Abstract
The effects of conjugated equine estrogens (CEE) and 17β-estradiol-based hormone replacement therapy (HRT) on atherosclerosis development remain controversial. Here, we investigated the effects of equilin, a major compound in CEE, and 17β-estradiol on atherosclerosis development in an atherosclerotic mouse model. Female B6.KOR/StmSlc-Apoeshl mice were ovariectomized and fed a high-fat diet for 9 and 12 weeks (early and late groups, respectively) and then treated with 17β-estradiol or equilin. Atherosclerotic lesions in the aortic arch and brachiocephalic artery (BCA) were assessed at the end of the experimental period. Compared with placebo, equilin and 17β-estradiol significantly inhibited atherosclerotic lesion formation in the aortic arch and BCA in both groups. However, 17β-estradiol had a significantly greater inhibitory effect than equilin in the late group. Although 17β-estradiol significantly inhibited atherosclerosis progression in the aortic root, no significant difference was observed between the equilin and placebo groups. Additionally, compared with equilin, 17β-estradiol significantly inhibited atherosclerotic plaque formation in the aortic root. Moreover, 17β-estradiol exerted a stronger inhibitory effect on atherogenesis than equilin and control. Both 17β-estradiol and equilin protect against atherosclerotic plaque formation in the vascular endothelium, with 17β-estradiol exhibiting a superior effect.
Similar content being viewed by others
Introduction
Hormone replacement therapy (HRT) is designed to treat climacteric disorders caused by estrogen deficiency and reduce the risk of osteoporosis1,2,3. Epidemiological studies have indicated that estrogen can curtail the risk of cardiovascular disease and maintain reproductive function in women4. Therefore, HRT is an effective strategy in the health care of postmenopausal women. However, HRT has also been associated with some unfavorable events, as observed in the Women’s Health Initiative (WHI) trial5.
The WHI trial was a large epidemiological study conducted in the United States in 2002, which examined the association between lifestyle and health habits and the development of cancer, cardiovascular disease, and osteoporosis in postmenopausal women5. Contrary to expectations, the trial showed that HRT was not effective in reducing the risk of coronary heart disease (CHD).
Similarly, the Heart and Estrogen/Progestin Replacement Study (HERS), which was a randomized trial on the use of estrogen plus progestin for preventing CHD, found that the treatment comprising 0.625 mg of conjugated equine estrogens (CEE) and 2.5 mg of medroxyprogesterone acetate (MPA) had no cardiovascular benefit and increased the risk of CHD events6. Furthermore, both the Women’s Angiographic Vitamin and Estrogen (WAVE) trial (CEE + MPA) and Papworth HRT atherosclerosis study (transdermal HRT) reported a higher risk of CHD in the HRT group compared with the control group7,8.
However, the above studies had some constraints, such as starting HRT in women over 60 years of age. Additionally, secondary analyses have indicated variations in the effects of HRT on atherosclerosis depending on the characteristics of each woman9,10. In contrast, the International Menopause Society (IMS) reported in 2016 that estrogen therapy reduces cardiovascular disease in women younger than 60 years or 10 years post-menopause and that HRT does not increase the risk of cardiovascular disease in women of the same age11. Considering the variations in the types, regimens, and timing of HRT initiation after menopause in previous studies, it is difficult to determine whether HRT increases the risk of cardiovascular disease.
Recently, some fundamental clinical studies have indicated that estradiol exerts protective effects against atherosclerosis. Some studies have also reported the potential of CEE in inhibiting atherosclerosis12. CEE is a mixture of about 10 different estrogens derived from the urine of pregnant horses, including equilin (Eq) and equilenin13. We hypothesized that significant differences in results observed in some studies might be due to Eq. In our previous in vitro study, we found that Eq, which is present in CEE but not physiologically present in humans, increases the levels of cell adhesion molecules and monocyte-endothelial cell adhesion, leading to an elevated risk of atherosclerosis onset14. Therefore, we suggested that Eq may contribute to atherosclerotic diseases in women treated with HRT. However, our study demonstrated the effect of Eq with only early stage of atherosclerosis and did not consider in vivo effects such as low-density lipoprotein cholesterol, nitric oxide-mediated vasodilation, and the response of blood vessels to injury. Furthermore, research on the comparison of the effects of each CEE component and natural 17β-estradiol (E2) on atherosclerosis is limited.
Therefore, the present study aimed to investigate the effect of Eq and E2 on atherosclerosis risk in Apoeshl mice, a commonly used murine model for examining atherosclerosis risk15. Specifically, we investigated the formation of atherosclerotic lesions in the aortic arch, brachiocephalic artery (BCA), and aortic sinus of mice at two different time points. Additionally, we assessed the lipid profiles of the experimental mice.
Methods
Animals
All animal experiments were approved by the Ethics Committee of the Kyoto Prefectural University of Medicine (approval no. ERB-C-1519-1) and conducted in accordance with the animal welfare guidelines of the Kyoto Prefectural University of Medicine and ARRIVE guidelines. Female B6.KOR/StmSlc-Apoeshl mice (4 weeks old) were purchased from Japan SLC Inc. (Hamamatsu, Japan). Apoeshl mice are congenic mice carrying Apoeshl, the gene associated with hyperlipidemia. As these mice mimic ApoE-knockout mice, they are used as a model for hyperlipidemia and atherosclerosis16. The mice were maintained in a specific pathogen-free environment at a constant temperature (22% ± 1 °C) and 50–60% humidity under a 12:12-h light/dark photoperiod. They were fed standard mouse chow and had ad libitum access to water.
At 6 weeks of age, the mice were weighed, ovariectomized (OVX) or sham-operated, and fed a high-fat and high-cholesterol (21% wt/wt saturated fat and 0.2% wt/wt cholesterol) diet (D12108C; Research Diets, New Brunswick, NJ, USA) for 9 or 12 weeks. OVX was performed via laparotomy with the mice under isoflurane anesthesia following previously reported techniques17. The sham procedure was also performed via laparotomy under similar conditions. The mice were weighed weekly during the experimental period and randomly assigned to three groups: E2, Eq, and control (C). After ovariectomy or sham-operation, mice in the E2 and Eq groups received E2 (#NE-121) and Eq (#NE-211) pellets, respectively, for 9 or 12 weeks (1.11 µg/day), and those in the control group received placebo pellets (#NC-111) (Innovative Research of America, Sarasota, FL, USA). All pellets were 3 mm in diameter; they were punctured with a subcutaneous trocar and implanted in the posterior neck of the mice subcutaneously. Thereafter, the mice were assigned to nine groups: early sacrificed (15-week-old) OVX + E2 (n = 3), early sacrificed OVX + Eq (n = 3), early sacrificed OVX + placebo (n = 3), late-sacrificed (18-week-old) OVX + E2 (n = 9), late-sacrificed OVX + Eq (n = 9), late-sacrificed OVX + placebo (n = 9), late-sacrificed sham-operated + E2 (n = 5), late-sacrificed sham-operated + Eq (n = 5), and late-sacrificed sham-operated + placebo (n = 5). After 9 or 12 weeks on a high-fat diet, the mice were fasted overnight and sacrificed using high concentrations of isoflurane. The protocol used in this study is illustrated in Fig. 1A.
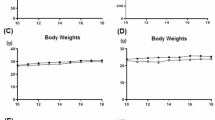
(A) Schematic illustration of the study protocol. (B,C); Body weight and uterine weight of mice at 18 weeks of age. (B) Comparison of body weight between the placebo (solid line), equilin (Eq; center line), and 17β-estradiol (E2; dashed line) groups. (C) Comparison of uterine weight between the placebo (solid bars), Eq (dotted bars), and E2 groups (open bars). *p < 0.01; n.s., not significant. (D–I); Lipoprotein profile of the serum of mice treated with placebo, equilin (Eq), or 17β-estradiol (E2). (D) Total cholesterol, (E) very-low-density lipoprotein (VLDL), (F) LDL, (G) high-density lipoprotein (HDL), (H) triglyceride, and (I) chylomicron levels. Solid bars indicate placebo, dotted bars indicate Eq, and open bars indicate E2.
Tissue preparation
At the end of the treatment, blood samples (500–800 µL) were collected from the right atrium of each mouse and centrifuged at 3000×g for 10 min to collect the serum, which was stored at − 20 °C for further analysis. After dissection, the uterine weight was recorded. Mouse tissues were prepared according to previously described procedures with some modifications15. After collecting the serum and making an incision in the right auricle to drain fluids, the heart, including the aortic root, aortic arch, and BCA, was perfused with PBS using a perfusion fixation tool set (Genostaff Co., Ltd., Tokyo, Japan) and dissected. The aortic arch and BCA were prepared for en face analysis; the heart, including the aortic root, was embedded in Tissue-Tek® optimal cutting temperature (OCT) compound (Sakura Finetek, Staufen, Germany) for Oil Red O staining.
En face analysis of atherosclerotic lesion in BCA and aortic arch
The en face procedure was performed as described previously, with some modifications15. Briefly, the aortic arch and BCA were fixed in 10%, 20%, and 30% sucrose for 24 h, opened and pinned to be flat, and stained with Oil Red O (Muto Pure Chemicals Co., Ltd., Tokyo, Japan) to assess fat deposition. Specifically, the tissue was washed with 60% isopropyl alcohol, immersed in the staining solution, incubated at 37 °C for 15 min, and promptly washed with 60% isopropyl alcohol. Quantification of lesions in the BCA and aortic arch was performed using the ImageJ software, as previously described15.
Measurements of atherosclerotic lesions in the aortic root
Following perfusion with PBS to remove circulating blood, the hearts and aortic roots were harvested and mounted in Tissue-Tek® OCT compound and rapidly frozen at − 80 °C. Thereafter, frozen aortic roots were cut into 10 μm-thick sections using a cryostat (Leica CM1950), fixed in cold acetone, incubated in Oil Red O solution for 20 min, and counterstained with hematoxylin. Lesion quantification was performed on 100-μm consecutive sections using the ImageJ software (version 1.54i).
Cholesterol and lipid profiling
Lipoprotein profiling of murine serum samples was conducted using LipoSEARCH (Skylight Biotech Inc., Akita, Japan).
Statistical analysis
Comparisons among groups were performed using Kruskal–Wallis test with Dunn’s multiple comparisons test. Data are presented as mean ± standard error of mean (SEM). Statistical significance was set at p < 0.05. All data analyses were performed using the GraphPad Prism (GraphPad Software, Boston, MA, USA) and R software.
Results
E2 and Eq did not alter lipid profiles in B6.KOR/StmSlc-Apoe shl mice
We examined the lipid profiles, body weights, and uterine weights of the mice at 18 weeks of age (long group). Notably, no mortality was recorded during the experimental period. Additionally, there was no significant difference in body weight between the groups at any age (Fig. 1B) or lipid profile (Fig. 1D–I). However, the uterine weight was significantly higher in the E2 group than in the placebo and Eq groups (Fig. 1C).
E2 protects against the development of atherosclerosis
Based on our previous in vitro study results, we hypothesized that Eq promotes atherogenesis. Therefore, we investigated the effects of E2 and Eq on the development of atherosclerotic lesions in the aortic arch and BCA of the experimental mice using en face analysis. In the short group (9 weeks after OVX and on the high-fat diet), Eq and E2 treatments significantly protected against the development of atherosclerosis in both the aortic arch and BCA, as evidenced by a significant decrease in lesion areas in the Eq and E2 groups compared with that in the placebo group. Additionally, there was no significant difference in lesion area in the BCA between the Eq and E2 groups (Fig. 2A,C,D).
En face analysis of the aortic arch and brachiocephalic artery (BCA) of a mouse model of atherosclerosis. (A,B); Atherosclerotic lesions in the aortic arch and BCA. Representative en face micrographs of aortic arches with lipid-rich plaques stained with Oil Red O (in red) in 15-week-old mice fed a high-fat diet for 9 weeks (A) and in 18-week-old mice fed a high-fat diet for 12 weeks (B). Scale bar, 2 mm. (C–H); The relative lesion area was calculated by dividing the total arch area by the plaque area. Given that there are no anatomical landmarks defining the aortic arch, the total arch area is usually measured from the beginning of the ascending aorta to the first intercostal branch. Similarly, the relative lesion area was calculated by dividing the total BCA area by the plaque area. In the short group (9 weeks on a high-fat diet), the bar graph shows the mean atherosclerotic lesion area in the (C) aortic arch (placebo, n = 3; Eq, n = 3; E2, n = 3) and (D) BCA (placebo, n = 3; Eq, n = 3; and E2, n = 3). In the long group (12 weeks on a high-fat diet), the bar graph shows the mean atherosclerotic lesion area in the (E) aortic arch (placebo, n = 9; Eq, n = 9; and E2, n = 9) and (F) BCA (placebo, n = 9; Eq, n = 9; and E2, n = 9). In the sham group, the bar graph shows the mean atherosclerotic lesion area in the (G) aortic arch (placebo, n = 5; Eq, n = 5; E2, n = 5) and (H) BCA (placebo, n = 5; Eq, n = 5; E2, n = 5). Comparison of lesions between the placebo (solid bars), Eq (dotted bars), and E2 groups (open bars). *p < 0.05, **p < 0.01.
In the long group (12 weeks after OVX and on the high-fat diet), although Eq treatment exerted a greater protective effect on the development of atherosclerosis in the aortic arch and BCA than the placebo treatment, E2 treatment significantly inhibited atherosclerosis progression in both the aortic arch and BCA compared with Eq and placebo treatments (Fig. 2B,E,F). Although there was no significant difference in the inhibitory effects of Eq and E2 on the formation of atherosclerotic lesions in the short group, E2 treatment exerted a significantly stronger inhibitory effect on atherosclerosis than Eq treatment in the long group (Fig. 2E,F). In the sham group (12 weeks after sham operation and on the high-fat diet), E2 treatment, compared with placebo treatment, significantly prevented atherosclerosis development in the aortic arch; however, there was no significant difference in lesion area in the BCA among the groups (Fig. 2G,H). There were no significant differences in lesion area in the BCA between the sham and OVX groups treated with the placebo, Eq, and E2 (Fig. 2E–H).
E2 protects against the development of atherosclerosis in the aortic root
Additionally, we examined the effects of Eq and E2 on atherogenesis in the aortic root in the mouse model. Specifically, we measured the plaque burden at 10 different locations on the aortic sinus to assess the progression of atherosclerosis. In the short group, there was no significant difference in the quantity of atherosclerotic plaque in the aortic root between the Eq and placebo groups at all locations except at the 0- and 300-µm sections of the aortic sinus (Fig. 3A,C). In contrast, E2 treatment significantly inhibited atherogenesis at all locations except at the 900-µm section of the aortic sinus compared with placebo (Fig. 3A,C). Importantly, E2 treatment had a significantly higher inhibitory effect on the formation of atherosclerotic plaques than placebo and Eq treatments in the aortic sinus at the 0–400-µm sections (Fig. 3C).
Atherosclerotic lesions in the aortic root. (A,B); Representative atherosclerotic lesions showing Oil Red O staining (in red color) of lipids in the aortic root at 300 µm from the aortic sinus (×50 magnification) in 15-week-old mice fed a high-fat diet for 9 weeks (A) and in 18-week-old mice fed a high-fat diet for 12 weeks (B). Scale bar, 500 µm. (C–E); Comparison the plaque burden. The graph shows the quantification of atherosclerotic lesion area from 10 consecutive sections. The relative lesion area can be calculated by dividing the atherosclerotic lesion by the total vessel area encircling the external elastic lamina of the aortic vessel wall. (C) 15-week-old mice fed a high-fat diet for 9 weeks after OVX. Solid, center, and dashed lines indicate the placebo (n = 3), Eq (n = 3), and E2 (n = 3) groups, respectively. (D) 18-week-old mice fed a high-fat diet for 12 weeks after OVX. Solid, center, and dashed lines indicate the placebo (n = 9), Eq (n = 9), and E2 (n = 9) groups, respectively. (E) 18-week-old mice fed a high-fat diet for 12 weeks after sham operation. Solid, center, and dashed lines indicate the placebo (n = 5), Eq (n = 5), and E2 (n = 5) groups, respectively. *p < 0.05 (placebo vs. E2); †p < 0.05 (Eq vs. E2); ♯p < 0.05 (placebo vs. Eq).
In the long groups, there was no significant difference in the quantity of atherosclerotic plaque between the placebo and Eq groups at the 0–400-µm sections (Fig. 3B,D). Compared with Eq treatment, E2 treatment significantly inhibited the formation of atherosclerotic plaque at all locations except at the 700–800-µm section (Fig. 3D). Moreover, E2 treatment had a significantly higher inhibitory effect on atherosclerotic plaque formation than placebo treatment at all positions (Fig. 3B,D). Furthermore, the mean values of atherosclerotic lesions in the aortic root for the long group was similar to those of the short group. In the sham groups, compared with placebo treatment, both Eq and E2 treatments significantly inhibited the formation of atherosclerotic plaque at the 400–700-µm sections (Fig. 3E). However, there were no significant differences between the sham and OVX groups treated with the placebo, Eq, and E2 (Fig. 3D,E).
Discussion
In this study, en face and aortic root analyses demonstrated that Eq and E2, the estrogens commonly used in HRT, inhibited atherosclerosis progression in a mouse model of atherosclerosis (B6.KOR/StmSlc-Apoeshl mice). However, E2 treatment had significantly higher inhibitory effect on the development of atherosclerosis than Eq treatment. To the best of our knowledge, this is the first in vivo study to compare the effect of Eq and E2 on the risk of atherosclerosis in female mice.
Although the incidence of atherosclerotic diseases, such as myocardial infarction and stroke, increases with age in both men and women, it is comparatively higher in postmenopausal women than in men of the same age18,19, which is attributable to a decrease in estrogen levels post-menopause. Dyslipidemia is an important risk factor for atherosclerosis, and low estrogen levels increase low-density lipoprotein (LDL) cholesterol levels, leading to the development of atherosclerosis20,21. Therefore, HRT may improve various symptoms associated with menopause and prevent atherosclerosis4. However, WHI reported that compared with the control group, the HRT group had an increased risk of CHD, stroke, and venous thrombosis5. Research findings suggest that the effects of HRT vary among patients depending on the timing of HRT initiation, steroid hormone dosage, and HRT regimen4,9,10,11,22,23.
Although randomized controlled trials did not reveal the inhibitory effect of estrogen on atherosclerosis development, several studies have suggested that E2 has a beneficial effect on vascular endothelial cells and protects against the development of atherosclerosis24,25. Recent studies have shown that E2 prevents atherosclerotic disease development and that E2 administration immediately after menopause significantly reduces the risk of mortality, heart failure, and myocardial infarction in postmenopausal women26. Additionally, CEE treatment, compared with transdermal E2 treatment, reportedly increases the levels of high-sensitivity C-reactive protein (CRP) and triglycerides but does not affect the formation of atherosclerotic plaques27. However, these studies are limited by poor participant selection, short observation period, and small samples. Overall, these results suggest that oral or transdermal E2 administration may be more effective than CEE in reducing the risk of atherosclerosis in postmenopausal women26,27,28. Although there is a consensus on the protective effect of E2 on vascular endothelial cells and the development of atherosclerosis in postmenopausal women, studies on the effects of estrogens, including CEE, on the risk of CHD are limited especially in basic research.
In the present study, we compared the effects of Eq and E2 on atherosclerosis in vivo in a murine model of atherosclerosis. Estrogen treatment suppressed the progression of atherosclerosis in a mouse model of atherosclerotic disease (B6.KOR/StmSlc-Apoeshl mice) without altering the lipid profile. Various systems have been developed to evaluate the progression of atherosclerotic disease in vivo, including en face and aortic root analyses15. Among the branches of the aortic arch, the BCA is the most specific branch for evaluating atherosclerotic plaque formation, and en face analysis enables direct evaluation of plaque formation in the BCA29. Aortic root analysis is superior for evaluating plaque formation, especially in the early stages, and is useful for both short- and long-term comparisons29. Here, Eq and E2 treatments effectively suppressed the formation of atherosclerotic lesions in both the short and long groups. However, E2 treatment was more effective than Eq treatment in suppressing atherosclerosis development, especially around the aortic root, which might be partially consistent with the results of a previous in vitro study14.
In the sham group, E2 treatment significantly inhibited the development of atherosclerosis; in contrast, Eq treatment did not significantly affect the formation of atherosclerotic plaque in the BCA. The aortic root analysis showed that Eq and E2 treatment effectively inhibited the formation of atherosclerotic lesions, with no significant difference between the groups. Furthermore, there were no significant differences between the sham and OVX groups treated with the placebo, Eq, and E2. It is likely that endogenous estrogen from the ovary alone was not sufficient to suppress atherosclerotic lesion formation in the high-fat diet-fed mouse model used in this study. Collectively, these results suggest that HRT may be effective in maintaining blood estrogen levels in postmenopausal women, which may reduce the risk of atherosclerosis. Like the WHI study5, we did not consider the effects of variation in the age of menopause initiation on the efficacy of Eq and E2. Therefore, the inhibitory effects of Eq and E2 treatments on atherosclerosis development at varying weeks after ovariectomy should be further examined.
In humans, elevated plasma cholesterol and triglyceride levels increase the risk of atherosclerotic disease and promote atherogenesis30,31. However, some reports suggest that the progression of atherosclerosis is not necessarily related to plasma lipid concentration32,33. In the present study, there was no significant difference in lipid profiles between the hormone-treated and placebo groups in the mouse model, which may be due to our small sample size (n = 3). Moreover, it has been suggested that LDL transferase and LDL oxidation may contribute more to atherosclerotic disease than plasma LDL concentrations34,35. However, further studies are warranted to determine how E2 and other estrogens suppress atherosclerosis development via lipid profile regulation and affect plaque composition, such as lipid, macrophage, and collagen content. In addition to reducing monocyte adhesion in the vascular endothelium, other factors, such as increased nitric oxide (NO) production or decreased oxidative stress and inflammation, may be involved in the preventive effect of estrogen on atherosclerosis36,37,38,39.
Despite the promising findings, this study has some major limitations. First, we used a murine model to examine atherosclerosis risk. Because the mice used were a hyperlipidemia model, the results might not be applicable to actual patients. Several studies have shown that CEE, E2, and other estrogens can improve cardiovascular endothelial function in older postmenopausal women12,22,40. On the other hand, some reports have indicated that estrogen’s protective effects do not extend to endothelial function in cases with high CHD risk such as hyperlipidemia or atherosclerotic lesions41. However, the present study suggests that both E2 and Eq may prevent atherosclerosis even in CHD high risk mice. These results imply that both E2 and Eq may be preferrable for women with low CHD risk without hyperlipidemia or atherosclerotic lesions in terms of reducing atherosclerosis risk. Second, the assessment time of the early group was set at 12 weeks (6 weeks after OVX) initially; however, the en face analysis showed that there was almost no atheroma formation in the aortic arch or BCA (data not shown), making comparisons impossible. Therefore, mice aged 15 weeks (9 weeks after OVX) were used as the short group. Furthermore, as the en face and aortic root analyses are very precise, focus on 12-week analysis resulted in a smaller sample size for the 9-week group. Third, the possible mechanism resulting in the different effects of E2 and Eq on atherosclerotic lesions remains unknown. Furthermore, the weight of uterus in the Eq group was lower than that in the E2 group. It is speculated that the differences in the effects of each estrogen on estrogen receptors in different organs in mice may be responsible for the above differences. Therefore, more detailed studies on the mechanism are needed in the future. Finally, Eq was administered subcutaneously, which differs from the actual HRT method. Contrary to previous in vitro study results, the inhibitory effects of Eq treatment on atherosclerosis may be due to the lack of a first-pass effect following transdermal administration42. Additionally, the elimination of the cholesterol clearance pathway in the mouse model resulted in a rapid disease course, whereas in humans the disease progresses slowly. In humans, the end-stage plaque rupture causes major complications, but this phenomenon does not occur in mouse models43. Therefore, the results of the present study may not fully reflect the clinical conditions in humans.
In conclusion, this study showed that subcutaneous administration of Eq and E2 inhibits the formation of atherosclerotic lesions without altering the lipid profile in mice, with E2 showing a superior effect. Overall, these results suggest that short- and long-term use of E2 rather than CEE containing Eq may have a better inhibitory effect on the development of atherosclerosis. However, it is necessary to investigate the effects of other estrogens included in CEE such as estrone-sulfate, a main component of CEE, and progestins, including MPA and natural progesterone, on atherosclerosis development to elucidate the actual effects of each HRT regimen in clinical settings. Additionally, further research is needed to elucidate the mechanisms of HRT regimens for the prevention of atherosclerosis.
Data availability
All data generated or analyzed in this study are included in this published article and are available from the corresponding author upon reasonable request.
References
Campbell, S. & Whitehead, M. Oestrogen therapy and the menopausal syndrome. Clin. Obstet. Gynaecol. 4, 31–47 (1977).
Nelson, H. D. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA 291, 1610–1620 (2004).
Marcus, R. et al. The relationship of biochemical markers of bone turnover to bone density changes in postmenopausal women: Results from the postmenopausal estrogen/progestin interventions (PEPI) trial. J. Bone Miner. Res. 14, 1583–1595 (1999).
Stampfer, M. J. et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N. Engl. J. Med. 325, 756–762 (1991).
Rossouw, J. E. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288, 321–333 (2002).
Hulley, S. et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and estrogen/progestin replacement study (HERS) research group. JAMA 280, 605–613 (1998).
Waters, D. D. et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: A randomized controlled trial. JAMA 288, 2432–2440 (2002).
Clarke, S. C., Kelleher, J., Lloyd-Jones, H., Slack, M. & Schofiel, P. M. A study of hormone replacement therapy in postmenopausal women with ischaemic heart disease: The Papworth HRT atherosclerosis study. BJOG 109, 1056–1062 (2002).
Hsia, J. et al. Conjugated equine estrogens and coronary heart disease: The Women’s health initiative. Arch. Intern. Med. 166, 357–365 (2006).
Manson, J. E. et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: The women’s health initiative randomized trials. JAMA 318, 927–938 (2017).
de Villiers, T. J. et al. Revised global consensus statement on menopausal hormone therapy. Maturitas 91, 153–155 (2016).
Appt, S. E., Clarkson, T. B., Lees, C. J. & Anthony, M. S. Low dose estrogens inhibit coronary artery atherosclerosis in postmenopausal monkeys. Maturitas 55, 187–194 (2006).
Bhavnani, B. R. Pharmacokinetics and pharmacodynamics of conjugated equine estrogens: chemistry and metabolism. Proc. Soc. Exp. Biol. Med. 217, 6–16 (1998).
Ito, F. et al. Equilin in conjugated equine estrogen increases monocyte-endothelial adhesion via NF-κB signaling. PLoS ONE 14, e0211462 (2019).
Centa, M., Ketelhuth, D. F. J., Malin, S. & Gisterå, A. Quantification of Atherosclerosis in Mice. J. Vis. Exp. 148, e59828 (2019).
Matsushima, Y., Hayashi, S. & Tachibana, M. Spontaneously hyperlipidemic (SHL) mice: Japanese wild mice with apolipoprotein E deficiency. Mamm. Genome 10, 352–357 (1999).
Souza, V. R. et al. Description of ovariectomy protocol in mice. Methods Mol. Biol. 2019(1916), 303–309 (2019).
Maas, A. et al. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: A consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur. Heart J. 42, 967–984 (2021).
Xiang, D., Liu, Y., Zhou, S., Zhou, E. & Wang, Y. Protective effects of estrogen on cardiovascular disease mediated by oxidative stress. Oxid. Med. Cell. Longev. 2021, 5523516 (2021).
Tribble, D. L., Holl, L. G., Wood, P. D. & Krauss, R. M. Variations in oxidative susceptibility among six low density lipoprotein subfractions of differing density and particle size. Atherosclerosis 93, 189–199 (1992).
Jensen, J., Nilas, L. & Christiansen, C. Influence of menopause on serum lipids and lipoproteins. Maturitas 12, 321–331 (1990).
Hodis, H. N. et al. Vascular effects of early versus late postmenopausal treatment with estradiol. N. Engl. J. Med. 374, 1221–1231 (2016).
Miller, V. M. et al. Using basic science to design a clinical trial: Baseline characteristics of women enrolled in the Kronos early estrogen prevention study (KEEPS). J Cardiovasc Transl Res 2, 228–239 (2009).
Li, P. et al. 17β-estradiol enhances vascular endothelial Ets-1/miR-126-3p expression: The possible mechanism for attenuation of atherosclerosis. J. Clin. Endocrinol. Metab. 102, 594–603 (2017).
Rodríguez, E., López, R., Paez, A., Massó, F. & Montaño, L. F. 17Beta-estradiol inhibits the adhesion of leukocytes in TNF-alpha stimulated human endothelial cells by blocking IL-8 and MCP-1 secretion, but not its transcription. Life Sci. 71, 2181–2193 (2002).
Schierbeck, L. L. et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: Randomised trial. BMJ 345, e6409 (2012).
Lacut, K. et al. Differential effects of oral and transdermal postmenopausal estrogen replacement therapies on C-reactive protein. Thromb. Haemost. 90, 124–131 (2003).
Vehkavaara, S. et al. Effects of oral and transdermal estrogen replacement therapy on markers of coagulation, fibrinolysis, inflammation and serum lipids and lipoproteins in postmenopausal women. Thromb. Haemost. 85, 619–625 (2001).
Andrés-Manzano, M. J., Andrés, V. & Dorado, B. Oil red O and hematoxylin and eosin staining for quantification of atherosclerosis burden in mouse aorta and aortic root. Methods Mol. Biol. 1339, 85–99 (2015).
Nordestgaard, B. G. & Varbo, A. Triglycerides and cardiovascular disease. Lancet 384, 626–635 (2014).
Burchfiel, C. M. et al. Combined effects of HDL cholesterol, triglyceride, and total cholesterol concentrations on 18-year risk of atherosclerotic disease. Circulation 92, 1430–1436 (1995).
Lepage, S. et al. Oxidizability of atherogenic low-density lipoprotein subspecies in severe familial hypercholesterolemia: Impact of long-term low-density lipoprotein apheresis. J. Cardiovasc. Pharmacol. Ther. 5, 87–103 (2000).
Chancharme, L. et al. LDL particle subclasses in hypercholesterolemia. Molecular determinants of reduced lipid hydroperoxide stability. J. Lipid Res. 43, 453–462 (2002).
Ishigaki, Y. et al. Impact of plasma oxidized low-density lipoprotein removal on atherosclerosis. Circulation 118, 75–83 (2008).
Khatana, C. et al. Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxid. Med. Cell. Longev. 2020, 5245308 (2020).
Guetta, V. et al. The role of nitric oxide in coronary vascular effects of estrogen in postmenopausal women. Circulation 96, 2795–2801 (1997).
Cossette, É., Cloutier, I., Tardif, K., DonPierre, G. & Tanguay, J. F. Estradiol inhibits vascular endothelial cells pro-inflammatory activation induced by C-reactive protein. Mol. Cell. Biochem. 373, 137–147 (2013).
Iorga, A. et al. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 8, 33 (2017).
Aryan, L. et al. The Role of estrogen receptors in cardiovascular disease. Int. J. Mol. Sci. 21, 4314 (2020).
Daugherty, A. et al. Recommendation on design, execution, and reporting of animal atherosclerosis studies: A scientific statement from the American heart association. Circ. Res. 121, e53–e79 (2017).
Yeboah, J., Reboussin, D. M., Waters, D., Kowalchuk, G. & Herrington, D. M. Effects of estrogen replacement with and without medroxyprogesterone acetate on brachial flow-mediated vasodilator responses in postmenopausal women with coronary artery disease. Am. Heart J. 153, 439–444 (2007).
Stevenson, J. C. Type and route of estrogen administration. Climacteric 12(Suppl 1), 86–90 (2009).
Schwartz, S. M., Galis, Z. S., Rosenfeld, M. E. & Falk, E. Plaque rupture in humans and mice. Arterioscler. Thromb. Vasc. Biol. 27, 705–713 (2007).
Acknowledgements
The authors thank Ayaka Miura and Dr. Masahiro Otani for technical assistance.
Funding
This work was partially supported by a Grant-in-Aid for Scientific Research (21K09546) from the Ministry of Education, Culture, Sports, Science, and Technology (Japan).
Author information
Authors and Affiliations
Contributions
AK, FI, and TM conceived and designed the experiments; AK, FI, OT, NT, MK, and KY performed the experiments; AK, NT, AK, EM, and YI analyzed the data and interpreted the results of the experiments; AK, FI, and TM edited and revised the manuscript; and all authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kakibuchi, A., Ito, F., Takaoka, O. et al. Effects of 17β-estradiol and equilin on atherosclerosis development in female Apoeshl mice. Sci Rep 15, 24922 (2025). https://doi.org/10.1038/s41598-025-10494-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10494-0