathway. Int. J. Mol. Med. 42(6), 3530–3541. https://doi.org/10.3892/ijmm.2018.3927 (2018).
Lee, C. M. et al. Modifiers of TGF-β1 effector function as novel therapeutic targets of pulmonary fibrosis. Korean J. Intern. Med. 29(3), 281–290. https://doi.org/10.3904/kjim.2014.29.3.281 (2014).
Wei, Y. et al. Fibroblast-specific inhibition of TGF-β1 signaling attenuates lung and tumor fibrosis. J. Clin. Investig. 127(10), 3675–3688. https://doi.org/10.1172/jci94624 (2017).
Tong, X. et al. Azithromycin attenuates bleomycin-induced pulmonary fibrosis partly by inhibiting the expression of LOX and LOXL-2. Front. Pharmacol. 12, 709819. https://doi.org/10.3389/fphar.2021.709819 (2021).
Guo, K. et al. Triptolide alleviates radiation-induced pulmonary fibrosis via inhibiting IKKβ stimulated LOX production. Biochem. Biophys. Res. Commun. 527(1), 283–288. https://doi.org/10.1016/j.bbrc.2020.04.023 (2020).
Peng, L. et al. NUDT21 regulates lysyl oxidase-like 2(LOXL2) to influence ECM protein cross-linking in silica-induced pulmonary fibrosis. Ecotoxicol. Environ. Saf. 290, 117572. https://doi.org/10.1016/j.ecoenv.2024.117572 (2025).
Adewale, A. T. et al. IGF-II regulates lysyl oxidase propeptide and mediates its effects in part via basic helix-loop-helix E40. Matrix Biol. 132, 24–33. https://doi.org/10.1016/j.matbio.2024.06.002 (2024).
Angelidis, I. et al. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat. Commun. 10(1), 963. https://doi.org/10.1038/s41467-019-08831-9 (2019).
Parimon, T. et al. Syndecan-1 promotes lung fibrosis by regulating epithelial reprogramming through extracellular vesicles. JCI insight https://doi.org/10.1172/jci.insight.129359 (2019).
Kadota, T. et al. Extracellular vesicles from fibroblasts induce epithelial-cell senescence in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 63(5), 623–636. https://doi.org/10.1165/rcmb.2020-0002OC (2020).
Waters, D. W. et al. Fibroblast senescence in the pathology of idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 315(2), L162-l172. https://doi.org/10.1152/ajplung.00037.2018 (2018).
Funding
This work was supported by funds from the National natural science foundation of China (Grant NO. 82370075), the Climbing Project for Medical Talent of Zhongnan Hospital of Wuhan University (Grant NO. PDJH202406 and PDJH202205), the research found from medical Sci-Tech innovation platform of Zhongnan Hospital of Wuhan University (Grant NO. PTXM2022001), the Science and technology innovation Cultivation Fund of Zhongnan Hospital of Wuhan University (Grant No. CXPY2023035) and Noncommunicable Chronic Diseases-National Science and Technology Major Project (Grant No. 2023ZD0506200).
Author information
Authors and Affiliations
Contributions
L.Y. conceived and designed research, performed experiments, analyzed data, and drafted the manuscript. R.W., Q.H. and F.Y. performed experiments, analyzed data, and drafted the manuscript. Y.L., M.P. and Y.D. conceived and designed research and edited and revised the manuscript. Y.L. and Z.C. conceived and designed research, analyzed data, drafted the manuscript, and edited and revised the manuscript. L.Y., R.W., Q.H., F.Y., Y.L., M.P., Y.D., Y.L. and Z.C. approved the final version of the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, L., Wang, R., Hou, Q. et al. Enhanced THBS2 promotes collagen synthesis and inflammatory secretome of fibroblasts in idiopathic pulmonary fibrosis. Sci Rep 15, 25926 (2025). https://doi.org/10.1038/s41598-025-09318-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09318-y