Abstract
While remifentanil, etomidate, and rocuronium are increasingly used for cesarean section due to their favorable hemodynamic stability and fetal safety profile, their pharmacokinetics and potential effects on neonates remain poorly understood. This study developed and validated a rapid, sensitive LC–MS/MS method for simultaneous quantification of the three anesthetics in microsamples of 10 μl serum, followed by a paired maternal and umbilical cord serum investigation. After protein precipitation with acetonitrile, analytes were separated within 4 min using positive electrospray ionization in MRM mode. Method validation demonstrated excellent linearity (R2 > 0.99) for all compounds, with LLOQs of 0.15 ± 0.02 ng/mL (remifentanil), 16.87 ± 0.51 ng/mL (etomidate), and 106.73 ± 8.63 ng/mL (rocuronium). Precision (intra-/inter-day < 15%) and minimal carry-over (< 5%) met bioanalytical standards. Applied to 20 maternal-newborn pairs, the method quantified differential drug distribution: maternal arterial concentrations (remifentanil 4.75 ± 0.19 ng/mL; etomidate 412.71 ± 35.29 ng/mL; rocuronium 7.08 ± 0.48 μg/mL) exceeded umbilical vein levels (2.43 ± 0.13 ng/mL; 302.15 ± 29.03 ng/mL; 0.86 ± 0.16 μg/mL), which were higher than umbilical artery concentrations (1.33 ± 0.15 ng/mL; 166.24 ± 21.53 ng/mL; 0.44 ± 0.77 μg/mL). Calculated placental transfer rates significantly differed among anesthetics (remifentanil 0.52 ± 0.02; etomidate 0.75 ± 0.04; rocuronium 0.13 ± 0.02; all P < 0.001), reflecting distinct pharmacokinetic behaviors. The validated method enables reliable microvolume analysis for perinatal pharmacokinetic studies, particularly valuable when sample availability is limited. Its rapid throughput and sensitivity make it suitable for clinical research applications investigating maternal–fetal drug transfer dynamics.
Similar content being viewed by others
Introduction
Recently, induction of anesthesia using propofol, rocuronium, and remifentanil are becoming popular1. Compared with propofol, etomidate has minimal impact to hemodynamics with low fluctuation of blood pressure and cardiac rhythm2. Therefore, the three anesthetics remifentanil, etomidate, and rocuronium are another choice for cesarean section, especially for pregnant women who have cardiovascular problems3,4,5. The three drugs operate through complementary mechanisms: remifentanil is an opioid analgesic; etomidate, a general anesthetic; and rocuronium, a muscle relaxant. Remifentanil is the preferred opioid analgesic for this situation: although it easily passes through the placental barrier, it is hydrolyzed in cord blood and rapidly metabolized in the fetus, and it is less likely to cause respiratory depression6. Etomidate and rocuronium are assumed to present minimal risk to fetal breathing because they are both administered at single dose7,8. However, the effect of the triple combination on fetal respiration has not been systematically analyzed, nor are the pharmacokinetics of the drugs in the mother and newborn well understood, which means their long-term safety is unclear.
Pharmacokinetic and safety studies require appropriate analytical methods for determining all three anesthetics in blood, yet published methods whether based on high-performance liquid chromatography or liquid chromatography-tandem mass spectrometry require large sample volumes and time- or labor-intensive procedures and have been validated for only one or two of the three drugs3,4. Therefore, we undertook the present study to develop and validate a single analytical method that could accurately determine all three anesthetics in extremely small volumes of serum. As a methodological application, we performed a pilot study using matched maternal-cord blood pairs to investigate transplacental drug transfer dynamics and maternal–fetal concentration gradients.
Results
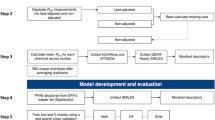
The outcomes present both method development/validation data and its successful application in paired maternal-neonatal serum specimens, outlined in Fig. 1.
Column chromatography lasting 4 min was sufficient to cleanly separate the three anesthetics of interest from their respective internal standards (Fig. 2). Retention time did not vary significantly when samples were analyzed on different days (data not shown). The method showed good linear response for all three anesthetics (Table 1), and LLOQs were 0.08 ± 0.01 ng/ml for remifentanil, 18.69 ± 0.14 ng/ml for etomidate, and 106.73 ± 8.63 ng/ml for rocuronium (Table 2). For all three anesthetics, intra- and inter-day imprecision was below 15% (Table 3), recovery was 85–115% (Table 4), and carry-over was below 15% (Table 5).
Representative total ion chromatograms (TICs) of (A) remifentanil and its internal standard sufentanil, (B) etomidate and its standard metomidate, or (C) rocuronium and its standard vecuronium. In all chromatograms, the x-axis shows the retention time (min), while the y-axis shows relative ion intensity. The exact ion intensity is indicated at the upper right of each chromatogram.
These results indicate that our analytical method is valid. Using this method, we determined levels of the three anesthetics in maternal arterial blood and in the blood from umbilical veins and arteries (Table 6). The mean concentration of remifentanil in maternal arterial blood was 4.75 ng/ml, in good agreement with the target-controlled infusion concentration of 5 ng/ml during anesthesia induction. The ratios of drug concentration in umbilical veins to the concentration in maternal arteries, which reflect rate of transport from mother to fetus, were 0.52 ± 0.02 for remifentanil, 0.75 ± 0.04 for etomidate, and 0.13 ± 0.02 for rocuronium (P < 0.001).
Discussion
Here we describe and validate an analytical method to simultaneously determine three anesthetics commonly given to pregnant women during cesarean section deliveries in maternal as well as umbilical blood. The method requires very small amounts of serum, which undergoes a straightforward protein precipitation procedure, followed by liquid chromatography and tandem mass spectrometry. With a measurement duration of under 4 min (faster than prior reports), the method offers significantly improved efficiency, making it particularly suitable for research involving large sample sizes9,10. Besides, this method also shows satisfactory LLOQs, linearity, precision and accuracy for determining remifentanil, etomidate and rocuronium.
Our protein precipitation approach is similar to that reported in other methods designed to analyze individual anesthetics in our study, but not all of them together11,12. The protein precipitation procedure in our method provides good sensitivity, with LLOQs as low as 0.08 ng/ml, even though it uses only 10 μl of serum for analysis. This gives our relatively straightforward method a strong advantage over other approaches that require more complicated processing, such as solid-phase extraction or microextraction in a packed syringe13,14. Our LLOQ for remifentanil is higher than that of another method involving liquid–liquid extraction (0.08 vs. 0.05 ng/ml), yet that method requires approximately 500 μl of serum15.
Rocuronium was nearly 4000 times more concentrated in maternal and umbilical serum than remifentanil or etomidate, which poses a challenge for their simultaneous determination. The success of our method depends on a suitably sensitive tandem mass spectrometry system that can accurately determine ions present at concentrations spanning five orders of magnitude16. We had to carefully optimize sample concentration and injection volume to prevent oversaturation by rocuronium.
Applying this method to a small sample of women giving birth by cesarean section at our hospital, we achieved precise quantification of drug concentrations at both nanogram (ng) and microgram (μg) levels, demonstrating the high sensitivity. More notably, we found rates of anesthetic transfer from mother to newborn varied significantly widely. These rates likely depend on several drug characteristics, such as size, lipophilicity, affinity for transporter proteins and extent of ionization17. The low transfer of rocuronium reflects its high water-solubility, which inhibits its passage through the placental barrier18. While rocuronium exhibits a significantly lower transfer rate compared to remifentanil and etomidate, its association with decreased 1-min Apgar scores (though 5-min scores remain largely unaffected) raises important clinical concerns19. Remifentanil is known to be distributed and metabolized faster in the newborn than in the mother because of newborns’ higher concentration of red blood cells in the circulation and their lower proportion of subcutaneous fat20. Whether the same is true for the other two anesthetics remains to be seen. In contrast to the different rates of transfer, rates of metabolism in the newborn were between 0.50 and 0.60 for all three anesthetics. Previous work reported a much higher rate of remifentanil transport from mother to newborn of 0.88 when the drug was used on its own, but only a slightly higher rate of 0.64 when it was used with propofol21,22. Combining remifentanil with propofol or etomidate may decrease its transport from mother to newborn, which should be explored in future research.
Our method should facilitate detailed studies of the pharmacokinetics of these drugs in the mother and newborn in order to verify their safety and identify any additive or antagonistic interactions among them. Still, the current study has several limitations worth noting: (1) the small cohort size may affect extrapolation of our pharmacokinetic observations to broader populations, and (2) restriction to single time-point measurements precludes evaluation of temporal metabolic patterns that could influence drug transfer ratios.
Methods
Study design
The study had two objectives: first, to develop and validate a sensitive LC–MS/MS assay for simultaneous measurement of remifentanil, etomidate, and rocuronium in serum; second, to implement this assay in paired maternal-neonatal serum analyses. Ethical approval was granted by the Ethics Committee of Chongqing Health Center for Women and Children (Approval No. 2021–011), and informed consent was obtained from all participating women. It was registered with the Chinese Clinical Trial Registry (registration ID: ChiCTR2100046547). All methods were performed in accordance with the relevant guidelines and regulations.
Reagents
HPLC-grade methanol and acetonitrile were purchased from Fisher Scientific (Geel, Belgium), and formic acid was purchased from Shanghai Aladdin Bio-Chem Technology (Shanghai, China). The following six narcotics were purchased from Toronto Research Chemicals (Toronto, Canada): remifentanil hydrochloride, sufentanil citrate, etomidate, metomidate hydrochloride, rocuronium bromide, and vecuronium bromide. Water in this study was purified using a Milli-Q apparatus (Millipore, Bedford, MA, USA).
Calibration standards, internal standards, and quality control samples
Stock solutions of remifentanil, etomidate, and rocuronium were prepared individually by dissolving solid compound into methanol to a concentration of 1 mg/ml, then vortexing at 1500 rpm for 5 min. The solutions were stored in brown vials at − 80 ℃ and used within 12 months. These stock solutions were combined and suitably diluted into serum from pregnant women unexposed to the three anesthetics to yield final concentrations of 10 ng/ml remifentanil, 20,000 ng/ml etomidate and 2000 ng/ml rocuronium. This working solution was stored in brown vials at − 20 °C and used within one month.
Stock solutions of the three internal standards sufentanil, metomidate and vecuronium were prepared individually by dissolving solid compound into acetonitrile to a concentration of 1 mg/ml, then vortexing at 1500 rpm for 5 min. These stock solutions were combined and suitably diluted into acetonitrile to yield final concentrations of 5 ng/ml sufentanil, 5 ng/ml metomidate, and 100 ng/ml vecuronium. This working solution was stored at − 20 °C in brown vials and used within one month.
Working solutions were diluted serially 1:2 in serum from pregnant women unexposed to the three anesthetics in order to determine calibration curves. Quality control samples were prepared by diluting working solutions into serum from unexposed pregnant women as follows: remifentanil, 0.5, 2.0 and 4.0 ng/ml; etomidate, 125.0, 500.0 and 1000.0 ng/ml; and rocuronium, 1000.00, 4000.00 and 8000.00 ng/ml.
Sample collection and preparation
Blood sample were taken from women giving birth by cesarean section under general anesthesia at our hospital. Blood for blank matrix was collected from pregnant women not yet exposed to anesthetics during venous access establishment. All women received anesthesia according to standard protocols at our hospital. General anesthesia was induced using target-controlled infusion of remifentanil (5 ng/ml), 5% sevoflurane inhalation, and intravenous etomidate (0.25 mg/kg), followed by intravenous rocuronium bromide (0.6 mg/kg). After rocuronium administration, the endotracheal tube was placed and connected to a ventilator. Sevoflurane was administered briefly and discontinued when the eyelash reflex disappeared. Immediately after ligation of the umbilical cord, blood was collected from the left radial artery of the mother as well as from the umbilical vein and artery. After umbilical cord ligation, anesthesia was maintained through target-controlled infusion of propofol at 3.5 μg/ml and of remifentanil at 5 ng/ml.
All the blood sample was centrifuged at 3000 rpm, and the serum was transferred to vials and stored at − 80 °C until analysis. Frozen samples were thawed at room temperature for about 20 min, and 10-μl volumes of the thawed samples, quality control solutions and standards were placed into fresh microcentrifuge tubes, diluted up to 50 μl with pure water and vortexed at 1500 rpm for 3 min. Then 150 μl of acetonitrile containing internal standards was added to the vials, the solution was mixed and incubated at − 20 °C for 20 min to precipitate proteins, and the solution was centrifuged at 13,000 g for 5 min at 4 °C. The supernatant (100 μl) was transferred into fresh microcentrifuge tubes.
An aliquot of supernatant (10 μl) was diluted with water (90 μl) to ensure accurate determination, and an aliquot of the dilution (3 μl) was injected into the liquid chromatography system for analysis as described below.
Liquid chromatography-tandem mass spectrometry
Samples were analyzed on an ultra-high performance liquid chromatography-tandem mass spectrometry system (Xevo TQ-S, Waters Corporation, Milford, MA, USA) equipped with an electrospray ionization source operating in positive mode. Samples were fractionated on a reverse-phase Acquity BEH C18 column (50 × 2.1 mm, 1.7 μm; Waters Corporation) under the following gradient of 0.1% (v/v) formic acid in water (solution A) and a 1:1 (v/v) mixture of methanol and acetonitrile in solution A (solution B): 0 to 0.6 min, 10% B; 0.6–2.8 min, 10–90% B; 2.8–3.6 min, 90% B; and 3.6–4 min, 10% B. The flow rate was 0.4 ml/min and column temperature was 45 °C.
Eluted compounds were analyzed in multiple reaction monitoring mode based on time-scheduled events with analyte-dependent parameters (Table 7) and the following additional parameters: ion source temperature, 150 °C; capillary voltage, 3.0 kV; desolvation temperature, 500 °C; gas flow rate for desolvation, 800 L/h; and gas flow rate in the cone, 150 L/h. Data were processed and quantified in MassLynx 4.2 software (Waters Corporation) based on the peak area and calibration with the internal standards. Amounts were quantified based on peak areas according to the internal standard method.
Validation of the analytical method
To assess linearity and the lower limit of quantitation (LLOQ), we prepared eight concentrations of each of the three anesthetics covering the following ranges: remifentanil, 0.08 to 10.00 ng/ml; etomidate, 15.63 to 2000 ng/ml; and rocuronium, 156.25 to 20,000 ng/ml. The ratio of each analyte’s peak area to the peak area of its corresponding internal standard was plotted against the nominal spiked concentration. A calibration curve was generated using linear least-squares regression. The method was considered valid if the correlation coefficient (R2) of the regression line exceeded 0.99.
The LLOQ was defined as the lowest concentration at which the signal-to-noise ratio was at least 10 and the coefficient of variation was below 20%. Accuracy was assessed in terms of the relationship between measured concentrations and nominal spiked concentrations in quality control samples. Imprecision was expressed as the relative standard deviation (RSD%) for quality control samples at low, intermediate and high concentrations. Twenty replicates of each concentration were determined within a single day (intra-day imprecision) or once daily on five consecutive days (inter-day imprecision). The method was considered valid if accuracy was 85–115% overall and 80–120% at the LLOQ, and if inter- and intra-day imprecision was below 15% overall and below 20% at the LLOQ.
Carry-over was assessed by analyzing three consecutive samples with high concentrations (H1, H2, H3) followed by three consecutive samples with low concentrations (L1, L2, L3). The carry-over rate was calculated as [(L1–L2) / (H3–L3)] × 100%. The method was considered valid if the carry-over rate was below 15% overall and below 20% at the LLOQ.
Statistical analysis
Data were reported as mean ± standard deviation, unless stated otherwise, as calculated in Microsoft Excel. Differences were assessed for significance using one-way ANOVA in SPSS 25 (IBM, Armonk, NY, USA).
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Sumikura, H. et al. Rethinking general anesthesia for cesarean section. J Anesth 30(2), 268–273 (2016).
Robertson, S. Advantages of etomidate use as an anesthetic agent. Vet Clin North Am Small Anim Pract 22, 277–280 (1992).
Van de Velde, M. The use of remifentanil during general anesthesia for caesarean section. Curr Opin Anaesthesiol 29, 257–260 (2016).
Eisa, B., Amir, A. K., Mehrnoosh, T. & Solmaz, F. Anesthesia with etomidate and remifentanil for cesarean section in a patient with severe peripartum cardiomyopathy–a case report. Middle East J Anaesthesiol 19, 1141–1149 (2008).
Demet, C. et al. Anaesthesia for caesarean section in the presence of multivalvular heart disease and severe pulmonary hypertension: A case report. Cases J. 2, 9383 (2009).
White, L. D. et al. Induction opioids for caesarean section under general anaesthesia: A systematic review and meta-analysis of randomised controlled trials. Int. J. Obstet. Anesth 40, 4–13 (2019).
Downing, J. W., Buley, R. J., Brock-Utne, J. G. & Houlton, P. C. Etomidate for induction of anaesthesia at caesarean section: Comparison with thiopentone. Br J Anaesth. 51, 135–140 (1979).
Blaha, J, Noskova, P, Hlinecka, K, Krakovska, V, Fundova, V, Bartosova, T, et al. 2020, Surgical conditions with rocuronium versus suxamethonium in cesarean section a randomized trial, Int J Obstet Anesth, 41: 14–21.
He, T.-F. et al. Detection of etomidate and etomidate acid in urine using HPLC-MS/MS method. Fa Yi Xue Za Zhi 40, 454–460 (2024).
Pei, L. et al. Characterization of etomidate and emerging analogs in human hair using UHPLC-MS/MS and confirmation in real forensic cases. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 15(1247), 124340 (2024).
Koster, R. A. et al. Analysis of remifentanil with liquid chromatography-tandem mass spectrometry and an extensive stability investigation in EDTA whole blood and acidified EDTA plasma. Anesth. Analg. 120, 1235–1241 (2015).
Yum, H. et al. Fast and reliable analysis of veterinary metomidate and etomidate in human blood samples by liquid chromatography–tandem mass spectrometry (LC–MS/MS) in a postmortem case. J. Forensic. Sci. 66, 2532–2538 (2021).
Bossù, E. et al. LC-MS Determination of remifentanil in maternal and neonatal plasma. J. Pharm. Biomed. Anal. 42, 367–371 (2006).
Said, R. et al. Determination of remifentanil in human plasma by liquid chromatography-tandem mass spectrometry utilizing micro extraction in packed syringe (MEPS) as sample preparation. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879, 815–818 (2011).
Cooreman, S. et al. A comprehensive LC-MS-based quantitative analysis of fentanyl-like drugs in plasma and urine. J. Sep. Sci. 33, 2654–2662 (2010).
Waters Corporation. Xevo TQ-S instrument specifications. 2017; 720003447EN TC-PDF.
Al-Enazy, S., Ali, S., Albekairi, N., El-Tawil, M. & Rytting, E. Placental control of drug delivery. Adv. Drug. Deliv. Rev. 116, 63–72 (2017).
Tang, M. et al. Advance in placenta drug delivery: Concern for placenta-originated disease therapy. Drug Deliv. 30, 2184315 (2023).
Kosinova, M. et al. Rocuronium versus suxamethonium for rapid sequence induction of general anaesthesia for caesarean section: Influence on neonatal outcomes. Int. J. Obstet Anesth. 32, 4–10 (2017).
Ross, A. K. et al. Pharmacokinetics of remifentanil in anesthetized pediatric patients undergoing elective surgery of diagnostic procedures. Anesth Analg 93, 1393–1401 (2001).
Kan, R. E. et al. Intravenous remifentanil: placental transfer, maternal and neonatal effects. Anesthesiology 88, 1467–1474 (1988).
Hu, L. et al. Propofol in combination with remifentanil for cesarean section: placental transfer and effect on mothers and newborns at different induction to delivery intervals. Taiwan J. Obstet. Gynecol 56, 521–526 (2017).
Funding
This study was supported by theChongqing Traditional Chinese Medicine Research Project (Joint project of Chongqing Health Commission and Science and Technology Bureau, 2023MSXM153), an institutional research grant from the Chongqing Health Center for Women and Children (2021YJMS03) andthe National Key Clinical Specialty Construction Project (Obstetrics and Gynecology).
Author information
Authors and Affiliations
Contributions
H L, raw data processing, manuscript writing and execution. M C, sample and clinical information collection. Y P: statistical analysis. JK M, table and figure preparation. J Y, manuscript revision and study design. The authors has accepted responsibility for the entire content of this manuscript and approved its submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Blood sampling and analysis were approved by the Medical Ethics Committee of Chongqing Health Center for Women and Children (2021–011). The women provided informed consent for their blood and umbilical blood to be sampled and analyzed in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, H., Cai, M., Peng, Y. et al. A pilot study on LC–MS/MS quantification of remifentanil, etomidate, and rocuronium in maternal and fetal serum microsamples. Sci Rep 15, 25876 (2025). https://doi.org/10.1038/s41598-025-09454-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09454-5