Abstract
Acute-on-chronic liver failure (ACLF) exhibits etiological heterogeneity across regions, with hepatitis B virus (HBV)-related ACLF predominant in China and alcohol-related ACLF dominating Western populations. This multicenter retrospective study systematically compared clinical profiles of HBV-related (n = 659) and alcohol-related ACLF (n = 296) stratified by the World Gastroenterology Organization (WGO) A/B/C classification, reflecting underlying chronic liver disease severity. Compared to HBV-related ACLF, alcohol-related ACLF showed higher systemic inflammation (leukocytosis, neutrophilia), bacterial infection (P < 0.001), extrahepatic organ failures (single-organ: renal, brain and respiratory, all P < 0.05; multi-organ: P < 0.001) and higher CLIF-C ACLF/COSSH-ACLF II scores. Conversely, HBV-related ACLF exhibited acute hepatocellular injury (elevated ALT/AST), and higher MELD/MELD-Na scores. These etiological disparities were most pronounced in type C ACLF. Despite these distinct profiles, mortality did not differ between etiologies. Type C ACLF demonstrated poorest profiles and uniformly high 90-day mortality (> 45%) regardless of etiology driven by cumulative organ failure burden. Importantly, CLIF-C ACLF and COSSH-ACLF II scores outperformed MELD and MELD-Na scores in predicting outcomes for type C patients. These findings underscore the critical influence of diverse etiologies and severity stages of underlying chronic liver diseases on ACLF profiles and outcomes, thereby necessitating stratified management approaches tailored to underlying chronic liver disease to ultimately improve patient outcomes.
Similar content being viewed by others
Introduction
Acute-on-chronic liver failure (ACLF) has emerged as a critical global health challenge, characterized by rapid progression and exceptionally high short-term mortality1. However, the lack of universally accepted diagnostic criteria substantially impedes early identification and timely intervention2. This diagnostic ambiguity stems not only from the complex pathophysiology of ACLF but also from apparent regional disparities in underlying etiologies1,2,3,4,5.
In Eastern countries, particularly in HBV-endemic China, HBV remains the leading cause of ACLF, despite a gradual rise in alcohol-related cases in recent years6,7,8. In contrast, alcohol-related liver disease predominates as the etiology in Western populations9,10,11. These regional differences in etiology have resulted in divergent diagnostic frameworks. For instance, the Asian Pacific Association for the Study of the Liver (APASL) defines ACLF as acute hepatic insults superimposed on chronic hepatitis or well-compensated cirrhosis, with a primary focus on liver failure itself6. In contrast, the European Association for the Study of the Liver (EASL) Chronic Liver Failure (CLIF) consortium incorporates both compensated and decompensated cirrhosis in defining chronic liver disease and concentrates much more on the presence of extrahepatic organ failure (EHOF) when defining ACLF9. To reconcile the global disparities in ACLF definitions, the World Gastroenterology Organization (WGO) has put forward the A/B/C classification system, aligning with the progressive severity of the underlying chronic liver disease12. However, the distinct disease trajectories exhibited by HBV-related and alcohol-related ACLF, based on the WGO classification, remain poorly characterized. A deeper understanding of these trajectory differences is crucial for optimizing treatment approaches and improving patient prognosis.
To address this unmet need, our multicenter study systematically characterized HBV-related versus alcohol-related ACLF through a comparative analysis based on the WGO classification. By elucidating the implications of diverse etiologies and chronic liver disease severity on the clinical profiles of ACLF, we aim to lay a foundation for stratified management strategies tailored to underlying chronic liver disease, ultimately improving patient survival and guiding future clinical practice.
Methods
Patients, study design and data collection
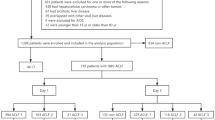
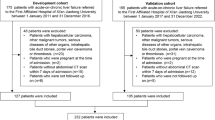
The study group selection process is outlined in Fig. 1. This retrospective multicenter study reviewed data from 1193 patients hospitalized for acute deterioration of liver function from January 2008 to October 2019 at Tianjin Third Central Hospital, as well as from November 2012 to June 2019 at Fifth Medical Center of Chinese PLA General Hospital, Beijing You’an Hospital, Shandong Provincial Hospital, First Hospital of Shanxi Medical University, and Third Hospital of Hebei Medical University. The inclusion criteria were occurrence of liver failure, manifesting as jaundice with a TBil ≥ 5 mg/dL and coagulation dysfunction (international normalized ratio [INR] ≥ 1.5 or prothrombin activity < 40%) within 4 weeks after an acute insult. Based on the WGO criteria12, all eligible patients were further categorized into three types based on the severity of chronic liver disease as follows: type A for patients without cirrhosis, type B for patients with well-compensated cirrhosis, and type C for patients with previous decompensated cirrhosis. The exclusion criteria as follows: (1) non-HBV and non-alcohol etiologies; (2) overlapping HBV and alcohol-related etiologies; (3) hepatocellular carcinoma or other malignant tumors; (4) severe chronic extra-hepatic disease, such as severe chronic kidney disease with renal failure [Estimated Glomerular Filtration Rate (eGFR) < 30 mL/min/1.73 m2 for ≥ 3 months], severe chronic obstructive pulmonary disease with respiratory failure [Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage III/IV], severe coronary heart disease with heart failure [New York Heart Association (NYHA) class III/IV] and severe coagulation failure caused by hematological system diseases; (5) post-liver transplantation; (6) concomitant human immunodeficiency virus infection; (7) patients with incomplete clinical indicator information.
All data were retrospectively collected from manual and electronic medical records. For each study patient, we collected baseline data from demographics (e.g., age and sex), etiology of chronic liver disease (e.g., HBV and alcohol), the nunber and types of acute precipitating events detected before the occurrence of liver failure [e.g.,HBV reactivation, active alcoholism, hepatotoxic drugs, bacterial Infection, gastrointestinal haemorrhage, others (overwork, surgery and non-HBV hepatotropic viral infection), none and multiple (two or more) precipitating events], acute precipitating events (e.g., no identifiable, hepatic insult, extra-hepatic insult or both), laboratory parameters [e.g., white blood cell (WBC) count, percentage of neutrophils (N%), hemoglobin (Hb), platelets (PLT), albumin (Alb), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (γ-GT), TBil, INR, creatinine (Cr) and serum sodium (Na)], complications during follow-up [e.g., ascites, bacterial infection, gastrointestinal haemorrhage, hepatic encephalopathy (HE)], the number and types of EHOF, and prognostic scoring systems scores [Model for End Stage Liver Disease (MELD)13, MELD-sodium (MELD-Na)14, Chronic Liver Failure-Consortium ACLF (CLIF-C ACLF)15 and Chinese Group on the Study of Severe Hepatitis B-ACLF II (COSSH-ACLF II)]16. In addition, the outcomes at 28-day and 90-day follow-up were collected for each study patient.
All study procedures complied with the ethical principles of the Declaration of Helsinki. This retrospective study was approved by the Ethics Committees of Tianjin Third Central Hospital, Beijing You’an Hospital, Shandong Provincial Hospital, First Hospital of Shanxi Medical University, Third Hospital of Hebei Medical University, and Fifth Medical Center of Chinese PLA General Hospital. Written informed consent was waived by the Ethics Committees of Tianjin Third Central Hospital, Beijing You’an Hospital, Shandong Provincial Hospital, First Hospital of Shanxi Medical University, Third Hospital of Hebei Medical University, and Fifth Medical Center of Chinese PLA General Hospital due to the retrospective design of the study.
Definitions
EHOF was diagnosed by the following criteria11,15: (1) brain failure: West-Haven grade III–IV, (2) renal failure: Cr ≥ 2 mg/dL or use of renal replacement therapy, (3) coagulation failure: INR ≥ 2.5, (4) circulatory failure: use of vasoactive drugs, (5) respiratory failure: PaO2/FiO2 ≤ 200 or SpO2/FiO2 ≤ 214 or the need for mechanical ventilation. According to the number of EHOF, multiple extrahepatic organ failure (MEHOF) was defined as two or more EHOF.
Statistical analysis
Continuous variables were described as means ± standard deviation or median [interquartile range (IQR)] and compared using the t-test, Mann–Whitney U test, one-way ANOVA or Kruskal–Wallis test, as appropriate. Categorical variables were expressed as frequency (%), and properly compared using chi-squared test or Fisher’s exact tests. We performed univariate analysis to compare characteristics between survivors and non-survivors. Variables with significant inter-group differences (P < 0.05) were entered into multivariable Cox proportional hazards regression models to identify independent predictors of 28- and 90-day mortality. Results are presented as hazard ratios (HR) with 95% confidence intervals. Kaplan–Meier analysis was used to plot 28- and 90-day survival curves, and survival rates were compared using the log-rank test. The areas under the receiver operating characteristic curve (AUROCs) of the various prognostic scoring systems were compared using the Z test with Delong’s method.
All the statistical analysis were performed by R software version 4.4.1. A two-sided P value < 0.05 was considered statistical significance.
Results
Description of the study population
As illustrated in Fig. 1, this study enrolled a total of 955 eligible patients who met the inclusion and exclusion criteria. Of these, 69.01% (659/955) had HBV-related ACLF, while 30.99% (296/955) had alcohol-related ACLF. According to the WGO classification, the HBV-related ACLF cohort comprised 122 patients (18.51%) with type A, 221 patients (33.54%) with type B, and 316 patients (47.95%) with type C ACLF. In contrast, the alcohol-related ACLF cohort included 20 patients (6.75%) classified as type A, 71 patients (23.99%) as type B, and 205 patients (69.26%) as type C ACLF.
Within the overall study cohort, 142 patients (14.87%) were classified as type A ACLF, 292 patients (30.57%) as type B ACLF, and 521 patients (54.55%) as type C ACLF. The cohort was predominantly male (83.46%), with a mean age of 48.66 years. In terms of organ failures, coagulation failure (32.25%) was the most common type of EHOF, followed by brain failure (8.06%), renal failure (7.75%), respiratory failure (6.49%), and circulatory failure (5.45%). Additionally, 278 (29.11%) patients developed single EHOF, 137 (14.35%) patients developed MEHOF. The overall 28-day and 90-day mortality rates were 24.50% and 36.65%, respectively (Supplementary Table S1).
Comparison of clinical characteristics between HBV-related and alcohol-related ACLF
As presented in Table 1, ACLF patients with alcohol-related etiology was more frequently precipitated by active alcohol consumption, bacterial infection and multiple precipitants compared to those with HBV-related etiology (all P ≤ 0.001), whereas HBV reactivation was the most common trigger in patients with HBV-related etiology. Alcohol-related ACLF patients demonstrated significantly elevated WBC, N%, γ-GT and Cr compared to those with HBV etiology (all P < 0.01), while HBV-related ACLF patients had significantly higher serum Hb, Alb, ALT, AST and Na levels (all P < 0.001). Moreover, alcohol-related ACLF patients had a significantly higher incidence of complications, including ascites (P = 0.001), HE (P = 0.001), bacterial infection (P < 0.001), gastrointestinal hemorrhage (P < 0.001). EHOF were also more significantly frequent in the alcohol-related cohort. Specifically, alcohol-related ACLF had higher rates of single renal failure (P < 0.001), brain failure (P = 0.003), and respiratory failure (P = 0.016), as well as MEHOF (P < 0.001).
In addition, alcohol-related ACLF demonstrated higher CLIF-C ACLF and COSSH-ACLF II scores (both P < 0.001 vs. HBV) On the other hand, HBV-related ACLF displayed higher MELD and MELD-Na scores (both P < 0.001 vs. alcohol). Despite these differences, the 28-day and 90-day mortality rates did not significantly differ between etiologies within the overall cohort or any WGO type (all P > 0.05) (Table 1). As illustrated in Supplementary Fig. S1, Kaplan–Meier survival analysis showed no 28- and 90-day survival difference between etiologies (log-rank test: P = 0.20 for 28-day; P = 0.24 for 90-day).
In ACLF patients with HBV-related etiology, WGO type C, elevated TBil, gastrointestinal hemorrhage as a complication, cerebral failure, as well as higher MELD and CLIF-C ACLF scores were significantly associated with 28-day and 90-day mortality. In patients with alcohol-related etiology, elevated N% and higher CLIF-C ACLF scores were significantly associated with 28-day mortality, while lower albumin levels, along with complications such as bacterial infection and HE, were significantly associated with 90-day mortality (Table 2). A detailed univariate analysis is provided in Supplementary Table S2.
Comparison of clinical characteristics across WGO ACLF types (A/B/C)
A detailed comparison of clinical profiles and outcomes across WGO ACLF types within the overall cohort, HBV-related subgroup, and alcohol-related subgroup is presented in Supplementary Table S3, Tables 3 and 4, respectively.
Within the overall cohort, type C ACLF exhibited distinct clinical profiles compared to types A and B. Type C ACLF were more frequently precipitated by bacterial infection, gastrointestinal hemorrhage and multiple precipitants (all P < 0.05 vs. types A/B). In addition, laboratory parameters including Hb, Alb, PLT, ALT, AST, γ-GT, TBil and Na were significantly lower in type C (all P < 0.001), whereas WBC, N%, and Cr levels were elevated (all P < 0.05). Type C also demonstrated higher incidences of ascites, bacterial infection, HE, gastrointestinal hemorrhage, and EHOF (all P < 0.001). Specifically, renal (11.13%), brain (11.90%), respiratory (9.40%), circulatory (8.45%) failures, and multi-organ failure (MEHOF, 22.07%) were most prevalent in type C (all P < 0.001 vs. types A/B) (Supplementary Table S3).
Within each etiology (HBV and alcohol), as compared to types A and B, type C ACLF consistently exhibited lower serum levels of Hb, Alb, ALT, AST, γ-GT, and Na (all P < 0.01) and had a higher incidence of complications, including ascites, infections, HE, and hemorrhage (all P < 0.05). Single brain, respiratory, and circulatory failures were more frequent in type C regardless of etiology (all P < 0.05 vs. types A/B). MEHOF incidence was highest in type C (P < 0.001 vs. types A/B) (Tables 3 and 4).
Furthermore, type C ACLF scored highest across all severity scores: MELD-Na , CLIF-C ACLF, and COSSH-ACLF II (all P < 0.01 vs. types A/B).
Mortality rates escalated with disease severity. In the overall cohort, type C had the highest 28-day (type C vs. types A/B: 30.33% vs. 16.20%/18.15%, P < 0.001) and 90-day mortality (type C vs. types A/B: 45.68% vs. 21.13%/28.08%; P < 0.001) (Supplementary Table S3). This trend persisted in etiology-stratified analyses: HBV-related type C showed the highest 28-day mortality (type C vs. types A/B: 30.06% vs. 16.39%/17.65%; P < 0.001) and 90-day mortality (type C vs. types A/B: 45.57% vs. 22.13%/28.51%; P < 0.001) (Table 3), while alcohol-related type C exhibited the worst 28-day (type C vs. types A/B: 30.73% vs. 15.00%/19.72%, P < 0.001) and 90-day outcomes (type C vs. types A/B: 45.85% vs. 15.00%/26.76%; P = 0.001) (Table 4). As illustrated in Fig. 2, Kaplan–Meier analysis confirmed significantly reduced 28-/90-day survival for type C in the overall and HBV cohorts (log-rank test: P < 0.001). Alcohol-related type C showed no significant 28-day survival difference but significantly worse 90-day survival (log-rank test: P = 0.10 for 28-day; P = 0.0031 for 90-day).
Kaplan–Meier analysis of three ACLF types. (a) Comparison of 28-day survival of type A/B/C ACLF in overall ACLF cohort (log-rank test: overall: A vs. B vs. C, P < 0.0001; A vs.C, P = 0.0011; B vs. C, P = 0.00016). (b) Comparison of 90-day survival of type A/B/C ACLF in overall ACLF cohort (log-rank test: A vs. B vs. C, P < 0.0001, A vs.C, P < 0.0001; B vs. C, P < 0.0001). (c) Comparison of 28-day survival of type A/B/C ACLF in HBV-related ACLF cohort (log-rank test: overall: A vs. B vs. C, P = 0.00046; A vs.C, P = 0.0038; B vs.C, P = 0.0012). (d) Comparison of 90-day survival of type A/B/C ACLF in HBV-related ACLF cohort (log-rank test: A vs. B vs. C, P < 0.0001; A vs.C, P < 0.0001; B vs. C, P < 0.0001). (e) Comparison of 28-day survival of type A/B/C ACLF in alcohol-related ACLF cohort (log-rank test: A vs. B vs. C, P = 0.10; A vs.C, P = 0.19; B vs.C, P = 0.07). (f) Comparison of 90-day survival of type A/B/C ACLF in alcohol-related ACLF cohort (log-rank test: A vs. B vs. C, P = 0.0031, A vs.C, P = 0.022, B vs. C, P < 0.0071). ACLF, acute-on-chronic liver failure; HBV, hepatitis B virus
Multivariate Cox regression analysis identified independent prognostic factors for 28-day mortality in total ACLF patients as N%, Tbil, gastrointestinal hemorrhage as a complication, and higher MELD and CLIF-C ACLF scores. For 90-day mortality, independent predictors included single EHOF, higher MELD and CLIF-C ACLF scores, and complications such as bacterial infection, gastrointestinal hemorrhage, and HE (Table 2). We subsequently performed multivariable analysis to identify independent predictors of 28-day and 90-day mortality within each WGO ACLF type. In type A, higher MELD and CLIF-C ACLF scores were independent prognostic factors. In type B, age, Tbil, coagulatory failure, circulatory failure, and higher MELD and CLIF-C ACLF scores were independent prognostic factors. In type C, higher N%, cerebral failure, complications including gastrointestinal hemorrhage and HE, as well as higher MELD and CLIF-C ACLF scores were independent prognostic factors (Table 5). Univariate analysis of factors associated with ACLF prognosis in the overall cohort and across WGO types are presented in Supplementary Tables S2 and S4, respectively.
Performance of the prognostic scoring systems for predicting 28-day and 90-day mortality of patients with type A, B and C ACLF
Figure 3 illustrate the predictive performance of prognostic scoring systems for 28-day and 90-day mortality in patients with three ACLF types.
Performance of prognostic scoring systems for predicting 28-day and 90-day mortality rates across WGO ACLF types. (a–f) Receiver operating characteristic curves for the abilities of prognostic scoring systems to predict the 28-day mortality of patients with type A (a), type B (b) and type C (c) ACLF, as well as the 90-day mortality of patients with type A (d), type B (e) and type C (f) ACLF. (g) Prognostic performance of scoring systems for 28-day and 90-day mortality in type A/B/C ACLF. ACLF, acute-on-chronic liver failure; MELDs, Model for End Stage Liver Disease score; MELD-Nas, MELD-sodium score; CLIF-C ACLFs, Chronic Liver Failure-Consortium ACLF score; COSSH-ACLF IIs, Chinese Group on the Study of Severe Hepatitis B-ACLF II score; AUROC, area under the receiver operating characteristic curve; CI, confidence interval; Ref, accuracy of this model as compared to the other models in predicting mortality.
Through receiver operating characteristic (ROC) curve analysis, in the types A and B ACLF cohorts, the CLIF-C ACLF score did not exhibit significant discriminative ability over the MELD and MELD-Na scores. However, in the type C ACLF cohort with a history of decompensated cirrhosis, the CLIF-C ACLF score demonstrated comparable prognostic performance to the COSSH-ACLF II score but was significantly superior to the MELD and MELD-Na scores for predicting 28-day and 90-day mortality (AUROC: 28-day: CLIF-C ACLF vs. MELD, 0.758 vs. 0.690, P = 0.021; CLIF-C ACLF vs. MELD-Na, 0.758 vs. 0.678, P = 0.004; 90-day: CLIF-C ACLF vs. MELD, 0.734 vs. 0.669, P = 0.016; CLIF-C ACLF score vs. MELD-Na score, 0.734 vs. 0.656, P = 0.001).
Discussion
Through a systematic comparison of HBV-related and alcohol-related ACLF across disease severity stages (types A/B/C), this multicenter study provides a comprehensive characterization of distinct ACLF phenotypes differentiated by underlying chronic liver disease for the first time. It underscores ACLF as a heterogeneous syndrome shaped by etiology-specific pathophysiological mechanisms and underlying chronic liver disease severity. These insights highlight the necessity for stratified therapeutic paradigms tailored to underlying chronic liver disease.
The divergence between HBV-related and alcohol-related ACLF reflects fundamentally distinct pathophysiological mechanisms4,7,8,16,17,18,19,20. HBV-related ACLF, often precipitated by viral reactivation, manifests as acute hepatocellular injury evidenced by elevated transaminases (ALT/AST) and higher MELD/MELD-Na scores, reflecting severe hepatic dysfunction21. In contrast, our study found that ACLF patients with alcohol-related etiology was more frequently triggered by bacterial infection than by active alcohol consumption. These patients also had a higher susceptibility to bacterial infections during follow-up, as indicated by leukocytosis and neutrophilia, a consequence of systemic inflammation, immune dysregulation, and gut barrier dysfunction17,18,20,22,23. Our study further demonstrated that a high N% and bacterial infection were independent predictors of 28-day and 90-day mortality in this alcohol-related ACLF cohort. The systemic inflammatory milieu in these patients promotes the development of EHOF17,18,24, including renal failure (15.54% vs. 4.25%), brain failure (12.16% vs. 6.22%), respiratory failure (9.46% vs. 5.16%), and multi-organ failure (MEHOF: 21.96% vs. 10.93%). These observations are consistent with the CANONIC study, which showed that alcohol ACLF patients were characterized by high prevalence of bacterial infection and EHOF11. Critically, our data underscores the imperative for etiology-specific interventions: HBV-related ACLF demands urgent antiviral therapy and robust hepatic support, while alcohol-related cases require intensive infection control and systemic organ protection.
The WGO classification stratifies risk based on the severity of underlying chronic liver disease12. Patients with type C ACLF, who have previously decompensated cirrhosis and severely compromised hepatic reserve, emerge as the most severe phenotype across both etiologies, with a 90-day mortality rate exceeding 45%. This group of patients exhibits particularly rapid progression to EHOF following acute precipitants, likely due to the cumulative effects of portal hypertension, severely diminished hepatic reserve, systemic inflammation, and immunosuppression1,4,11,24,25,26. Additionally, the occurrence of cerebral failure, as well as higher MELD and CLIF-C ACLF scores were identified as independent risk factors for adverse outcome in this cohort. These findings indicate that patients with type C ACLF require aggressive surveillance for multiple organ failures, rather than monitoring for isolated liver failure.
Furthermore, this study, utilizing ROC curve analysis, demonstrates that the CLIF-C ACLF and COSSH-ACLF II scores significantly outperform the MELD and MELD-Na scores in predicting the prognosis of type C ACLF patients at high risk of developing EHOF. These findings align with emerging evidence suggesting that MELD and MELD-Na, which primarily assess liver function, may underestimate mortality in ACLF cases complicated by EHOF13,14,27. In contrast, the CLIF-C ACLF and COSSH-ACLF II scores15,16, by incorporating extrahepatic organ failure/dysfunction and WBC count, exhibit superior prognostic accuracy over the MELD/MELD-Na scores, particularly in subgroups with a high likelihood of developing EHOF, such as type C patients, as reported by Shi et al.28. Moreover, alcohol-related cases in our study demonstrated significantly higher CLIF-C ACLF scores (44.33 vs. 40.56 for HBV-related cases, P < 0.001) and COSSH-ACLF II scores (6.10 vs. 4.99 for HBV-related cases, P < 0.001), consistent with their predisposition to EHOF. Considering COSSH-ACLF II score was originally developed for HBV-related ACLF16, its potential applicability to non-HBV-related ACLF remains to be confirmed in further studies specifically designed to address this question.
Notably, despite distinct clinical profiles, there was no significant difference in 28- and 90-day mortality between etiologies. Multivariable analysis further confirmed that mortality risk was independently associated with MELD and CLIF-C ACLF scores, as well as the presence of EHOF and specific complications, but not with the underlying etiology (HBV or alcohol). These findings underscore that survival in ACLF is primarily determined by the cumulative organ failure burden rather than the specific etiology of chronic liver disease. The hypothesis is further supported by the observation that patients with type C ACLF, characterized by a high incidence of organ failures, demonstrate uniformly high mortality rates (> 45% at 90 days) regardless of the underlying etiology, advocating for standardized protocols for multi-organ support.
This study is limited by incomplete emerging etiologies [e.g., metabolic dysfunction-associated steatotic liver disease (MASLD)], the lack of liver transplantation data, potential exclusion of ACLF grade 1 patients based on CLIF-C ACLF criteria, and the retrospective design. However, the strength of multicenter recruitment, the use of standardized criteria for diagnosis and classification, and minimal data loss helped to mitigate these potential limitations.
In conclusion, this multicenter study successfully provides the first granular characterization of the heterogeneity in clinical profiles between HBV-related and alcohol-related ACLF across diverse severity stages, underscoring the critical importance of stratified treatment strategies tailored to underlying chronic liver disease. Our findings bridge the gap between regionally dominant etiologies and globally harmonized therapeutic standards, thereby paving the critical way for precision treatment approaches to ultimately enhance patient survival.
Data availability
All data generated or analyzed in this study are available from the corresponding author for the reasonable request.
References
Moreau, R., Gao, B., Papp, M., Banares, R. & Kamath, P. S. Acute-on-chronic liver failure: A distinct clinical syndrome. J. Hepatol. 75(Suppl 1), S27–S35. https://doi.org/10.1016/j.jhep.2020.11.047 (2021).
Kulkarni, A. V. & Sarin, S. K. Acute-on-chronic liver failure-steps towards harmonization of the definition!. J. Hepatol. 81, 360–366. https://doi.org/10.1016/j.jhep.2024.03.036 (2024).
Aggarwal, A. et al. Definitions, etiologies, and outcomes of acute on chronic liver failure: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 22, 2199-2210 e2125. https://doi.org/10.1016/j.cgh.2024.04.018 (2024).
Zaccherini, G., Weiss, E. & Moreau, R. Acute-on-chronic liver failure: Definitions, pathophysiology and principles of treatment. JHEP Rep. 3, 100176. https://doi.org/10.1016/j.jhepr.2020.100176 (2021).
Bajaj, J. S. et al. Acute-on-chronic liver failure clinical guidelines. Am. J. Gastroenterol. 117, 225–252. https://doi.org/10.14309/ajg.0000000000001595 (2022).
Sarin, S. K. et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): An update. Hepatol. Int. 13, 353–390. https://doi.org/10.1007/s12072-019-09946-3 (2019).
Wu, T. et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut 67, 2181–2191. https://doi.org/10.1136/gutjnl-2017-314641 (2018).
Liver, F., Artificial Liver Group, C. S. o. I. D. C. M. A., Severe Liver, D. & Artificial Liver Group, C. S. o. H. C. M. A. [Guideline for diagnosis and treatment of liver failure]. Zhonghua Gan Zang Bing Za Zhi 27, 18–26, https://doi.org/10.3760/cma.j.issn.1007-3418.2019.01.006 (2019).
European Association for the Study of the, L. EASL Clinical Practice Guidelines on acute-on-chronic liver failure. J. Hepatol. 79, 461–491, https://doi.org/10.1016/j.jhep.2023.04.021 (2023).
Karvellas, C. J. et al. AASLD practice guidance on acute-on-chronic liver failure and the management of critically ill patients with cirrhosis. Hepatology 79, 1463–1502. https://doi.org/10.1097/HEP.0000000000000671 (2024).
Moreau, R. et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 144, 1426–1437, 1437 e1421–1429, https://doi.org/10.1053/j.gastro.2013.02.042 (2013).
Jalan, R. et al. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology 147, 4–10. https://doi.org/10.1053/j.gastro.2014.05.005 (2014).
Kamath, P. S., Kim, W. R., Advanced Liver Disease Study, G. The model for end-stage liver disease (MELD). Hepatology 45, 797–805. https://doi.org/10.1002/hep.21563 (2007).
Biggins, S. W. et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology 130, 1652–1660. https://doi.org/10.1053/j.gastro.2006.02.010 (2006).
Jalan, R. et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J. Hepatol. 61, 1038–1047. https://doi.org/10.1016/j.jhep.2014.06.012 (2014).
Li, J. et al. Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J. Hepatol. 75, 1104–1115. https://doi.org/10.1016/j.jhep.2021.05.026 (2021).
Macdonald, S. et al. Cell death markers in patients with cirrhosis and acute decompensation. Hepatology 67, 989–1002. https://doi.org/10.1002/hep.29581 (2018).
Claria, J. et al. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology 64, 1249–1264. https://doi.org/10.1002/hep.28740 (2016).
Li, J. et al. PBMC transcriptomics identifies immune-metabolism disorder during the development of HBV-ACLF. Gut 71, 163–175. https://doi.org/10.1136/gutjnl-2020-323395 (2022).
Liu, S. Y., Tsai, I. T. & Hsu, Y. C. Alcohol-related liver disease: Basic mechanisms and clinical perspectives. Int. J. Mol. Sci. 22, 5170. https://doi.org/10.3390/ijms22105170 (2021).
Li, H. et al. Submassive hepatic necrosis distinguishes HBV-associated acute on chronic liver failure from cirrhotic patients with acute decompensation. J. Hepatol. 63, 50–59. https://doi.org/10.1016/j.jhep.2015.01.029 (2015).
Ballester, M. P., Sittner, R. & Jalan, R. Alcohol and acute-on-chronic liver failure. J. Clin. Exp. Hepatol. 12, 1360–1370. https://doi.org/10.1016/j.jceh.2021.12.010 (2022).
Louvet, A. & Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 12, 231–242. https://doi.org/10.1038/nrgastro.2015.35 (2015).
Bajaj, J. S. et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology 60, 250–256. https://doi.org/10.1002/hep.27077 (2014).
Arroyo, V., Moreau, R. & Jalan, R. Acute-on-chronic liver failure. N. Engl. J. Med. 382, 2137–2145. https://doi.org/10.1056/NEJMra1914900 (2020).
Shi, Y. et al. Increased delayed mortality in patients with acute-on-chronic liver failure who have prior decompensation. J. Gastroenterol. Hepatol. 30, 712–718. https://doi.org/10.1111/jgh.12787 (2015).
Kamath, P. S. et al. A model to predict survival in patients with end-stage liver disease. Hepatology 33, 464–470. https://doi.org/10.1053/jhep.2001.22172 (2001).
Shi, Y. et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology 62, 232–242. https://doi.org/10.1002/hep.27795 (2015).
Acknowledgements
The authors wish to thank all China Network for Severe Liver Diseases members for their continued support with data collection.
Funding
This work was supported by research grants from the National 12th 5-year Plan for Hepatitis Research (No.2012ZX10002004-011) and the National 13th 5-Year Plan for Hepatitis Research (No.2017ZX10203201-007).
Author information
Authors and Affiliations
Contributions
QZ and JXH collected data, performed statistical analysis, and drafted the final manuscript. STQ, JLB and AMG helped to collect data. FL, CYZ, LYZ, WHR, SJX, YC and ZPD helped to collect data and drafted the final manuscript. TH conceived and designed this study, collected data, and contributed to the critical revision of the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Q., Hu, J., Qiu, S. et al. Clinical differences between HBV and alcohol related ACLF in a WGO classification multicenter study. Sci Rep 15, 25292 (2025). https://doi.org/10.1038/s41598-025-09620-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09620-9