Abstract
Modern horticulture relies heavily on chemical inputs to ensure plant growth and health. While the benefits of plant-associated microbes in promoting drought tolerance are well established, their consistent performance under field-like conditions remains limited. We hypothesize that a minimal, drought-adapted bacterial community—composed of strains isolated from resilient plants—can not only outperform single-strain inoculants, but also confer drought tolerance across plant cultivars when transplanted into a sensitive host such as clove basil. To test this hypothesis, three drought-tolerant bacterial strains from the Pseudomonas fluorescence genus were selected and their effects on clove basil (Ocimum gratissimum) plants were evaluated under severe drought stress conditions. The protectiveness of bacteria and their efficacy increased by growing the bacterial consortium size from one to three strains. A minimal community of three bacterial strains increased clove basil growth, nutrient content, stress tolerance and essential oil constituents under drought conditions. We propose that the minimal bacterial community enhance drought resilience in clove basil by boosting nutrient uptake and increasing the production of key secondary metabolites such as linalool and caryophyllene. By leveraging the potential of a minimal bacterial community, this research presents a pathway toward enhancing vegetable drought tolerance, contributing to a more sustainable and resilient horticultural practice.
Similar content being viewed by others
Introduction
Modern horticulture confronts significant challenges in preserving the quality, resistance, and overall health of plants, particularly under stressful environmental conditions1,2. Among these stressors, drought is a major threat, representing a physiological form of water deficiency in which the soil’s available water to the plant becomes insufficient, adversely affecting its metabolism3. Drought stress detrimentally impacts plant growth including photosynthesis, nutrient contents, respiration, translocation, ion uptake, water potential, and stomatal closure4. It is essential to develop approaches to mitigate the adverse effects and support agriculture in the course of climate change.
Efforts to reduce drought stress in plants traditionally rely on methods such as plant breeding and the application of agrochemicals. However, breeding encounters limitations due to a narrow genetic pool, low heritability of drought-resistance genes, the intricate nature of tolerance, and extensive environmental interactions5. Simultaneously, the use of agrochemicals raises social and ecological concerns6,7,8. This calls for sustainable and effective alternatives to current agrochemicals, including molecular and physiological advance to improve the genes invloved in water use efficiency and overall growth under water deficiency9,10.
Recent studies have revealed a promising avenue through the exploration of plant-associated microbes, known as plant microbiomes for environmentally friendly, low-cost, and safe alternatives to common agrochemicals. These microbes, regarded as the second genome of plants, offer a multitude of services. Recent studies have demonstrated that the application of plant-associated bacteria can have broader effects than plant growth promotion, namely altering different aspects of plant growth and development (Ravanbakhsh et al., 2018). Interestingly, the same plant phenotype was achieved either by modification of the bacterial genome or genetic modification of the plant11. This discovery suggests that the incorporation of plant microbiomes in agricultural systems could be an effective approach for stress tolerance and nutrient supplements. The same approach can potentially enhance plant health and reduce the excessive application of chemical inputs such as fertilizers and pesticides8,12.
Plant-associated microorganisms play a vital role in enhancing plant drought tolerance3,13,14. They contribute to improved nutrient uptake, water-use efficiency, and reduced oxidative stress in host plants15thereby significantly enhancing drought resilience (Mathur and Roy, 2021). Additionally, these microorganisms can alter the composition of essential oils16,17. Many vegetable crops are highly sensitive to drought stress, and their quality is especially important due to the direct impact of vegetable consumption on human health. Water stress can alter plant biochemistry, affecting not only taste but also nutritional and medicinal properties. Therefore, plant-associated microorganisms hold potential to mitigate these effects by supporting plant growth and improving both flavor and nutritional value under drought conditions.
Clove basil (Ocimum gratissimum) holds considerable medicinal, economic, industrial, and nutritional value18. However, it is highly sensitive to drought, which can significantly reduce its quality, nutrient content, biomass, and shelf life19. Despite its importance, there is limited information on how clove basil responds to drought stress, particularly in relation to interactions with beneficial microorganisms. In this study, we examine the effects of drought-protective microorganisms on clove basil drought tolerance. The results of using plant-associated microbes to confer drought resistance in the laboratory are promising. However, an important challenge lies in translating these results from controlled laboratory settings to complex and dynamic field conditions20.
One promising strategy involves the use of a minimal bacterial community—defined here as a small consortium of 1–3 strains that, together, can reproduce the drought-protective effect seen in more complex communities. This approach is intended to balance functional effectiveness with practical applicability in real field conditions (Ocimum gratissimum), a plant known for its sensitivity to drought, and by testing bacterial inoculations in natural soil conditions. Our approach combines both individual and community-based inoculations of three strains belonging to the Pseudomonas fluorescens genus, bacteria previously isolated from dryland environments and known for their plant-growth-promoting potential under stress21. This study presents two key novel aspects: first, the implementation of a transplanting approach to bridge lab-to-field translation, and second, the evaluation of a minimal bacterial consortium in a realistic plant-soil system, offering insights into both drought mitigation and essential oil composition, a relatively unexplored area. Clove basil (Ocimum gratissimum) is a medicinal and aromatic plant valued for its essential oil content. However, it is sensitive to water deficit, with drought stress leading to reduced biomass, leaf wilting, and alterations in essential oil composition and yield (Crisálida Machado Vilanova, 2018; Sharma et al., 2019). Despite its economic and pharmacological importance, there is limited research on strategies to improve drought tolerance in clove basil, particularly through microbial inoculation. We inoculated these bacteria, individually or as a minimal community, on clove basil plants, to examine two hypotheses: (1) Bacterial isolated from a drought resistance plants can confer drought tolerance to clove basil plants, leading to enhanced quantity and quality of clove basil under drought stress, and (2) A minimal community of the three selected bacteria from the Pseudomonas fluorescence genus can reliably support clove basil plants, compared to control, in natural soil and under field conditions. (3) Effect of bacteria and drought on essential oil composition is an area that has received limited scientific investigation. Through this investigation, we aim to shed light on the potential of harnessing plant microbiomes for improving drought tolerance in clove basil and other crops, thereby presenting a promising and sustainable approach to advance modern horticulture.
Materials and methods
Plant materials
Clove basil (O. gratissimum) seeds were germinated in a seed germination tray and placed in the greenhouse (28 °C, 50% relative humidity, 16 h of light). In the four-leaf stage, two plants were transferred to 10 kg pots containing pasteurized soil (90 °C, 30 min) and kept in greenhouse conditions. Following a growth period of four weeks, uniform seedlings were selected for further experimentation.
Bacterial strains
Three drought-protective bacteria from the Pseudomonas genus were isolated from Alcea aucheri, a naturally drought-resistant plant, in a dryland area in the south of Iran (K31, K32 and K33). These strains were kept at − 80 °C. Some selected bacterial metabolites are reported in Table S4. At first, one single colony of bacterial strains was grown on 30 g l−1 tryptic soy broth medium (TSB) (Ibresco) (Rotary shaker, IKA, KS 130 basic; 28 °C, 100 rpm, 24 h). Three days before applying bacteria, strains were streaked to solid TSB from glycerol stocks. The plates were kept at 28 °C for 2 days then transferred to TSB and kept in a shaker incubator (28 °C, 100 rpm) for 24 h. Bacteria were centrifuged (4000 rpm) for 20 min and pellets were resuspended into autoclaved, distilled water, and adjusted on 106 cells. per ml (density 0.5 at 600 nm) before inoculation. Preparation was done for all bacterial strains and used as a baseline for designing the synthetic community. The synthetic bacterial community was constructed by using equal amounts of bacterial strains (K31, K32, and K33), either single bacteria, double, or triple including K31, K32, K33, K31 + 32, K32 + 33, K31 + 33, K31 + 32 + 33. Non-inoculated treatment (autoclaved water) served as a control. The three Pseudomonas strains (K31, K32, K33) were selected for their complementary traits associated with drought tolerance, including IAA and EPS production, ACC deaminase activity, and nutrient solubilization (see Table S4). Their overlapping abilities suggest functional redundancy, enhancing the stability and effectiveness of the bacterial consortium under varying conditions. Cell growth was measured at OD600 and stop when OD value reaches 0.5. Two hundred microliter of bacterial suspension was added to the surface of the soil near plant stems.
Drought stress treatments
Drought treatments were started 30 days after transplantation. The water regime was adjusted to 50% of the field capacity (FC) and considered as drought treatment. To appropriately choose the drought levels applied to the plants, we estimated the FC of the soil in the laboratory. First, soil water content was measured by saturating 120 g of oven-dried soil samples with water for 24 h. Then, pots were covered with plastic foil to prevent evaporation. After saturation, the pots were placed in a petri dish with a paper base to absorb the gravitationally draining excess water for 72 h. This amount of time represents the usual period for excess soil water to drain off. The resulting soil water content represents 100% FC. The amount of soil water (SW) at FC was calculated as the weight loss while oven-drying for 24 h at 105 ℃ using the following formula: SW = Wet Weight (g) – Dry Weight (g).
Plant phenotype: fresh and dry weight
Plants were harvested at the flowering stage (three months after transplanting) and transferred to the laboratory. Fresh weight was measured immediately. Then plants were put in an oven at 70 °C for 72 h to measure dry weight.
Water use efficiency (WUE)
For measurement WUE, we recorded total water consumption by plants, and calculated based on the following equations:
In this formula, “Y” is the yield (kg/plant) of clove basil plants, and “I” is water usage (m3/plant) during the experiment.
Relative leaf water content (RWC)
Relative leaf water content (RWC) was measured according to Ritchie, et al.22 Half a gram of fresh leaf (FW) was separated and immersed in distilled water for 1 day. Following this, the leaves were weighed (TW) and subsequently placed in an oven (70 °C, 1 day) (DW). The plant material was weighed, and considered as a dry weight. The RWC calculation was calculated as follows:
Photosynthesis pigments
Photosynthetic pigments were measured based on Lichtenthaler23 methods, in which first 0.1 g of fresh leaves and 10 ml of 80% acetone round together and centrifuged (6000 rpm, 10 min). Then the absorbance of the samples was read using a spectrophotometer (Photonix Ar5, Iran) at 470, 646, and 664 nm wavelengths. The following equations were used for measuring photosynthesis pigments:
In these formulas, Chl a, Chl b, Total Chl, and Car represent chlorophyll a, chlorophyll b, total chlorophyll, and Carotenoid, respectively. The unit of all photosynthesis pigments was mg/gFW. The concentration is determined in terms of mg/liter of plant extract. The results of measuring the amount of photosynthetic pigment are calculated and presented in terms of mg/gFW.
Vitamin C
First, one gram of the powdered samples was digested in 1 ml of 10% trichloroacetic acid and centrifuged (2100 rpm, 20 min). Ten µL of supernatant were added to one milliliter of dinitrophenylhydrazine-copper sulfate-thiourea reagent and the mixture was incubated at 31 °C for 3 h. Then 110 µL of 41% sulfuric acid was added to the samples and the absorbance was measured at 120 nm with a spectrophotometer (Photonix Ar5, Iran). The unit reported as mg/g fresh weight of the plant sample24.
Proline
Briefly, 0.5 g of plant leaves were ground in 3 ml of sulfosalicylic acid 3% and filtered with filter paper. In this stage, samples were mixed with 1 ml of acid ninhydrin and glacial acetic acid and put in a water bath (100 °C, 1 h). Finally, after cooling toluene was added to the mixture, and the absorbance was measured spectrophotometrically (Photonix Ar5, Iran) at 520 nm25.
Antioxidant capacity
Aerial parts of the plants were dried in the shade at room temperature immediately after being transferred to the laboratory, and grounded with a pestle and mortar. Then 10% methanol extract was prepared by soaking. The plant extracts were stored in the fridge at −20 °C26. This extract used for antioxidant capacity and total phenol content.
Fifteen microliters (µL) of the plant methanolic extract were mixed with two mL of DPPH (2,2-diphenyl-1-picrylhydrazyl) methanolic solution (0.01 M) and kept at room temperature (darkness, 30 min). Finally, the absorbance was measured at 517 nm using a spectrophotometer (Photonix Ar5, Iran)27,28. The following equation was used for total antioxidant activity:
where I is the rate of DPPH radical trapping, and Ai and At are the absorption of control and tested samples, respectively.
Total phenol content
Briefly, 100 µL of the extract and 750 µL of Folin Ciocalteu reagent (diluted with a ratio of 1:10) were mixed. Then four mL of sodium carbonate solution (7.5%) was added to the mixture after 5 min. Following 90 min of incubation (room temperature and dark) absorbance was read at 750 using a spectrophotometer (Photonix Ar5, Iran). The unit presented in mg GAE/g DW29,30.
Nutrient concentration
Dried shoot samples were used for nutrient measurement. Nitrogen concentration was measured using the Kjeldahl method31 with standard procedure. Briefly, 0.1 g of clove basil dry weight was sampled. The powdered materials were digested with concentrated sulfuric acid and copper sulfate. The resulting mixture is heated until organic matter is oxidized to ammonium sulfate. The digested solution is distilled to liberate ammonia gas, which is collected in a boric acid solution. Finally, titration with standardized hydrochloric acid determines the nitrogen content in the plant materials. Phosphorus, potassium, zinc, manganese, and iron elements were measured by inductively coupled plasma mass spectroscopy (plasma ICP device, Plasma Quant 9100, Analytik Jena, Germany)32. In this method, 0.125 g of dry leaf tissue were kept in 5 ml of concentrated nitric acid for 24 h. Then, the volume of the solution was increased to 50 mL with distilled water and filtered through filter paper. The transparent solution was used for injection in the ICP.
Essential oil content and gas chromatography-mass spectrometry (GC-MS) analysis
Essential oil content extracted via hydro-distillation methods using Clevenger. At first 300 g dried samples were added to 700 ml water and after 3 h essential oil was harvested.
GC analysis and GC-MS analysis were carried out using a Thermo-UFM ultra-fast gas chromatograph and Varian 3400 GC-MS system equipped with a DB-5 fused silica column, respectively. A computer library and n-alkanes (C6–C24) were used for compound identification based on GC retention indices. The oil compounds were identified by matching retention indices to Wiley and Adams Mass Spectral libraries as well as by comparison of mass spectra with those published in the literature (Adams, 2007; NIST, 2008).
Drought tolerance indexing
Two stress tolerance indexes were calculated, including Geometric mean productivity (GMP) and Stress tolerance index (STI)33. These indexes were calculated based on the following equations:
Ys is biomass under stressful conditions and Yp is biomass in control.
Statistical analysis
Data analysis was done using SAS 9.1 (SAS Institute/USA). The experimental design was conducted as a completely randomized design with different bacterial treatments (K31, K32, K33, K31 + 32, K32 + 33, K31 + 33, K31 + 32 + 33) under drought stress, with three replicates per treatment. The drought treatment is considered 50% of the moisture at the FC level. The mean values of traits were compared using ANOVA, followed by Duncan’s test (p ≤ 0.05). Principal component analysis (PCA) and weighted average plant growth and nutrients index calculated with all measured traits rarefied using the “multifunc” package in R studio (v.4.3.2).
Results
Effect of bacteria on plant growth and nutrients
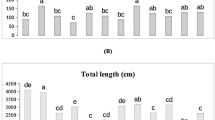
The effects of Pseudomonas bacteria (single, double, and triple inoculation) on plant weight, and biochemical index of clove basil plants (O. gratissimum) are depicted in Fig. 1. Plant fresh weight in clove basil plants was affected by bacteria (Table S1 Fig. 1a, b, and c). The effect of treatments on fresh weight was greater than dry weight. The Bacterial consortium size (double and triple bacteria combinations) increased almost 23% and 24% plant fresh weight significantly compared to other treatments. There was no significant difference between control and single inoculation (Fig. 1a). Bacteria had also an effect on clove basil’s dry weight and the inoculation overall increased the dry weight compared to the no-inoculating control (Fig. 1b, Table S1).
Bacterial minimal community inoculation significantly affected the biochemical index of clove basil plants (Table S1, Fig. 1d-i). Chlorophyll a was significantly affected by bacterial treatments (Fig. 1d, Table S1), K31 + 32 was higher than others. Also, plants inoculated with the K31 + 32 produced more chlorophyll b (Fig. 1e) but there was no significantly difference between double and triple inoculation treatments. The inoculation increased the antioxidant capacity. There is, however, no difference in antioxidant capacity between different Bacterial consortium size (1, 2 and 3). For phenol contents plants inoculated with single and dual inoculation showed the highest value and control plants recorded the lowest values (Fig. 1f, Table S1). Vitamin C contents were same under different bacterial treatment and just K31 + 32 and K31 + 33 were lower (Fig. 1g; Table S1 and S2). Proline content as a stress indicator in the control plant was high compared to others and also plants inoculated with K31 + 32 + 33 and K31 + 32 produced the lowest proline content almost 27 and 37% compare to control respectively (Fig. 1h, Table S2). Also, RWC showed significant differences (Table S1, Fig. 1i). Double and triple inoculation (K31 + 32, K32 + 33, K31 + 33 and K31 + 32 + 33) increased RWC by approximately 15, 11, 7 and 11% respectively significantly compared to the others but no difference between them. Carotenoid content in plant treated with K32 + 33 was high significantly and other treatments have not significant differences.
Effects of bacterial treatments on clove basil plant (O. gratissimum) parameters and biochemical indices. Panel (a) fresh weight (g), (b) dry weight (g), (c) chlorophyll a, e) chlorophyll b, f) antioxidant capacity, g) phenol content, h) proline content, i) Relative Water Content (RWC). The experimental design was randomized complete design with 3 replications. Data analysis was performed using SAS 9.1. Mean values were compared using ANOVA, followed by Duncan’s test (p ≤ 0.05). Different colors represent specific treatments: K31, K32, K33, K31 + 32, K32 + 33, K31 + 33, K31 + 32 + 33. K1, K2 and K3 bacteria from the Pseudomonas genus were isolated from Alcea aucheri, a naturally drought-resistant plant, in a dryland area in the south of Iran.
Effect of bacterial treatments on nutrient concentration in clove basil under drought stress. Nutrient content analysis of clove basil plants (O. gratissimum) depicted in six panels (a-f): (a) Nitrogen (N) content (%), (b) Phosphorus (P) content (%), (c) Potassium (K) content (%), (d) Manganese (Mn) content (µg mg−1), (e) Iron (Fe) content (µg mg−1), and (f) Zinc (Zn) content (µg mg−1). The experimental design was a randomized complete design with 3 replications. Data analysis was performed using SAS 9.1. Mean values were compared using ANOVA, followed by Duncan’s test (p ≤ 0.05). Each black circular represents specific treatments: K31, K32, K33, K31 + 32, K32 + 33, K31 + 33, K31 + 32 + 33. Each treatment was replicated three times. K1, K2 and K3 bacteria from the Pseudomonas genus were isolated from Alcea aucheri, a naturally drought-resistant plant, in a dryland area in the south of Iran.
Bacteria treatments increased the macro and micronutrient content in the clove basil plants under drought. Further statistical tests revealed that all nutrients measured in this experiment were significantly affected by bacterial treatments (Table S1). Specifically, increasing bacteria led to a significant increase in the macro and micro nutrient content of clove basil plants. Concentration of phosphorus, potassium, manganese, iron, and zinc increased by 13, 7, 145, 24, and 39% at triple inoculation levels compared to control plants, respectively. Single inoculation in basil plants showed higher nitrogen concentration (Fig. 2a). Phosphorus concertation under K31 + 32 + 33 treatment was highest and lowest amount shown in single inoculation of bacteria and control without significant differences (Fig. 2b), but in potassium concentration there is no differences between bacterial treatments and just the control plants and K31 + 33 were different statically (Fig. 2c, Table S2). Manganese values also under K31 + 32 + 33 treatments were highest compared to the control plant (Fig. 2d, Table S2). Iron and Zinc content were statically different in plants treated with the triple combined bacteria (Fig. 2e and f, Table S2).
Effect of bacterial treatments on essential oil constituent
In this project, major compounds of clove basil essential oil were linalool, anethol and methyl cis-cinnamate (Table S3, Fig. 3). The other minor constituent was eucalyptol, terpinen-4-ol, bornyl acetate, eugenol, germacrene D, caryophyllene, cis-α-bergamotene, γ-elemene and γ-murolene. K33, K31 + 32, K31and K31 + 32 + 33 resulted in higher linalool content, with percentages of 49.9, 46.2, 42.1 and 42%, respectively. Anethole, as second most compound, was higher under plant treated with K32, K33 and K31 + 32 + 33. Also, eucalyptol was detected more in K31 + 32 + 33 (4.7%) and K31 (4%). Eugenol, as important compound, increased under K31 + 32 + 33 treatments almost 3.1%. K31 + 32 + 33 produced plants with higher γ-elemene content (1.5%) compared to other treatments.
Effect of bacterial increasing the bacterial consortium size on plant resistance and advance analysis
PCA analysis of the data revealed strong evidence of significant separation based on bacterial consortium size (Fig. 4a). The first two principal component scores (PC1 and PC2) explained 78.5% and 19.3% of the variance, respectively. Different consortium size levels (1, 2, and 3) showed a separation, the significant separation between size 3 and control was observed.
The weighted average plant growth index is calculated for all measured traits of plants as one index, this parameter clove basil plants increased with bacterial consortium size. This index is calculated for all measured traits of planta as one index, this parameter of clove basil plants increased with bacterial consortium size (Fig. 4b, Table S2). Weighted average nutrients are calculated for all nutrients measurement. For size 3 this index was significant and higher than others, but no differences between 1 and 2, and for control plants recorded the lowest value (Fig. 4c, Table S2).
WUE of clove basil plants increased with bacterial consortium size (Fig. 4d, Table S2). Increasing WUE with high size shown plant inoculated with size 3 absorbed water better than other treatments under drought stress and produced more biomass and high-quality plants (more macro and microelement concentration).
In our study, we pooled the data to create two different drought tolerance indexes; GMP and STI. (Fig. 4e & f, Table S2). GMP and STI indexes increased with increasing bacterial consortium size. The GMP value increased 3, 39 and 121% in size 3 compared to control and 1, 17, and 49% increases was recorded for STI in size 1, 2, and, 3 compared to control, respectively.
Effect of f bacterial consortium size on some parameter in O. gratissimum under drought stress. (a) PCA analysis of different bacterial consortium size on clove basil plants. (b) Weighted average growth index, (c) Weighted average nutrients index, (d) Water Use Efficiency (WUE), (e) Stress Tolerance Index (STI), (f) Geometric Mean Productivity (GMP). Consortium sizes were categorized as follows: 0 (Control), 1 (Individual bacteria), 2 (Combination of two bacteria), and 3 (Combination of three bacteria). Bacteria treatments included three strains of Pseudomonas fluorescence (K31, K32, K33). The experimental design was a randomized complete design with 3 replications. Each black circular above indicates treatments, including K31, K32, K33, K31 + 32, K32 + 33, K31 + 33, K31 + 32 + 33. K1, K2 and K3 bacteria from the Pseudomonas genus were isolated from Alcea aucheri, a naturally drought-resistant plant, in a dryland area in the south of Iran.
Discussion
The plant response to the environment depends on the plant genome and its associated microbiome34. Microbiome engineering is a rapidly evolving frontier for solutions in health, agriculture, and climate management35. However, despite increased efforts, microbiome inoculants frequently fail to establish or confer long-lasting modifications to ecosystem function. One underlying cause of these shortfalls lies is in the failure to consider barriers to organism establishment.
This study introduces a transformative approach to fortify plant resilience against drought stress by transplanting drought-protective bacteria sourced from Alcea aucheri, a naturally drought-resistant plant, into clove basil, which is susceptible to drought stress during growth. This transplantation exposes clove basil to a specific set of microbial partners that can confer drought resistance. The three Pseudomonas strains used in this study were isolated from the rhizosphere of A. aucheri and selected based on their known drought-protective mechanisms, including enhanced exopolysaccharide and indole acetic acid (IAA) production (see Table S4). The strains were selected based on complementary and overlapping functional traits known to contribute to drought tolerance, including IAA and EPS production, and solubilization of phosphorus and zinc (see Table S4). Their shared abilities to promote plant stress resilience suggest functional redundancy, which increases the stability and reliability of the microbial consortium under variable environmental conditions. We investigated this hypothesis by subjecting the resulting plant-bacteria combination to drought conditions.
Principal Component Analysis (PCA) reveals clear differentiation in term of bacterial consortium sizes effects (Fig. 3c). This distinction sets the stage for understanding the subsequent positive effects of bacterial consortia on drought tolerance and plant phenotype under stress. Drought-induced reductions in weight, water use efficiency, and antioxidant capacity were evident (Figs. 1a, amp and f and 3e). The application of bacterial consortia, especially at the highest size, mitigated the effects of drought on the plant traits. The highest consortium size significantly enhanced plant height (Fig S1c) and resulted in significant increases in both dry and fresh weight (Fig S2a and b). Bacterial consortium size significantly increased antioxidant capacity, phenol content, total chlorophyll (Figure S1c, d, and e), and the content of elements including iron, phosphorus, potassium, manganese, and zinc (Fig. 2). We consider two indexes for evaluating drought resistance. These indexes (GMP and STI) also increased with bacterial consortium size. The highest size showed a significant effect compared to double and single inoculation levels, confirmed by a positive correlation between bacterial consortia and drought resistance (Fig. 4). The results align with previous studies showing the effectiveness of creating synthetic community in improving the yield and physiological traits in maize plants36sorghum and Arabidopsis plants37 and Mexican ornamental plants, Amaranthus hypochondrius L38. and Pearl millet39.
The “weighted growth index” serves as a comprehensive metric encapsulating various plant characteristics, providing a holistic view of overall plant performance. In our study, we amalgamated multiple factors under this index to gauge the collective impact of bacterial consortia on clove basil (O. gratissimum) plants. Notably, our results indicate a substantial increase in the weighted growth index under the influence of bacterial consortia, particularly at higher consortium size, a combination of K31 + 32 + 33. This suggests that the use of bacterial consortia has a beneficial and synergistic effect on multiple plant traits, contributing to enhanced growth and resilience under drought stress conditions.
Water Use Efficiency (WUE) plays an important role in facilitating improved plant growth under minimal available water in drought conditions40. WUE is a measure of how effectively plants utilize water for growth and development41. We observed a notable improvement in WUE with increasing the bacterial consortium size, with the highest size exhibiting the highest efficiency. This enhancement in WUE implies that the bacterial consortia help the plants optimize water usage, allowing them to thrive and grow better even when faced with limited water availability during drought conditions. A previous study by Akhtar, et al.42 presented increased WUE in plants upon inoculation of maize plants with Bacillus licheniformis. Therefore, the positive impact of bacterial consortia on the weighted growth index and WUE collectively underscores their potential as an effective strategy for bolstering plant resilience and productivity under water scarcity. Also, we focused on two key factors defining the drought resilience in the plants, namely STI and GMP43. We observed that individual and combining two bacteria (size 1 and 2) did not yield significant results on these indexes. The consortium size of bacteria, specifically the highest consortium size (K31 + 32 + 33) significantly increased both STI and GMP indexes (Fig. 4e and f). The STI and GMP indicate the extent to which plants can withstand drought conditions, and both indexes show a positive correlation with bacterial consortium size (Fig. 4, b, and e). STI and GMP show a positive correlation with yield and water-deficient stress in other plants such as barley44 and potato45. One of the reasons for better STI, GMP and WUF under higher bacterial consortium size can be connected to more nutrient (e.g. phosphorus and potassium) content. For example potassium is important because of effect on cell elongation, aquaporin activity and water uptake46,47.
Our findings have important implications for global agriculture and food security, particularly in addressing the challenges of drought stress. Clove basil, a plant vulnerable to drought-induced damage during production48 and post-transportation49, benefited significantly from the bacterial-mediated plant drought tolerance. This fortification is not only vital for the economic viability of clove basil in retail but also crucial for ensuring consistent and high-quality produce for consumers50. Moreover, the concurrent improvement in vegetable like clove basil can address also a pressing global problem of hidden hunger51. As a substantial portion of the world’s population suffers from nutrient deficiencies52, our approach presents a promising solution by enhancing the nutrient profile of a staple crop, potentially mitigating hidden hunger on a broader scale. We also investigate the essential oil constituents in clove basil plants. In this project the most constituent of clove basil was linalool53,54,55. Based on the major oil constituents, six chemotypes (thymol, eugenol, citral, ethyl cinnamate, linalool, and geraniol) of O. gratissimum were reported56,57. Linalool synthase is highly sensitive to environment condition58. Bacterial consortium size changes the amount of essential oil content and alters essential oil constituents reported in other plants59. In addition, increasing essential oil in clove basil plants on the presence of bacteria and especially linalool tendencies reported by Monfort, et al.60. Previous studies have shown that IAA-producing bacteria can enhance biomass and essential oil production by improving nutrient uptake, which is crucial for enzyme activity and organic compound synthesis 5961. IAA-producing bacteria also help protect plants against stress and can alter the main constituents of essential oils in medicinal plants, such as nerol and geraniol in lemon balm62, thymol in Lippia63.
In this study, a higher content of effective substances was recorded in the bacterial treatments compared to control. The role of different plant essential oil constituents in resistance to abiotic stresses and plant physiology has rarely been studied. Linalool, as the most important compound in the studied basil plant, showed a higher content in bacterial treatments. One hypothesis is that the increase in linalool content contributes to drought resistance in basil by elevating calcium and H2O2 levels. These compounds raise ROS levels, which act as signaling molecules, triggering processes that enhance plant resistance. Additionally, the increase in salicylic acid induced by linalool in other plants has been shown to improve resistance to both biotic and abiotic stresses64. Another important compound in basil essential oil is eugenol, which has been proven to increase resistance to drought and salt stress in Camellia sinensis plants through the regulation of ROS scavenging and ABA homeostasis65.
The bacterial strains examined in this study exhibit the capability to produce Indole acetic acid (IAA), ACC deaminase enzyme (AcdS), and Exopolysaccharides (EPS) (Table S4). Future investigations using bacterial functional mutants for these specific traits can aid in unraveling these effects. Additionally, technical limitations prevented us from directly measuring the colonization of the transplanted bacteria within the clove basil plants. We, however, measured the bacterial respiration within different size and showed an increased bacterial respiration with increasing the consortium size of bacteria (Fig S3). While this study demonstrates the potential of the introduced Pseudomonas strains for improving drought tolerance, we acknowledge two key limitations. First, the interactions between these strains and indigenous microbial communities were not explored. Investigating these interactions in future research is important, as they could influence the strains’ effectiveness and persistence in the field. Second, a comparison with well-watered (control) plants was not included due to experimental constraints. Evaluating bacterial effects under both drought-stressed and non-stressed conditions would help determine whether the observed benefits are specific to drought mitigation or represent broader plant growth promotion. These remain valuable areas for future investigation.
Conclusion
Plant-associated microorganisms play an important role in plant drought tolerance. This study not only contributes to the understanding of the positive effects of plant-associated microbes on increasing the quantity and quality of the clove basil plant under drought but also showed success in transplanting the beneficial bacteria in agricultural practices. By harnessing the holistic potential of bacterial transplantation, we propel agriculture into a future where plant resilience against environmental stressors is fortified by the persistent collaboration of plants and their associated bacteria. We think probably via increasing nutrient absorption and some essential oil constitute increased drought resistance of basil plants. For knowing more about mechanism in clove basil and these bacteria we need more experiments about gene expression, investigation of these bacteria in different situation such as well water condition compared to water deficient.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Xu, X. Major challenges facing the commercial horticulture. Front. Horti. 1, 980159 (2022).
Behdad, A., Mohsenzadeh, S., Azizi, M. & Moshtaghi, N. Salinity effects on physiological and phytochemical characteristics and gene expression of two Glycyrrhiza glabra L. populations. Phytochem 171, 112236 (2020).
Kumar, A. & Verma, J. P. Does plant—microbe interaction confer stress tolerance in plants: a review? Microbiol. Res. 207, 41–52. https://doi.org/10.1016/j.micres.2017.11.004 (2018).
Oguz, M. C., Aycan, M., Oguz, E., Poyraz, I. & Yildiz, M. Drought stress tolerance in plants: interplay of molecular, biochemical and physiological responses in important development stages. Physiol 2, 180–197 (2022).
Kaur, H., Kohli, S. K., Khanna, K. & Bhardwaj, R. Scrutinizing the impact of water deficit in plants: transcriptional regulation, signaling, photosynthetic efficacy, and management. Physiol. Plant. 172, 935–962. https://doi.org/10.1111/ppl.13389 (2021).
Heidari, S., Azizi, M., Soltani, F. & Hadian, J. Foliar application of Ca (NO3) 2 and KNO3 affects growth, essential oil content, and oil composition of French Tarragon. Ind. Crops Prod. 62, 526–532 (2014).
Singh, H. et al. Recent advances in the applications of nano-agrochemicals for sustainable agricultural development. Environ. Sci. Process. Impacts. 23, 213–239. https://doi.org/10.1039/D0EM00404A (2021).
Taghizadeh, S. F., Azizi, M., Rezaee, R., Giesy, J. P. & Karimi, G. Polycyclic aromatic hydrocarbons, pesticides, and metals in olive: analysis and probabilistic risk assessment. Environ. Sci. Pollut Res. 28, 39723–39741 (2021).
Hura, T., Hura, K. & Ostrowska, A. MDPI, Vol. 23 4698 (2022).
Korgaonkar, S. & Bhandari, R. Drought stress in plants: effects and tolerance. J. Stress Physiol. Biochem. 20, 5–23 (2024).
Ravanbakhsh, M., Kowalchuk, G. A. & Jousset, A. Targeted plant hologenome editing for plant trait enhancement. New. Phytol. 229, 1067–1077. https://doi.org/10.1111/nph.16867 (2021).
Antar, M. et al. Biomass for a sustainable bioeconomy: an overview of world biomass production and utilization. Renew. Sus Energ. Rev. 139, 110691. https://doi.org/10.1016/j.rser.2020.110691 (2021).
Chieb, M. & Gachomo, E. W. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant. Biol. 23, 407 (2023).
Raza, A. et al. Fighting to thrive via plant growth regulators: green chemical strategies for drought stress tolerance. Physiol. Plant. 176, e14605 (2024).
Hamid, B. et al. Bacterial plant biostimulants: a sustainable way towards improving growth, productivity, and health of crops. Sustainability 13, 2856. https://doi.org/10.3390/su13052856 (2021).
Eshaghi Gorgi, O., Fallah, H. & Niknejad, Y. Barari tari, D. Effect of plant growth promoting rhizobacteria (PGPR) and mycorrhizal fungi inoculations on essential oil in Melissa officinalis L. under drought stress. Biologia 77, 11–20 (2022).
Yilmaz, A. & Karik, Ü. AMF and PGPR enhance yield and secondary metabolite profile of Basil (Ocimum Basilicum L). Ind. Crops Prod. 176, 114327 (2022).
Carović-Stanko, K. et al. Genetic relations among Basil taxa (Ocimum L.) based on molecular markers, nuclear DNA content, and chromosome number. Plant. Syst. Evol. 285, 13–22. https://doi.org/10.1007/s00606-009-0251-z (2010).
Kalamartzis, I., Menexes, G., Georgiou, P. & Dordas, C. Effect of water stress on the physiological characteristics of five Basil (Ocimum Basilicum L.) cultivars. Agron 10, 1029. https://doi.org/10.3390/agronomy10071029 (2020).
Lin, Y., Zhang, H., Li, P., Jin, J. & Li, Z. The bacterial consortia promote plant growth and secondary metabolite accumulation in Astragalus mongholicus under drought stress. BMC Plant. Biol. 22, 475 (2022).
Ravanbakhsh, M., Ronaghi, A. M., Taghavi, S. M. & Jousset, A. Screening for the next generation heavy metal hyperaccumulators for dryland decontamination. J. Environ. Chem. Eng. 4, 2350–2355. https://doi.org/10.1016/j.jece.2016.04.013 (2016).
Ritchie, S. W., Nguyen, H. T. & Holaday, A. S. Leaf water content and gas-exchange parameters of two wheat genotypes differing in drought resistance. Crop Sci. 30, 105–111 (1990).
Lichtenthaler, H. K. In Methods in EnzymologyVol. 148350–382 (Elsevier, 1987).
Omaye, S. T., Turnbull, J. D. & Sauberlich, H. E. in Methods in enzymology Vol. 62 3–11Elsevier, (1979).
Bates, L. S., Waldren, R. & Teare, I. Rapid determination of free proline for water-stress studies. Plant. Soil. 39, 205–207 (1973).
Lien, E. J., Ren, S., Bui, H. H. & Wang, R. Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Rad Biol. Med. 26, 285–294 (1999).
Behdad, A., Mohsenzadeh, S. & Azizi, M. Growth, leaf gas exchange and physiological parameters of two Glycyrrhiza glabra L. populations subjected to salt stress condition. Rhizosphere 17, 100319 (2021).
Akowuah, G., Ismail, Z., Norhayati, I. & Sadikun, A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 93, 311–317 (2005).
Marinova, D., Ribarova, F. & Atanassova, M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. JU Chem. Metal. 40, 255–260 (2005).
Araghi, A. M. et al. Assessment of phytochemical and agro-morphological variability among different wild accessions of Mentha longifolia L. cultivated in field condition. Ind. Crops Prod. 140, 111698 (2019).
Barbano, D. M., Clark, J. L., Dunham, C. E. & Flemin, R. J. Kjeldahl method for determination of total nitrogen content of milk: collaborative study. J. AOAC Int. 73, 849–859. https://doi.org/10.1093/jaoac/73.6.849 (1990).
Shahhoseini, R., Azizi, M., Asili, J., Moshtaghi, N. & Samiei, L. Effects of zinc oxide nanoelicitors on yield, secondary metabolites, zinc and iron absorption of feverfew (Tanacetum parthenium (L.) Schultz Bip). Acta Physiol. Plant. 42, 1–18 (2020).
Fernandez, G. C. Effective selection criteria for assessing plant stress tolerance. (1993).
Ravanbakhsh, M., Sasidharan, R., Voesenek, L. A., Kowalchuk, G. A. & Jousset, A. Microbial modulation of plant ethylene signaling: ecological and evolutionary consequences. Microbiome 6, 1–10. https://doi.org/10.1186/s40168-018-0436-1 (2018).
Jiang, G. et al. Exploring rhizo-microbiome transplants as a tool for protective plant-microbiome manipulation. ISME Commun. 2 https://doi.org/10.1038/s43705-022-00094-8 (2022).
Armanhi, J. S. L., de Souza, R. S. C., Biazotti, B. B., Yassitepe, J. E. & Arruda, P. d. C. T. Modulating drought stress response of maize by a synthetic bacterial community. Front. Microbiol. 12, 747541, (2021). https://doi.org/10.3389/fmicb.2021.747541
Qi, M. et al. Identification of beneficial and detrimental bacteria impacting sorghum responses to drought using multi-scale and multi-system Microbiome comparisons. ISME J. 16, 1957–1969. https://doi.org/10.1038/s41396-022-01245-4 (2022).
Devi, R., Kaur, T., Kour, D. & Yadav, A. N. Microbial consortium of mineral solubilizing and nitrogen fixing bacteria for plant growth promotion of Amaranth (Amaranthus hypochondrius L). Biocatal. Agric. Biotechnol. 43, 102404. https://doi.org/10.1016/j.bcab.2022.102404 (2022).
Kaur, T., Devi, R., Kumar, S., Kour, D. & Yadav, A. N. Plant growth promotion of Pearl millet (Pennisetum glaucum L.) by novel bacterial consortium with multifunctional attributes. Biologia 78, 621–631 (2023).
Yu, L., Gao, X. & Zhao, X. Global synthesis of the impact of droughts on crops’ water-use efficiency (WUE): towards both high WUE and productivity. Agri Syst. 177, 102723. https://doi.org/10.1016/j.agsy.2019.102723 (2020).
Hatfield, J. L. & Dold, C. Water-use efficiency: advances and challenges in a changing climate. Frontiers in plant science 10, 103, doi:Water-use efficiency: advances and challenges in a changing climate (2019).
Akhtar, S. S. et al. Bacillus licheniformis FMCH001 increases water use efficiency via growth stimulation in both normal and drought conditions. Front. Plant. Sci. 11, 297. https://doi.org/10.3389/fpls.2020.00297 (2020).
Sabouri, A. et al. Screening of rice drought-tolerant lines by introducing a new composite selection index and competitive with multivariate methods. Sci. Rep. 12, 2163. https://doi.org/10.1038/s41598-022-06123-9 (2022).
Mariey, S. & Khedr, R. A. Evaluation of some Egyptian barley cultivars under water stress conditions using drought tolerance indices and multivariate analysis. J. Sustain. Agri Sci. 43, 105–114. https://doi.org/10.21608/jsas.2017.1061.1005 (2017).
Cabello, R., Monneveux, P., De Mendiburu, F. & Bonierbale, M. Comparison of yield based drought tolerance indices in improved varieties, genetic stocks and landraces of potato (Solanum tuberosum L). Euphytica 193, 147–156 (2013).
Wang, M., Zheng, Q., Shen, Q. & Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 14, 7370–7390 (2013).
Das, S., Chakdar, H., Kumar, A., Singh, R. & Saxena, A. K. Chasmophyte associated stress tolerant bacteria confer drought resilience to Chickpea through efficient nutrient mining and modulation of stress response. Sci. Rep. 14, 12189 (2024).
Damalas, C. A. Improving drought tolerance in sweet Basil (Ocimum Basilicum) with Salicylic acid. Sci. Hortic. 246, 360–365. https://doi.org/10.1016/j.scienta.2018.11.005 (2019).
Lazarević, B. et al. Application of phenotyping methods in detection of drought and salinity stress in Basil (Ocimum Basilicum L). Front. Plant. Sci. 12, 629441. https://doi.org/10.3389/fpls.2021.629441 (2021).
Thakur, S., Singh, A., Insa, B. & Sharma, S. Food fortification in India as malnutrition concern: a global approach. Sustain. Food Technol. https://doi.org/10.1039/D3FB00079F (2023).
Consentino, B. B. et al. Current acquaintance on agronomic biofortification to modulate the yield and functional value of vegetable crops: A review. Hortic 9, 219 (2023).
Bouis, H. In Hidden Hunger: Strategies To Improve Nutrition QualityVol. 118112–122 (Karger, 2018).
Dung, P. et al. in IOP Conference Series: Materials Science and Engineering. 012092 (IOP Publishing).
Kumar, A. et al. Delineation of Ocimum gratissimum L. complex combining morphological, molecular and essential oils analysis. Ind. Crops Prod. 139, 111536 (2019).
Saran, P. L. et al. Identification of suitable chemotype of Ocimum gratissimum L. for cost effective Eugenol production. Ind. Crops Prod. 191, 115890 (2023).
Charles, D. J. & Simon, J. E. A new geraniol chemotype of Ocimum gratissimum L. J. Essent. Oil Res. 4, 231–234 (1992).
Padalia, R. C. & Verma, R. S. Comparative volatile oil composition of four Ocimum species from Northern India. Nat. Prod. Res. 25, 569–575 (2011).
Pinto, J. et al. Chemical characterization of the essential oil from leaves of Basil genotypes cultivated in different seasons. Bol. Latinoam. Caribe Plantas Med. Aromat. 18 (1), 58–70 (2019).
Çakmakçı, R., Mosber, G., Milton, A. H., Alatürk, F. & Ali, B. The effect of auxin and auxin-producing bacteria on the growth, essential oil yield, and composition in medicinal and aromatic plants. Curr. Microbiol. 77, 564–577 (2020).
Monfort, L. E. F. et al. Effects of plant growth regulators, different culture media and strength MS on production of volatile fraction composition in shoot cultures of Ocimum basilicum. Ind. Crops Prod. 116, 231–239 (2018).
Santoro, M. V., Zygadlo, J., Giordano, W. & Banchio, E. Volatile organic compounds from rhizobacteria increase biosynthesis of essential oils and growth parameters in peppermint (Mentha piperita). Plant. Physiol. Biochem. 49, 1177–1182 (2011).
Silva, S. et al. Essential oil composition of Melissa officinalis L. in vitro produced under the influence of growth regulators. J. Brazil Chem. Soc. 16, 1387–1390 (2005).
Castilho, C. V. et al. In vitro propagation of a carvacrol-producing type of Lippia Origanoides kunth: A promising oregano-like herb. Ind. Crops Prod. 130, 491–498 (2019).
Jiang, Y. et al. Linalool induces resistance against tobacco mosaic virus in tobacco plants. Plant. Dis. 107, 2144–2152 (2023).
Zhao, M. et al. Eugenol functions as a signal mediating cold and drought tolerance via UGT71A59-mediated glucosylation in tea plants. Plant. J. 109, 1489–1506 (2022).
Acknowledgements
This work has been done under support of Iran National Science Foundation (INSF) by project number 4000645.
Author information
Authors and Affiliations
Contributions
Hamide Fatemi: Writing – original draft, Review & editing, Data Curation, Investigation, Formalanalysis, Data curation, Conceptualization, Visualization. Majid Azizi: Review & editing, Investigation, Funding acquisition, Supervision, Methodology, Conceptualization. Amirali Salavati NiK: Methodology, Data Curation, Formal analysis. Mohammadhossein Ravanbakhsh: Writing, Review & editing, Investigation, Methodology, Formal analysis, Supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This research complies with the ethical rules applicable for this journal.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fatemi, H., Azizi, M., Salavati Nik, A. et al. Transplanting drought-Protective Bacteria to enhance clove basil’s (Ocimum gratissimum) drought tolerance. Sci Rep 15, 25863 (2025). https://doi.org/10.1038/s41598-025-09941-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09941-9