Abstract
Social attention, including shared attention and social orienting, is essential for positive social interactions. Although early visual social attention is often quantified using eye tracking, these indices may not consistently reflect cognitive engagement. Heart rate defined sustained attention (HRDSA) is a physiological measure that can index cognitive engagement alongside visual attention, leading to more comprehensive assessments of attentional processes that are particularly important in young, neurodiverse children with high support needs, including those with autism and fragile X syndrome (FXS). The present study examined visual and heart-defined measures of social attention to the Selective Social Attention task, a video-based assay of social attention, in children with autism, FXS, and neurotypical development. Linear mixed models examined group and condition effects in multiple cardiac indices and overall looking at the scene. Findings suggest that, overall, children across all groups engaged similarly across the experiment in most dimensions of HRDSA, and consistent with previous work, autistic children spent less time visually attending to the scene than either other group. HRDSA was positively associated with visual social attention. Combining physiological and visual attention measures may elucidate the complex nature of social attention and be especially valuable for neurodiverse children when typical assessments are inaccessible.
Similar content being viewed by others
Introduction
Social attention, including social orienting and shared social gaze, is essential for effective social interaction. Social attention emerges within the first few months of life1 and can have cascading impacts on higher-order social skills including social cognition, language, theory of mind, joint attention, social motivation, and social learning, throughout development2,3,4,5. Given its importance for functional outcomes, several studies have evaluated how social attention, primarily indexed through eye-tracking methodologies, manifests in neurodevelopmental disorders characterized by social differences, such as autism or fragile X syndrome (FXS)6,7,8,9,10,11. Furthermore, eye-tracking has a long history of measuring social attention in infants and young children with autism and FXS, especially those with high support needs, due in large part to the fact that eye-tracking is relatively non-invasive and experiments can require very few task demands12,13,14,15,16,17. This work is extremely valuable, as much autism research has excluded individuals who have high support needs (e.g., are nonspeaking or have intellectual disability), leading to a dearth of validated outcome measures for use in these populations15,18,19. While eye-tracking methodologies have been highly useful in characterizing social attention in autistic children and children with FXS6indexing visual attention alone has some limitations20,21. As such, multimethod studies of social attention and engagement are essential to objectively quantify and understand social attention, particularly in children who have high support needs.
Autism spectrum disorder (ASD, or autism) is a neurodevelopmental condition defined by differences in social communication and the presence of restricted and repetitive interests and behaviors22. Autism is one of the most common neurodevelopmental conditions, with current prevalence estimates of 1:31 children diagnosed23although the causes of autism are largely unknown. Fragile X syndrome, a heritable condition affecting roughly 1:7,000 males and 1:11,000 females24is a single gene condition caused by a CGG repeat expansion mutation on the Fragile X Messenger Ribonucleoprotein 1 (FMR1) gene, suppressing the production of the fragile X messenger ribonucleoprotein (FMRP), a protein critical for neurodevelopment25,26. There is a diagnostic co-occurrence with autism of approximately 60%27, but both autism and FXS are characterized by a multitude of overlapping features, even when co-occurrence is absent27,28,29,30. Atypical social attention is a prominent overlapping feature across non-syndromic autism and FXS that has been an important topic of research to date because of its potential cascading effects on important functional and developmental outcomes13,31.
Given the complex and multi-faceted nature of social attention, there are numerous measurement approaches for young children with neurodevelopmental disorders29,30,31 with automatic eye tracking13,32,33 among the most common. Eye tracking provides an objective and efficient measure of attention with relatively high temporal and spatial precision34. The Selective Social Attention (SSA) task is one of the most commonly used and validated eye-tracking assays in neurodevelopemental research35. It is a brief, dynamic video that depicts an actress surrounded by four non-social distractors engaging in experimental probes that use child-directed speech and eye contact as bids for attention. Multiple studies using the SSA in infant through school-age populations have indexed unique profiles of social attention in autism and FXS as compared to neurotypical development or other non-autistic developmental delays13,36,37. Furthermore, studies have associated visual social attention measured by the SSA with measures of observable social behavior, including autism features, adaptive behavior, naturalistic social attention, and play skills13,32,36,38.
Few studies to date have employed a multimethod approach to indexing social attention; that is, one combining eye-tracking with other objective measures of attention39. This is particularly important, as visual attention does not consistently index cognitive engagement40and gaze aversion has been deployed in neurodiverse persons as a strategy for managing cognitive load41. Thus, the development and implementation of physiological indices of attention, such as heart activity, coupled with eye-tracking, may provide multidimensional insight into the cognitive states of social attention42. Heart activity has immense promise as part of a multimethod index of social attention42 given the clear evidence that cardiac indices of physiological regulation contribute to cognitive and social competency and hypotheses that physiological hyperarousal may underly difficulties in social, cognitive, and affective engagement27,43,44,45. Cardiac indices of physiological regulation are also passive and relatively non-invasive measures, optimizing them for use in neurodevelopmental populations with high support needs39,46.
Heart rate-defined sustained attention (HRDSA) is a well-validated physiological measure of sustained attention40. It is defined by a period of sustained decrease in heart rate and heart rate variability following attentional orienting to a stimulus and is separate from phases of attention orienting and attention termination47. This deceleration in heart rate occurs as activation of the sympathetic nervous system is withdrawn and/or the parasympathetic nervous system is activated to cognitively attend to a particular stimulus. Periods of sustained attention are associated with cognitive engagement43,48. When paired with observed looking behaviors, HRDSA combines behavioral and physiological measures to index cognitive engagement alongside visual attention, increasing validity in capturing states of sustained visual attention. Previous research suggests that, compared to visual attention alone, HRDSA may provide differential insight into the development of social attention in neurotypical infants49leading to a more comprehensive measurement of attentional processes40. Reflecting cognitive engagement during states of sustained visual attention, HRDSA offers a multidimensional and precise account of attentional processes, which, in a social context, are critical for social learning and the later development of higher-order social skills. Thus, including HRDSA paradigms may increase insight into the development and understanding of social attention in young children with high support needs, including those with autism and FXS.
Previous research with children with neurodevelopmental disorders supports the utility of HRDSA as a measure of social attention in this population. For example, shallower heart rate decelerations were associated with autistic features in a longitudinal sample of infant siblings of autistic children during an engaging, nonsocial viewing task50 and marginally associated with autistic features in infants with FXS during a non-social visual attention task51. Tonnsen and colleagues50 found that HRDSA was able to predict group status (i.e., infant siblings of autistic children and neurotypical infants) better than behavioral looking alone. It is important to note, however, that while these studies provide a unique insight into HRDSA as an index of attention, they primarily utilized manual coding of eye gaze to index behavioral states of attention rather than eye-tracking methods, which is a time- and labor-intensive process. Eye-tracking, on the other hand, has the potential to provide an automatic and precise estimate of time spent looking at a screen, although it does not record time that a child spends looking away. Nevertheless, a multi-method approach including eye-tracking and physiological indices offers the potential for quickly gathering precise insight into processes of social attention, greatly facilitating more widespread use of multimethod approaches49. For example, Helminen and colleagues found a lack of orienting response to direct gaze in young autistic children with high support needs using both eye-tracking and heart rate-defined measures of orienting, highlighting the utility of this particular approach in neurodiverse populations39.
Present study
In sum, although much existing eye-tracking research has assessed social attention in neurodevelopmental disorders using visual gaze, relatively few have incorporated heart activity as a supplemental measure. Also, there are limited multimethod studies of social attention in neurodiverse populations with high support needs, including those with low cognitive ability15,39. Therefore, the present study aimed to (1) evaluate the potential difference in HRDSA between preschoolers with non-syndromic autism and intellectual disability (AS), FXS, and neurotypical (NT) development during a social eye-tracking task, and (2) determine whether HRDSA relates to visual social attention captured with eye-tracking. In line with prior eye-tracking work, we hypothesized that children with non-syndromic autism and those FXS would engage in less physiological social attention than their neurotypical peers13. Relatedly, we hypothesized that physiological social attention would be closely linked in all groups48.
Linear mixed models were estimated using the lmer package in R52,53. Four within-child, between-group models of each of four primary variables were estimated: proportion of time spent in periods of HRDSA (%HRDSA); the change in inter-beat interval (IBI) during periods of HRDSA in milliseconds (ΔIBI); average IBI during periods of HRDSA (IBI); and the proportion of time visually attending to the eye-tracking experiment (%Looking). Effects of group, condition, and the group*condition interaction were also modeled. To evaluate the association between HRDSA and eye-tracked social attention, we estimated an additional mixed effects model. Specifically, %HRDSA was estimated with effects of %Looking, group, condition, and their two-way and three-way interactions. The estimated trend of the relationship between %HRDSA and %Looking was the primary outcome of interest.
Statistical power was estimated using G*Power54. An estimated effect size of d = 0.9 was used based on results reported in Tonnsen and colleagues’ study of heart rate defined sustained attention in children at high and low likelihood of neurodevelopmental disabilities50. Alpha was set to 0.05 and power to 0.80. With these parameters, the present study was powered to detect a significant between group difference with a sample of n = 19 in each group, or n = 22 in one group and n = 16 in another, with an allocation of 1.4. These proposed sample sizes are comparable or larger than previous studies in this area39,50,51.
Results
Research question 1
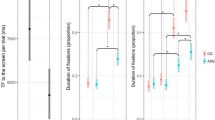
Full model results are presented in Table 1. Results from linear mixed models indicated that there were no group or condition effects in %HRDSA, nor was there a significant interaction (all ps > 0.05; Fig. 1). There was a significant main effect of condition on ΔIBI that was driven by the Sandwich condition (χ2(df) = 15.2(3), p = .002). That is, children exhibited shallower IBI decelerations during the Sandwich condition relative to all other conditions (Fig. 2). There were significant main effects of group and condition for average IBI. Specifically, for the main effect of group, autistic children t(df) = -4.17(52.1) ; p = .001) and FXS t(df) = -3.52(51.9); p = .000) had significantly shorter average IBI during HRDSA periods relative to NT children, but not each other (t(df) = -0.194(52.0); p = .846); Fig. 3). For the main effect of condition, average IBI was shorter for all children during the Sandwich condition relative to the Dyadic Bid (t(df) = -3.20(92.6); p = .0019) and Moving Toys conditions (t(df) = 3.024(92.4); p = .003), but not for the Joint Attention Condition (t(df) = 1.734(92.3); p = .086; Fig. 4). Finally, for %Looking, there was a significant group effect, such that autistic children spent significantly less time visually attending to the scene throughout the experiment relative to NT children (t(df) = -3.27(61.9.6); p = .002). Children with FXS did not differ from either the AS t(df) = -1.21(61.6), p = .232) or NT t(df) = -1.77(61.8), p = .081) groups in their visual attention to the scene (Fig. 1).
Research question 2
Results from the model evaluating associations between HRDSA and eye-tracked visual attention across groups revealed a significant positive relationship between %HRDSA and %Looking (B = 0.569, χ2(df) = 4.71(1), p = .03), with no other significant model effects (all ps > 0.05), suggesting an association between HRDSA and eye-tracked visual attention independent of group status and experimental condition (Table 2).
Discussion
Despite a wide body of literature demonstrating reduced visual social attention in children with non-syndromic autism and FXS relative to neurotypical controls, limited work has taken a biobehavioral, mixed methods approach to measuring social attention in this population. Hence, the present study had two primary aims. First, to evaluate potential differences in HRDSA between preschoolers with non-syndromic autism and ID, FXS, and neurotypical development during a social eye-tracking task. Second, to determine whether HRDSA relates to visual social attention to quantify and describe alternative approaches to understand social attention and engagement in these populations. Overall, our findings demonstrated unique patterns of social attention in children with autism and FXS relative to their neurotypical peers.
First, our findings did not indicate overall differences in the proportion of time spent in HRDSA in either clinical sample relative to neurotypical children. Our results also revealed distinct patterns of physiological differences in social attention across the course of our experiment that highlight the vulnerabilities present in children with autism and FXS. Interestingly, our finding of a condition effect, but not a diagnosis effect in change in IBI during periods of HRDSA, suggests that all children modulated their HRSDA in expected ways based on the experimental video content. That is, they exhibited significantly shallower IBI deceleration during silent, non-social conditions (e.g., when the actress was looking down and engaged in an activity) relative to the other experimental conditions, which were more social or visually/auditorily salient. However, both autistic children and those with FXS had shorter average IBI during periods of HRDSA relative to neurotypical children throughout the experiment. It is worth noting that the current study did not take into account overall baseline differences in IBI, so it is unknown whether the differences in IBI observed are task-specific or reflect global differences in arousal in autism and FXS. Nevertheless, our current findings are in line with an established “hyperarousal hypothesis” in children with FXS, as shorter IBI is indicative of overall ANS dysfunction45. Although the research on hyperarousal in non-syndromic autism is mixed55the present study provides some evidence for physiological vulnerabilities in autistic children, particularly during periods of sustained attention.
In terms of visual social attention, our results align with prior work and suggest that autistic children, but not those with FXS, spend less time visually attending to social information than their neurotypical peers13,35,36. That is, children with FXS did not differ from neurotypical or autistic children in their overall visual attention to social scenes. This finding is particularly interesting in light of the lack of observed group differences in physiological social attention (i.e., HRDSA). Nevertheless, our findings do provide evidence that these two measures are moderately related in all groups and experimental conditions and thus may provide complementary information about overall social engagement. Taken together, these findings provide evidence for the utility of integrated measurements of attention and engagement. Future studies should continue to look beyond pure measures of looking behavior to index social attention. This is particularly important in light of research aligned with supporting neurodiverse perspectives highlighting the negative cognitive and social consequences of sustained eye contact21,41,56. The present study could thus offer a supplemental measure of social attention that is potentially more affirming of neurodivergent strategies and not reliant on looking behavior alone.
The present study takes an integrated biobehavioral approach to measuring social attention using eye-tracking and physiological measures, and findings offer an important extension of our current understanding of social attention in autism and FXS. Nevertheless, it is not without its limitations. First, we took a categorical approach to defining our diagnostic groups; future work could consider examining continuous measures of autism features and other aspects of phenotypic variability (e.g., intellectual ability, language) as correlates of social attention. Second, the present study examined only global measures of social attention across the duration of the experiment and therefore could not draw specific conclusions about how shifts in visual social attention or attention to specific regions (e.g., faces) manifest as changes in HRDSA beyond broader between-condition effects. Future research should take a more fine-grained approach to examine the temporal aspects of social attention, especially considering evidence that there are temporal differences in visual attention in FXS specifically57. Additionally, the present study was unable to address potential confounds related to heart rate variability and respiration, which could impact the interpretation of the findings. Future research should include measures such as respiratory sinus arrhythmia or respiration to better distinguish and isolate attentional processes from general autonomic reactivity.
Taken together, the present study demonstrates that HRDSA can provide unique and valuable information about social attention in neurodevelopmental disorders and suggests that looking behavior is correlated with physiological measures of attention. Combining physiological and behavioral measures of attention may be particularly important in children with neurodevelopmental disabilities for whom typical assessments may not be accessible.
Methods
Participants
Data were collected from preschoolers with non-syndromic autism (AS; n = 23), FXS (n = 16), and neurotypical controls (n = 22) who were participants in longitudinal studies of early development at the University of South Carolina (National Institutes of Health R01MH90194; R01MH107573; PI: Roberts). Children were included in the present study if they had complete data on relevant measures. Inclusion criteria included gestational age ≥ 37 weeks, English as the primary language in the home, and no uncorrected hearing or vision impairments. Recruitment was primarily conducted through research medical sites and social media networks specializing in autism or FXS. A majority of the current sample (80%) overlaps with that of a previous study, which did not include HRDSA measures or examine visual social attention across the entire SSA task13. A detailed description of participant demographics can be found in Table 3.
Group assignment was made at intake using existing genetic or psychological reports. Those in the FXS group were confirmed through a review of a genetic report documenting > 200 repeats of the CGG sequence on the Fragile X messenger ribonucleoprotein 1 (FMR1) gene. Those in the AS group had an existing community diagnosis of autism and evidence of developmental delays. Although no formal genetic testing was done to rule out underlying genetic syndromes in the AS group, participants in the AS group were required to have no known genetic syndromes. Intellectual ability was assessed using the Mullen Scales of Early Learning (MSEL) Early Learning Composite58,59 and autism diagnoses were informed by the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2)60. Autism diagnosis and the presence of co-occurring intellectual disability (i.e., Mullen Early Learning Composite score of 70 or below) in the FXS and AS groups were confirmed at enrollment using Clinical Best Estimate (CBE) procedures27. Participants enrolled in the neurotypical group were required to have no family history of autism and were confirmed not to have autism or developmental delay (i.e., Mullen Early Learning Composite scores greater than 70) through study participation.
Procedures
As a part of their participation, children completed a battery of measures including intellectual/developmental ability, autism features, and multiple indices of social attention. All procedures were approved by the Institutional Review Board at the University of South Carolina, and parents provided written informed consent before enrollment in the study, and all experiments were performed in accordance with relevant guidelines and regulations.
Measures
Heart-rate defined sustained attention (HRDSA)
Heart activity was collected during the eye-tracking presentation via electrocardiogram (ECG) with an Actiwave Cardio Monitor (Alive Technologies; CamNtech Ltd., Cambridge, UK) at 1024 Hz. Trained research assistants edited ECG data to correct false heart periods and artifacts using CardioEdit software, and only ≤ 10% of beats were edited61. Interbeat interval (i.e., IBI, the time in milliseconds between consecutive heartbeats) series data were extracted from CardioEdit and used to determine HRDSA.
The start of the eye-tracking task was synced to the heart activity data using audio cues from behavioral video. Baseline IBI was defined as the median IBI of the first 5 beats of the eye-tracking video. The start of HRDSA began after 5 successive beats with IBIs longer than baseline IBI, and HRDSA terminated after 5 successive beats with IBIs shorter than baseline IBI. HRDSA was quantified using three primary variables: proportion of time in HRDSA (%HRDSA), average heart rate deceleration during HRDSA in milliseconds (ΔIBI), quantified as the average change in IBI from baseline during periods of HRDSA, and average IBI during HRDSA overall (IBI). While measures of HRDSA have typically investigated phasic heart rate decelerations time-locked to discrete stimuli over short durations39,40,41,42,43,44,45this application was grounded in prior literature demonstrating that extended heart rate deceleration can reflect sustained attention during continuous, engaging stimuli50,62.
Visual social attention
Visual social attention was measured as the total time looking at any point on the screen (%Looking) during the presentation of the Selective Social Attention (SSA) task35. The experimental stimulus depicted a 3-minute dynamic video scene depicting an adult female actress seated at a table surrounded by four mechanical toys35. Interspersed throughout the video were four experimental conditions designed to evoke different patterns of looking behavior: (1) the actress engaged the viewer in child-directed speech (Dyadic Bid); (2) the actress looked down at the table and made a sandwich without direct gaze or speech (Sandwich); (3) the actress looked up briefly at the camera and said ‘uh-oh’ as she turned toward one of the toys (Joint Attention); and (4) after the actress looked up at the camera, a toy began to move and make noises (Moving Toys).
Eye-tracking data were collected on an SR Eyelink 1000 Plus eye tracker. The stimulus was built and presented with SR Experiment Builder using monocular, remote eye tracking on a 24-inch widescreen LCD monitor with a 60-Hz refresh rate, 16:9 (1366 × 768 pixel) aspect ratio, and a 65 cm participant eye-to-monitor distance. Children were shown a cartoon to adjust to the experimental setting. A five-point calibration preceded the experiment for all participants, and five-point validation and calibration quality were assessed through a manufacturer-defined five-point validation63. A behavioral camera recorded the child’s face and the monitor to allow for syncing of heart rate data with the eye-tracking stimulus presentation. Participants were excluded from analyses if the amount of valid eye-tracking data was less than 20%.
Eye-tracking data were processed with custom Matlab software that accommodated standard techniques for processing eye-tracking data including blink detection, data calibration, and Region of Interest (ROI) analysis64. Data reduction and analysis were carried out through programs written in R53.
Data availability
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
References
Jones, W. & Klin, A. Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature 504, 427–431 (2013).
Charman, T. et al. Testing joint attention, imitation, and play as infancy precursors to Language and theory of Mind. Cogn. Dev. 15, 481–498 (2000).
Mundy, P. & Newell, L. Attention Joint attention, and social cognition. Curr. Dir. Psychol. Sci. 16, 269–274 (2007).
Salley, B. & Colombo, J. Conceptualizing social attention in developmental research. Soc. Dev. 25, 687–703. https://doi.org/10.1111/sode.12174 (2016).
Vallorani, A. et al. Relations between social attention, expressed positive affect and behavioral Inhibition during play. Dev. Psychol. 58, 2036–2048 (2022).
Falck-Ytter, T., Kleberg, J. L., Portugal, A. M. & Thorup, E. Social attention: developmental foundations and relevance for autism spectrum disorder. Biol. Psychiatry 94, 8–17. https://doi.org/10.1016/j.biopsych.2022.09.035 (2023).
Frazier, T. W. et al. A Meta-Analysis of gaze differences to social and nonsocial information between individuals with and without autism. J. Am. Acad. Child. Adolesc. Psychiatry 56, 546–555 (2017).
Chita-Tegmark, M. Social attention in ASD: a review and meta-analysis of eye-tracking studies. Res. Dev. Disabil. 48, 79–93. https://doi.org/10.1016/j.ridd.2015.10.011 (2016).
Tonnsen, B. L., Grefer, M. L., Hatton, D. D. & Roberts, J. E. Developmental trajectories of attentional control in preschool males with fragile X syndrome. Res. Dev. Disabil. 36, 62–71 (2015).
Chernenok, M., Burris, J. L., Owen, E. & Rivera, S. M. Impaired attention orienting in young children with fragile X syndrome. Front. Psychol. 10, 1567 (2019).
Scerif, G. et al. To look or not to look? Typical and atypical development of oculomotor control. J. Cogn. Neurosci. 17, 591–604 (2005).
Bradshaw, J. et al. The use of eye tracking as a biomarker of treatment outcome in a pilot randomized clinical trial for young children with autism. Autism Res. 12, 779–793 (2019).
Wall, C. A., Shic, F., Varanasi, S. & Roberts, J. E. Distinct social attention profiles in preschoolers with autism contrasted to fragile X syndrome. Autism Res. https://doi.org/10.1002/aur.2857 (2022).
Frazier, T. W. et al. Social attention as a cross-cultural transdiagnostic neurodevelopmental risk marker. Autism Res. 14, 1873–1885 (2021).
Tager-Flusberg, H. et al. Conducting research with minimally verbal participants with autism spectrum disorder. Autism 21, 852–861 (2016).
Klin, A., Shultz, S. & Jones, W. Social visual engagement in infants and toddlers with autism: early developmental transitions and a model of pathogenesis. Neurosci. Biobehav. Rev. https://doi.org/10.1016/j.neubiorev.2014.10.006 (2014).
Shic, F. Eye tracking as a behavioral biomarker for psychiatric conditions: the road ahead. J. Am. Acad. Child. Adolesc. Psychiatry. 55, 267–268 (2016).
McKinney, A. et al. Overcoming hurdles to intervention studies with autistic children with profound communication difficulties and their families. Autism 25, 1627–1639. https://doi.org/10.1177/1362361321998916 (2021).
Tager-Flusberg, H. & Kasari, C. Minimally verbal school-aged children with autism spectrum disorder: the neglected end of the spectrum. Autism Res. 6, 468–478. https://doi.org/10.1002/aur.1329 (2013).
Richards, J. E. The development of sustained visual attention in infants from 14 to 26 weeks of age. Psychophysiology 22, 409–416 (1985).
Hadjikhani, N. et al. Look me in the eyes: constraining gaze in the eye-region provokes abnormally high subcortical activation in autism. Sci Rep 7, 1245 (2017).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM 5 (American Psychiatric Pub, 2013).
Shaw, K. A. et al. Prevalence and early identification of autism spectrum disorder among children aged 4 and 8 Years-Autism and developmental disabilities monitoring network, 16 sites, united states, 2022. MMWR Surveil. Summar. 74, 1423 (2025).
Hunter, J. et al. Epidemiology of fragile X syndrome: a systematic review and meta-analysis. Am. J. Med. Genet. A. 164, 1648–1658 (2014).
Loesch, D. Z., Huggins, R. M. & Hagerman, R. J. Phenotypic variation and FMRP levels in fragile X. Ment Retard. Dev. Disabil. Res. Rev. 10, 31–41 (2004).
Tassone, F., R. H.-A. journal of & 2000. Transcription of the FMR1 gene in individuals with fragile X syndrome (Wiley Online Library, 2024).
Roberts, J. E. et al. Emergence and rate of autism in fragile X syndrome across the first years of life. Dev. Psychopathol. 32, 1335–1352 (2020).
Hall, S., DeBernardis, M. & Reiss, A. Social escape behaviors in children with fragile X syndrome. J. Autism Dev. Disord. 36, 935–947 (2006).
Hall, S. S., Lightbody, A. A., Huffman, L. C., Lazzeroni, L. C. & Reiss, A. L. Physiological correlates of social avoidance behavior in children and adolescents with fragile X syndrome. J. Am. Acad. Child. Adolesc. Psychiatry. 48, 320–329 (2009).
Hall, S. S., Lightbody, A. & Reiss, A. Compulsive, self-injurious, and autistic behavior in children and adolescents with fragile X syndrome. Am. J. Ment. Retard. 113, 44–53 (2008).
Hong, M. P. et al. Differentiating social preference and social anxiety phenotypes in fragile X syndrome using an eye gaze analysis: a pilot study. J. Neurodev Disord. 11, 1 (2019).
Wall, C. A. et al. Eye-tracking measures of social versus nonsocial attention are related to level of social engagement during naturalistic caregiver‐child interactions in autistic children. Autism Res. https://doi.org/10.1002/aur.2920 (2023).
Falck-Ytter, T., Bölte, S. & Gredebäck, G. Eye tracking in early autism research. J. Neurodev Disord. 5, 1–13 (2013).
Venker, C. E. et al. Comparing automatic eye tracking and manual gaze coding methods in young children with autism spectrum disorder. Autism Res. 13, 271–283 (2020).
Chawarska, K., Macari, S. & Shic, F. Context modulates attention to social scenes in toddlers with autism. J. Child. Psychol. Psychiatry. https://doi.org/10.1111/j.1469-7610.2012.02538.x (2012).
Shic, F. et al. The selective social attention task in children with autism spectrum disorder: results from the autism biomarkers consortium for clinical trials (ABC-CT) feasibility study. Autism Res. https://doi.org/10.1002/aur.3026 (2023).
Chawarska, K., Macari, S. & Shic, F. Decreased spontaneous attention to social scenes in 6-month- old infants later diagnosed with ASD Katarzyna. Biol. Psychiatry. 74, 195–203 (2013).
Murias, M. et al. Validation of eye-tracking measures of social attention as a potential biomarker for autism clinical trials. Autism Res. 11, 166–174 (2018).
Helminen, T. M. et al. Atypical physiological orienting to direct gaze in low-functioning children with autism spectrum disorder. Autism Res. 10, 810–820 (2017).
Richards, J. E. & Casey, B. J. Heart rate variability during attention phases in young infants. https://doi.org/10.1111/j.1469-8986.1991.tb03385.x (1991).
Doherty-Sneddon, G., Riby, D. M. & Whittle, L. Gaze aversion as a cognitive load management strategy in autism spectrum disorder and Williams syndrome. J. Child. Psychol. Psychiatry. 53, 420 (2012).
Brez, C. C. & Colombo, J. Your eyes say no, but your heart says yes: behavioral and psychophysiological indices in infant quantitative processing. Infancy 17, 445–454 (2012).
Porges, S. W. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin. J. Med 76, 412 (2009).
Patriquin, M. A., Scarpa, A., Friedman, B. H. & Porges, S. W. Respiratory sinus arrhythmia: a marker for positive social functioning and receptive Language skills in children with autism spectrum disorders. Dev. Psychobiol. 55, 101–112 (2013).
Hogan, A. et al. Trajectories of heart activity across infancy to early childhood differentially predict autism and anxiety symptoms in fragile X syndrome. Front. Psychiatry. 12, 1–13 (2021).
Wall, C. A. & Roberts, J. E. Negative affect and respiratory sinus arrhythmia are differentially related to social anxiety and autism features in autistic preschoolers contrasted to fragile X syndrome. Front. Psychiatry 14, 1452 (2023).
Reynolds, G. D. & Richards, J. E. Infant heart rate: a developmental psychophysiological perspective. In Developmental Psychophysiology: Theory, Systems, and Methods (eds. Schmidt, L. A. & Segalowitz, S. J.) 173–212 (Cambridge University Press, 2008).
Porges, S. W. The polyvagal perspective. Biol. Psychol. 74, 116–143 (2007).
Bradshaw, J., Fu, X. & Richards, J. E. Infant sustained attention differs by context and social content in the first 2 years of life. Dev. Sci. E. 2024, 13500. https://doi.org/10.1111/DESC.13500 (2024).
Tonnsen, B. L., Richards, J. E. & Roberts, J. E. Heart rate-defined sustained attention in infants at risk for autism. J. Neurodev Disord. 10, 1–13 (2018).
Roberts, J. E., Hatton, D. D., Long, A. C. J., Anello, V. & Colombo, J. Visual attention and autistic behavior in infants with fragile X syndrome. J. Autism Dev. Disord. 42, 937–946 (2012).
Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. lmerTest package: tests in linear mixed effects models. J Stat. Softw 82, 1456 (2017).
R Core Team. R: A language and environment for statistical computing (2021). https://www.r-project.org/.
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 39, 175–191 (2007).
Klusek, J., Roberts, J. E. & Losh, M. Cardiac autonomic regulation in autism and fragile X syndrome: a review. Psychol. Bull. 141, 141–175 (2015).
Trevisan, D. A., Roberts, N., Lin, C. & Birmingham, E. How do adults and teens with self-declared autism spectrum disorder experience eye contact? A qualitative analysis of first-hand accounts. PLoS One. 12, e0188446 (2017).
Wall, C. A., Shic, F., Will, E. A., Wang, Q. & Roberts, J. E. Similar Gap-Overlap profiles in children with fragile X syndrome and IQ-Matched autism. J. Autism Dev. Disord. https://doi.org/10.1007/s10803-024-06245-1 (2024).
Mullen, E. Mullen scales of early learning: AGS edition. Scales of Early Learning.docx. http://www.v-psyche.com/doc/special-cases/Mullen (1995).
Elliott, C. D., Murray, G. J. & Pearson, L. S. Differential ability scales-II (1990).
Lord, C. et al. Autism Diagnostic Observation Schedule: ADOS-2 (Western Psychological Services, 2012).
Brain-Body Center U. of I. at C. CardioEdit software (2007).
Roberts, J. E., Tonnsen, B., Robinson, A. & Shinkareva, S. V. Heart activity and autistic behavior in infants and toddlers with fragile X syndrome. Am. J. Intellect. Dev. Disabil. 117, 90–102 (2012).
SR Research Ltd. EyeLink ® 1000 User Manual Tower, Desktop, LCD Arm, Primate and Long Range Mounts Remote, 2000 Hz and Fiber Optic Camera Upgrades (2005).
Shic, F. Computational methods for eye-tracking analysis: applications to autism. Dissertation Abstr. Int. Sect. B: Sci. Eng. 70, 420 (2009).
Acknowledgements
This work was funded by the National Institute of Mental Health: R01MH90194 (PI: Roberts), R01MH090194 (PI: Roberts), 1R01MH107573 (PI: Roberts), NICHD F32HD097877 (PI: Will), NICHD K99HD105980 (PI: Will), and the University of South Carolina’s Support to Promote Advancement of Research and Creativity (SPARC; PI: Wall). We sincerely thank the families that contributed to this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the study. KS, FS, and BK developed the procedures for analyzing the data. CAW made extensive contributions to the analysis and interpretation of the data, and CAW and KS drafted the manuscript. The remaining authors were extensively involved in revisions. All authors have given final approval for the version to be published.
Corresponding author
Ethics declarations
Competing interests
FS consults for Roche, Janssen, and MindMed pharmaceutical companies. The remaining authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wall, C.A., Smith, K., Shic, F. et al. Heart rate defined sustained attention relates to visual attention in autism and fragile X syndrome. Sci Rep 15, 25389 (2025). https://doi.org/10.1038/s41598-025-09537-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09537-3