Abstract
In this study, we determined whether gypenosides exert a therapeutic effect on colitis. We then explored the underlying mechanism of gypenosides in the treatment of ulcerative colitis (UC) via multiomics analyses. Dextran sulfate sodium (DSS) was used to establish a UC model in mice, and a subgroup of colitis model mice was subjected to gypenosides intervention. The inflammatory manifestations of the model and drug groups were determined via histological and molecular experiments. In addition, tissue and blood samples were collected for transcriptome and metabolomics analysis, respectively. Immunohistochemistry was used to detect the key proteins involved in UC pathogenesis and drug intervention. By combining transcriptomics and metabolomics assays, we explored one of the possible mechanisms by which gypenosides ameliorate UC in mice. Gypenosides treatment significantly alleviated clinical symptoms, prevented colon shortening, and decreased the disease activity index (DAI) in an 3% DSS-induced UC mouse model. Transcriptome sequencing revealed that the intestinal stem genes Ascl2 and Lgr5 ranked forefront in terms of differential expression before and after intervention with gypenosides. The metabolomics results suggest that the tricarboxylic acid cycle (TCA) and amino acid metabolism (AM) were the main metabolic pathways associated with UC and gypenosides treatment. Taken together, these results suggest that gypenosides may affect the biological activity of stem cells by regulating the tricarboxylic acid cycle and glutamine metabolism, promoting the repair of the damaged mucosa. In this study, we demonstrated that gypenosides alleviate colitis, likely by regulating the expression of genes associated with stemness and modulating the tricarboxylic acid cycle and amino acid metabolism, in a DSS-induced colitis mouse model.
Similar content being viewed by others
Introduction
Ulcerative colitis (UC) is a chronic and recurrent autoimmune disease. The incidence of UC has been increasing globally over the past few decades, particularly in newly industrialized countries in Asia, such as China and India1. Although the exact cause of UC remains unclear, several studies indicate that various factors play important roles in its pathogenesis. These factors include genetics, environmental exposures, immune dysregulation, alterations in the gut microbiota, barrier dysfunction, and endoplasmic reticulum stress2. 5-Aminosalicylic acid drugs, steroids, immunosuppressants, immunomodulators, biologics, and surgery are the most widely used treatments for UC3. Although effective means of treatment have been applied in controlling UC, the management of UC has remained a challenge until now. Further studies are needed to discover new therapeutic drugs and preventive strategies.
Traditional Chinese Medicine (TCM) is recognized for its multitarget approach and low toxicity, making it increasingly important in research on the prevention and treatment of UC. Traditionally, Gynostemma pentaphyllum was prepared as a tea and valued for its delightful flavor and weight loss benefits. Researchers have further identified numerous bioactive compounds in Gynostemma pentaphyllum, including saponins (gypenosides), polysaccharides, flavonoids, and phytosterols. Studies indicate that the active components in Gynostemma pentaphyllum significantly inhibit the production and release of inflammatory mediators, thereby reducing intestinal inflammation. For example, one study demonstrated that Gynostemma pentaphyllum extract alleviates colitis symptoms in a rat model through inhibiting the nuclear factor-kappa B (NF-κB) signalling pathway. The herb has immunomodulatory effects, balancing the immune system and reducing autoimmune damage to the intestines4. Gynostemma pentaphyllum can also regulate the functions of T cells and B cells, promoting immune responses in vivo5. Moreover, gypenosides, the primary active components of Gynostemma pentaphyllum, have been reported to have significant anti-inflammatory and antioxidant effects6,7,8,9,10,11. Previous studies have demonstrated that gypenosides are capable of reducing oxidative stress damage to the intestines, and alleviating intestinal inflammation by inhibiting the release of inflammatory mediators, thereby suppressing inflammatory signalling pathways12. In terms of their anti-inflammatory and antioxidant effects, gypenosides show multifaceted potential in the treatment of intestinal diseases. Several studies have further demonstrated that it enhances intestinal barrier function and reduces intestinal permeability13. However, the potential therapeutic effects of gypenosides on UC have not been reported. Therefore, we aimed to evaluate the potential impact of gypenosides on UC and the underlying mechanisms in this study.
A series of studies have reported that high-throughput transcriptomics and metabolomics are effective approaches for elucidating the mechanisms of TCM active compounds14. Transcriptomic analysis reveals the relationships between gene expression patterns and cellular functions, biological processes, and disease mechanisms, and has been widely used to identify TCM-affected genes15,16.These genes directly participate in or regulate metabolic pathways, thereby influencing the production of and changes in metabolites. Metabolites can also influence gene expression through feedback mechanisms. When TCM is effective, integrated analysis helps establish causal networks from gene expression to metabolite production, revealing the underlying biological mechanisms17. In this study, we created an animal model of UC with dextran sulfate sodium (DSS) to explore the anti-UC effect of gypenosides. Additionally, we used a multiomics approach to screen potential targets and explore the mechanism of gypenosides. To confirm the relevant mechanism, we detected the expression of candidate genes via molecular methods. In conclusion, we investigated the anti-UC effects of gypenosides and the underlying mechanisms.
Methods
Animals
Six-to eight-week-old male C57BL/6J mice were purchased from Hunan SJA Laboratory Animal Co., Ltd. The mice were housed in a specific pathogen-free (SPF) facility at the Animal Center of South China University. The environmental conditions were maintained at a temperature of 22 ± 2 °C and a humidity of 55 ± 5%, with a 12 h light/dark cycle. All the mice were acclimated for 1 week prior to the experiments. All procedures will be approved by the First Hospital of South China University (2023LL0908001), in line with the Guide for ARRIVE to decrease animal number and suffering as much as possible. All methods were carried out in accordance with relevant regulations and ARRIVE guidelines.
Construction of UC animal models
C57BL/6J mice were first stratified into blocks of three based on their initial body weight to ensure homogeneity within blocks. Then mice were randomly divided into three groups: the control (NC) group, the model (DSS) group, and the gypenosides (GP) group, with 10 mice in each group. Except for those in the control group, all the mice were administered 3.0% DSS for 7 days to induce acute colitis. The mice in the gypenosides (GP) group were gavaged with a 400 mg/kg gypenosides solution until the DSS treatment was stopped. During the modeling period, monitoring of body weight, stool consistency, and the presence of occult or gross blood in the stool was conducted to assess the progression of colitis18.
Histopathological validation
On the 8th day of the experiment, the mice were anaesthetized prior to sacrifice using carbon dioxide. The length of each colon was measured. Colon tissues were harvested, fixed in 10% neutral-buffered formalin, embedded in paraffin, and sectioned. The tissue sections were stained with haematoxylin and eosin (H&E) to evaluate histopathological changes. Histopathological evaluation were performed via a standard scoring system to quantify the severity of inflammation and tissue damage19.
Enzyme-linked immunosorbent assay (ELISA)
The levels of TNF-α, and IL-6 in colon tissues were quantified using ELISA kits following the manufacturer’s protocols. The concentrations in the colonic tissues were normalized using a bicinchoninic acid (BCA) assay.
Transcriptomic sequencing
Total RNA was extracted from colon tissues using TRIzol reagent following the manufacturer’s protocol. The RNA quality and concentration were assessed using a NanoDrop spectrophotometer and Agilent Bioanalyzer. RNA-Seq libraries were prepared using the Illumina TruSeq RNA Sample Preparation Kit. After library construction, PCR amplification was employed to enrich library fragments, selecting a library size of approximately 450 bp. The library was then quality checked via an Agilent 2100 Bioanalyzer, and both the total and effective concentrations of the library were measured. The raw sequencing data underwent quality control using FastQC, and then were filtered using HISAT2 to remove low-quality sequences. These high-quality sequences were then aligned to the reference genome of the species. The remaining high-quality sequences were then aligned to the reference genome of the species. This alignment allows us to determine the genomic position of each sequence and calculate the expression level of each gene. Differentially expressed genes (DEGs) were subsequently identified via DESeq2 with the criteria of |log2-fold change| > 1 and an adjusted p value < 0.05.
Gene ontology (GO) and kyoto encylcopedia of genes and genomes (KEGG) pathway enrichment analysis
All identified genes were subjected to KEGG pathway and GO enrichment analyses via the “clusterProfiler” and “topGO”, respectively, with annotations from “org.Mm.eg.db” in R. The significance thresholds were set at a p value < 0.01 and a q-value < 0.05.
Pathway impact diagram
A pathway impact diagram combines the centrality of metabolites within a network and pathway enrichment results. It calculates the sum of importance measures for each matched metabolite, then divides this sum by the total importance measures of all metabolites within each pathway. For the importance measures, we utilized both betweenness centrality and out-degree centrality.
Immunohistochemical validation
Paraffin-embedded colon sections were deparaffinized, rehydrated, and subjected to antigen retrieval. The sections were incubated with the primary antibody at 4 °C overnight. After the samples were washed, they were incubated with an HRP-conjugated secondary antibody at room temperature for 1 h. Freshly prepared DAB substrate solution was added for colour development. The slides were counterstained with haematoxylin and examined via a light microscope.
Metabolomic analysis
Blood samples were delivered to Bioprofile for the metabolomics analysis. Blood samples were collected by enucleating the eyes via sterile surgical tweezers. After allowing the blood to sit for 30 min, it was centrifuged (3500 rpm, 4 °C, 30 min) to obtain the serum, which was then stored at -80 °C for future use. Metabolomic analysis was conducted using a UPLC-ESI-Q-Orbitrap-MS system. QC samples were used to equilibrate the chromatography-mass spectrometry system, monitor instrument performance, and assess overall system stability throughout the experimental process. For liquid chromatography, samples were separated on an Acquity UPLC column from Waters. The flow rate was maintained at 0.3 mL/min with mobile phase A (0.1% formic acid in water) and mobile phase B (100% acetonitrile). The gradient program was as follows: 0% B for 2 min, increased to 48% B over 4 min, ramped up to 100% B over 4 min, held at 100% B for 2 min, decreased to 0% B in 0.1 min, and then re-equilibrated for 3 min. Mass spectrometry data were acquired in both positive and negative modes using electrospray ionization (ESI). The HESI source conditions were: spray voltage at 3.8 kV (positive) and 3.2 kV (negative); capillary temperature at 320 °C; sheath gas flow rate at 30 ARB; auxiliary gas flow rate at 5 ARB; probe heater temperature at 350 °C; and S-lens RF level at 50. The full MS scan range was 70–1050 m/z, with a resolution of 70,000 at m/z 200 for full MS scans and 17,500 at m/z 200 for MS/MS scans. The maximum injection time was set to 100 ms for MS and 50 ms for MS/MS. The MS2 isolation window was 2 m/z, with stepped normalized collision energies of 20, 30, and 40. Processed data underwent rigorous quality control and transformation. Metabolic features exhibiting > 50% missingness within any experimental group were excluded, followed by independent total peak area normalization of positive and negative ionization mode datasets. Subsequently, integrated cross-mode features were subjected to multivariate pattern recognition using Python.The variable importance for the projection (VIP) scores obtained from the OPLS-DA model were used to assess the impact and explanatory power of each metabolite’s expression pattern in distinguishing between different sample groups. This approach helps to identify biologically significant differentially abundant metabolites. One-way ANOVA test was used to analyse the significance of metabolites across multiple groups. Metabolites with a p value less than 0.05 and an OPLS-DA VIP score greater than 1 were considered statistically significant. The pathway impact plot is a combination of the center of the metabolite network and pathway enrichment results. The sum of importance measures for each matching metabolite is calculated and then divided by the sum of importance measures for all metabolites with in each pathway. We used betweenness centrality and out degree centrality to measure importance.
Integrated transcriptomic and metabolomic analysis
Integrated analysis of transcriptomic and metabolomic data were performed to identify correlations between gene expression and metabolite levels. Correlation analysis refers to the examination of two or more correlated variables to measure the degree of association between them. The Spearman correlation coefficient can be used to assess the relationships between species diversity and metabolites in environmental samples. In this study, the Spearman correlation method was used to calculate the correlation coefficients between significantly different metabolites and significantly different genes in R (version 4.0.5). To streamline the data for visualization, we ranked the elements on the basis of the absolute value of their correlation coefficients between the two omics methods and selected the top 60 elements. These elements were used for subsequent correlation coefficient matrix heatmaps, hierarchical clustering heatmaps, and correlation network diagrams.
Statistical analysis
The data are presented as the means ± standard deviation (SDs). Comparisons between groups were statistically analysed using Student’s t-test or ANOVA (post-test: Tukey), depending on the experimental design. Statistical significance was set at a p value < 0.05. All analyses were performed using GraphPad Prism or SPSS software.
Results
Gypenosides alleviate symptoms in DSS-induced UC model mice
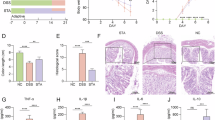
To evaluate the therapeutic effect of gypenosides on UC, DSS-induced UC model mice were treated with gypenosides(Fig. 1A). Compared with their initial body weights, the body weights of the mice treated with gypenosides were greater (Fig. 1B), and the disease activity indexes (DAIs) were lower (Fig. 1C). Treatment with gypenosides also led to a statistically significant trend towards increased colon length (Fig. 2A). H&E stained sections revealed that gypenosides restored the morphology and architecture of the colonic villi damaged by DSS (Fig. 2B). The pathological state of the GP group significantly improvement. The expression levels of colon inflammatory factors in the GP group were significantly lower than those in the DSS group (Fig. 2C). Overall, these results demonstrate that gypenosides treatment can alleviate symptoms in a DSS-induced UC mouse model.
Effects of gypenosides on the transcriptome of the colon
To differentially expressed genes between the two groups, a volcano plot was generated. A total of 2099 DEGs were identified between the NC and DSS groups, with 1243 upregulated and 856 downregulated genes (Fig. 3A). A total of 356 DEGs were identified between the DSS and GP groups, with 254 upregulated and 102 downregulated genes (Fig. 3B). A total of 1657 DEGs were identified between the DSS and GP groups, with 1081 upregulated genes and 576 downregulated genes (Fig. 3C). Venn diagrams show the shared and unique genes among the three groups (Fig. 3D). The DEGs were predominantly associated with signal transduction and the immune system, such as the response to external stimuli, the inflammatory response, the defence response, immune system processes, the immune response, the response to other organisms, the response to external biotic stimuli, the regulation of immune system processes, the response to biotic stimuli, and the regulation of the response to external stimuli (Fig. 4A–D). These findings suggest that gypenosides may exert their anti-UC effects primarily through immune regulation, cell signal transduction, stress response, and adaptation mechanisms.
Effects of gypenosidess treatment on intestinal transcript levels in DSS-induced colitis model mice. Differentially abundant transcripts were compared between the NC group and the DSS group (A), the DSS group and the GP group (B), and the NC group and the GP group (C), with differences defined by a fold change ≥ 1.5 and a P value < 0.05. (D Venn diagram showing differentially abundant transcripts among the three groups (n = 10).
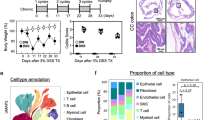
Gypenosides increase amino acid metabolite levels in DSS-induced UC model mice
OPLS-DA was performed on all metabolic profiles to study the overall metabolic changes in each group of mice. The three-dimensional results show that the samples were clearly distributed in three regions according to the processing method (Figure S1). Most of the changes were observed between the DSS and the NC group (Fig. 5A), as well as the comparison between the GP group and the model group (Fig. 5B), indicating that both UC modelling and gypenosides treatment significantly altered the metabolic components in mouse serum. All replicates of each processed organism were clustered together and significantly separated from the other samples. Using the projection variable importance (VIP) of the OPLS-DA model, as well as univariate analysis (T-test P value) and multiple change (FC), we determined the differentially abundant metabolites in the serum of the DSS group compared with the NC group. The screening criteria were VIP > 1, FC > 1.5 or FC < 1/1.5, and a P value < 0.05. We generated a chord diagram, and further pathway impact analysis revealed that the differentially abundant metabolites were associated with amino acid biosynthesis, aminoacyl tRNA synthesis, arginine biosynthesis, and glutamate metabolism (Fig. 5C, D). Furthermore, a total of 245 different metabolites were identified among the three groups. The number of differentially abundant metabolites between the DSS group and the NC group, as well as between the GP group and the DSS group, was 249 and 86, respectively (Fig. 6A, B). Pathway analysis suggested that saponins from Gynostemma pentaphyllum may alleviate inflammatory symptoms by regulating amino acid metabolism pathways (Fig. 6C-D).
Effect of gypenosidess on intestinal metabolism in DSS-induced colitis model mice. Differentially abundant metabolites between the NC group and the DSS group (A), the DSS group and the GP group (B), the NC group and the GP group (C). The criteria for determining differences were a fold change ≥ 1.5 and a p value < 0.05. Pathway impact analysis between the NC group and the DSS group (C), and between the DSS group and the GP group (D).
Changes in intestinal metabolism caused by gypenosides treatment. Volcano plot of metabolism between the NC group and the DSS group (A), and between the DSS group and the GP group (B). KEGG analysis revealed potential pathways associated with metabolites involved in regulation between the NC group and the DSS group (C), and between the DSS group and the GP group (D).
Immunohistochemical analysis
The highly expressed differentially expressed genes are listed in Table 1. To confirm the reliability of the RNA sequencing results, immunohistochemistry was conducted. Compared with those in the NC group mice, the protein expression levels of Ascl2 and Lgr5 were significantly lower in the colons of the DSS group mice, and gypenosides administration restored the altered gene expression levels to levels similar to those in the NC group.
Lgr5 and Ascl2 are key markers and regulators of intestinal stem cells, and play crucial roles in the maintenance and regeneration of the intestinal epithelium. Our transcriptome sequencing analysis revealed that Lgr5 and Ascl2 were among the top 20 DEGs. Therefore, we selected Lgr5 and Ascl2 as differential genes for immunohistochemical validation. As shown in Fig. 7A, the expression levels of Lgr5 and Ascl2 were significantly lower in the DSS group than in the NC group. However, after the DSS group mice were treated with gypenosides, the expression levels of these two genes increased significantly (P < 0.05). This trend is consistent with the transcriptome sequencing data, indicating that gypenosides may alleviate colitis in mice by modulating the expression of Lgr5 and Ascl2.
Spearman’s correlation of Lgr5, Ascl2, and differentially abundant metabolites
To determine the correlations among Lgr5, Ascl2, and differentially abundant metabolites, Spearman’s correlation analysis was conducted using spearman’s rank correlation coefficient. We performed Spearman correlation hierarchical clustering analysis on the two omics elements and presented the results in the form of a correlation hierarchical clustering heatmap. The results revealed that Lgr5 and Ascl2 levels were positively correlated with the relative abundance of glutamine, whereas Lgr5 and Ascl2 levels were negatively correlated with the level of arginine, which plays a role in intestinal homeostasis (Fig. 7B).
Discussion
Current medications for UC often lead to multiple side effects and complications with prolonged use. Hence, new treatments derived from natural products for the clinical management of this disease are urgently needed. In this study, we validated the intestinal protective effect of gypenosides and explored the underlying mechnisms via transcriptomics and metabolomics, providing experimental evidence for its potential as a novel therapeutic agent against UC. We used a DSS-induced mice model to simulate the typical clinical conditions of UC, and elucidated the drug-transcriptomic/ metabolomic-disease interactions. Our findings confirm the intestinal protective effects of gypenosides. Treatment with a 400 mg/kg gypenosides solution alleviated colitis symptoms in UC model mice, as evidenced by increased body weight and colon length, and reduced histological tissue inflammation. Moreover, drug intervention effectively reduced the expression levels of inflammatory cytokines (IL-6 and TNF-α) in UC model mice. As a natural product, gypenosides are both cost-effective and relatively safe, making them a promising alternative or supplementary therapy for UC treatment. To elucidate the therapeutic mechanisms of gypenosides in UC, we conducted comprehensive metabolomic and transcriptomic analyses. Transcriptomic analysis revealed that the intestinal stem cell markers Ascl2 and Lgr5 were restored to normal levels by gypenosides treatment. The expression of Ascl2 and Lgr5 in the DSS mice was significantly lower than that in the NC group. However, the administration of gypenosides abrogated this downwards trend in expression. In summary, our findings offer substantial evidence demonstrating the beneficial effects of gypenosides in alleviating UC and clarify the underlying mechanisms that support its clinical application.
Previously, Ascl2 and Lgr5 were shown to be critical markers for intestinal stem cells (ISCs) and to play important roles in maintaining intestinal homeostasis and regeneration17. Lgr5 is expressed primarily on the cell surface of the intestine, and has been identified as a specific marker for crypt-base columnar cells (CBCs) in mice20. Lgr5 + intestinal stem cells (ISCs) are intricately regulated by the Wnt/β-catenin and Notch signalling pathways to maintain their self-renewal and differentiation functions, thereby sustaining crypt homeostasis and repairing intestinal mucosal damage21. This process is crucial for the homeostatic regeneration of the small intestine and colon. Moreover, Ascl2 is a transcription factor crucial for the maintenance and proliferation of intestinal stem cells, which that drives expression of Lgr5 gene22. The interaction between Ascl2 and Lgr5 is crucial for sustaining a population of active intestinal stem cells, which are vital for the ongoing renewal of the intestinal lining23,24,25. The increased interest in intestinal dryness has opened new avenues for the clinical treatment of UC. We performed immunohistochemistry validation and demonstrated that the ameliorative effect of gypenosides on colitis in mice might occur through the regulation of Lgr5 and Ascl2 expression, thereby affecting intestinal stem cell function.
Previous studies have shown that Ascl2 and Lgr5 together regulate the metabolic pathways of intestinal stem cells, including glycolysis, lipid metabolism, and amino acid metabolism26. These pathways provide the energy and building blocks necessary to support the efficient proliferation and differentiation of stem cells. In this study, the metabolomic analysis revealed significant pattern differences between UC model mice and those treated with gypenosides, indicating that metabolic disturbances during UC progression were abrogated by gypenosides intervention. Compared with UC model mice, mice treated with gypenosides presented significant differences in metabolic pathways related to amino acid metabolism. Previous studies have indicated that amino acid metabolism is associated with the development of colitis in UC patients, primarily involving pathways such as ‘aminoacyl-tRNA biosynthesis’, ‘arginine biosynthesis’, ‘alanine, aspartate, and glutamate metabolism’, ‘biosynthesis of unsaturated fatty acids’, ‘valine, leucine, and isoleucine biosynthesis’, and ‘pantothenate and CoA biosynthesis’. Glutamine is a vital fuel source for enterocytes (intestinal epithelial cells) and plays a crucial role in maintaining the integrity and function of the intestinal barrier27. It supports the proliferation and differentiation of enterocytes, thereby reinforcing the mucosal lining of the gut. The combined analysis results further revealed a positive correlation between intestinal stem cell markers and glutamine levels. The rapid proliferation of intestinal stem cells requires a large supply of glutamine, which serves not only as a crucial amino acid for protein synthesis but also for energy metabolism and cell signalling. Glutamine acts as a nutrient sensor, influencing intestinal stem cell proliferation and differentiation by regulating pathways such as mTOR signalling28,29,30. Additionally, glutamine exerts strong antioxidant properties effects in the intestine, helping to reduce oxidative stress damage to intestinal stem cells and protecting them from environmental stimuli31,32,33,34. Although positively correlated with glutamine, Lgr5 and Ascl2 are negatively correlated with arginine. Arginine metabolism may lead to changes in the cellular metabolic state, affecting the transcriptional activity of Ascl2 21. For example, metabolites of arginine may inhibit Ascl2 expression by regulating the intracellular environment and signalling pathways35,36. Arginine plays a crucial role in immune cell function and inflammatory responses, which may indirectly regulate Lgr5 expression by affecting the homeostasis of the intestinal microenvironment37. Moreover, changes in the inflammatory response and immune status can influence the expression of genes, including Lgr5, in intestinal stem cells38. The relevant mechanisms are worth further exploration.
We have preliminarily confirmed the mechanism by which gypenosides in treat UC. However, our study also has certain limitations. First, integrated analyses of transcriptomics and metabolomics often rely on existing databases. These databases may not fully cover all genes and metabolites. Second, individual biological differences can affect the reliability of transcriptomic and metabolomic data. Large sample sizes are needed to control and correct for this variability. Besides, gypenosides may confer intestinal protection through the modulation of intestinal stem cells, the potential secondary regulatory effects on gut function mediated by their central anti-inflammatory actions via the brain-gut axis remain poorly characterized.Finally, gypenosides reduces colonic inflammation and improves UC symptoms. However, direct evidence for remission induction and the efficacy and safety require further validation in acute-phase models or clinical trials with earlier intervention.
Conclusion
In conclusion, we observed that gypenosides treatment alleviated DSS-induced colitis, with this effect correlating with altered expression of intestinal stem cell markers and amino acid metabolism profiles in a DSS-induced colitis mouse model.
Data availability
Data is provided within the manuscript or supplementary information files only for review. The sequencing datasets generated and analyzed during the current study are not publicly available as the research team is still conducting further data analysis and exploring underlying mechanisms. However, the data are available from the corresponding author upon reasonable request.
References
Mak, W. Y., Zhao, M., Ng, S. C. & Burisch, J. The epidemiology of inflammatory bowel disease: East Meets West. J. Gastroenterol. Hepatol. 35, 380–389 (2020).
Xavier, R. J. & Podolsky, D. K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 (2007).
Torres, J., Mehandru, S. & Colombel, J. F. Peyrin-Biroulet, L. Crohn’s disease. Lancet 389, 1741–1755 (2017).
Su, C. et al. Progress in the medicinal value, bioactive compounds, and Pharmacological activities of gynostemma pentaphyllum. Molecules 26, 6249 (2021).
Huang, W. C. et al. Extract of gynostemma pentaphyllum enhanced the production of antibodies and cytokines in mice. Yakugaku Zasshi-J Pharm. Soc. Jpn. 127, 889–896 (2007).
Xia, X. et al. Gypenosides pretreatment alleviates the cerebral ischemia injury via inhibiting the Microglia-Mediated neuroinflammation. Mol. Neurobiol. 61, 1140–1156 (2024).
Zhang, X. et al. Anti-inflammatory, cardioprotective effect of gypenosides against isoproterenol-induced cardiac remodeling in rats via alteration of inflammation and gut microbiota. Inflammopharmacology 31, 2731–2750 (2023).
Zhang, H. K., Ye, Y., Li, K. J., Zhao, Z. N. & He, J. F. Gypenosides prevent H2O2-Induced retinal ganglion cell apoptosis by concurrently suppressing the neuronal oxidative stress and inflammatory response. J. Mol. Neurosci. 70, 618–630 (2020).
Wu, H. et al. Gypenosides induces apoptosis by inhibiting the PI3K/AKT/mTOR pathway and enhances T-cell antitumor immunity by inhibiting PD-L1 in gastric cancer. Front. Pharmacol. 15, 1243353 (2024).
Zhang, H. et al. Gypenosides improve diabetic cardiomyopathy by inhibiting ROS-mediated NLRP3 inflammasome activation. J. Cell. Mol. Med. 22, 4437–4448 (2018).
Li, H., Xi, Y., Xin, X., Tian, H. & Hu, Y. Gypenosides regulate farnesoid X receptor-mediated bile acid and lipid metabolism in a mouse model of non-alcoholic steatohepatitis. Nutr. Metab. (Lond). 17, 34 (2020).
Shen, S., Wang, K., Zhi, Y. & Dong, Y. Gypenosides counteract hepatic steatosis and intestinal barrier injury in rats with metabolic associated fatty liver disease by modulating the adenosine monophosphate activated protein kinase and Toll-like receptor 4/nuclear factor kappa B pathways. Pharm. Biol. 60, 1949–1959 (2022).
Huang, X. et al. Gypenosides improve the intestinal microbiota of non-alcoholic fatty liver in mice and alleviate its progression. Biomed. Pharmacother. 118, 109258 (2019).
Zhu, X. et al. Multi-omics approaches for in-depth Understanding of therapeutic mechanism for traditional Chinese medicine. Front. Pharmacol. 13, 1031051 (2022).
Jiashuo, W. U., Fangqing, Z., Zhuangzhuang, L. I., Weiyi, J. & Yue, S. Integration strategy of network Pharmacology in traditional Chinese medicine: a narrative review. J. Tradit. Chin. Med. 42, 479–486 (2022).
Zhu, Y. et al. New opportunities and challenges of natural products research: when target identification Meets single-cell multiomics. Acta Pharm. Sin. B. 12, 4011–4039 (2022).
Leung, C., Tan, S. H. & Barker, N. Recent advances in Lgr5+ stem cell research. Trends Cell. Biol. 28, 380–391 (2018).
Zhang, J. et al. Vitexin protects against dextran sodium Sulfate-Induced colitis in mice and its potential mechanisms. J. Agric. Food Chem. 70, 12041–12054 (2022).
Erben, U. et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 7, 4557–4576 (2014).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Schuijers, J. et al. Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell. Stem Cell. 16, 158–170 (2015).
Strubberg, A. M. et al. The zinc finger transcription factor PLAGL2 enhances stem cell fate and activates expression of Ascl2 in intestinal epithelial cells. Stem Cell. Rep. 11, 410–424 (2018).
Zheng, L., Duan, S. L., Wen, X. L. & Dai, Y. C. Molecular regulation after mucosal injury and regeneration in ulcerative colitis. Front. Mol. Neurosci. 9, 996057 (2022).
Murata, K. et al. Ascl2-Dependent cell dedifferentiation drives regeneration of ablated intestinal stem cells. Cell. Stem Cell. 26, 377–390 (2020).
Wu, L. et al. Ascl2 affects the efficacy of immunotherapy in Colon adenocarcinoma based on Single-Cell RNA sequencing analysis. Front. Immunol. 13, 829640 (2022).
Yan, K. S. et al. Intestinal enteroendocrine lineage cells possess homeostatic and Injury-Inducible stem cell activity. Cell. Stem Cell. 21, 78–90 (2017).
Liu, M., Guo, S. & Wang, L. Systematic review of metabolomic alterations in ulcerative colitis: unveiling key metabolic signatures and pathways. Th. Adv. Gastroenterol. 17, 17562848241239580 (2024).
Moore, S. R. et al. Glutamine and alanyl-glutamine promote crypt expansion and mTOR signaling in murine enteroids. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G831–839 (2015).
Boukhettala, N. et al. Effects of essential amino acids or glutamine deprivation on intestinal permeability and protein synthesis in HCT-8 cells: involvement of GCN2 and mTOR pathways. Amino Acids. 42, 375–383 (2012).
Tian, Y., Wang, K., Wang, Z., Li, N. & Ji, G. Chemopreventive effect of dietary glutamine on colitis-associated colon tumorigenesis in mice. Carcinogenesis 34, 1593–1600 (2013).
Xu, C. L. et al. Protective effect of glutamine on intestinal injury and bacterial community in rats exposed to hypobaric hypoxia environment. World J. Gastroenterol. 20, 4662–4674 (2014).
Soares, A. D. et al. Dietary glutamine prevents the loss of intestinal barrier function and attenuates the increase in core body temperature induced by acute heat exposure. Br. J. Nutr. 112, 1601–1610 (2014).
Gu, A., Yang, L., Wang, J., Li, J. & Shan, A. Protective effect of glutamine and alanyl-glutamine against zearalenone-induced intestinal epithelial barrier dysfunction in IPEC-J2 cells. Res. Vet. Sci. 137, 48–55 (2021).
Wang, B. et al. Glutamine and intestinal barrier function. Amino Acids. 47, 2143–2154 (2015).
Baier, J. et al. Arginase impedes the resolution of colitis by altering the Microbiome and metabolome. J. Clin. Invest. 130, 5703–5720 (2020).
Hou, Q. et al. Regulation of the Paneth cell niche by exogenous L-arginine couples the intestinal stem cell function. FASEB J. 34, 10299–10315 (2020).
Hou, Q. et al. Exogenous L-arginine increases intestinal stem cell function through CD90 + stromal cells producing mTORC1-induced Wnt2b. Commun. Biol. 3, 611 (2020).
Martí, I. L. A. A. & Reith, W. Arginine-dependent immune responses. Cell. Mol. Life Sci. 78, 5303–5324 (2021).
Funding
This study was supported by Scientific Research Fund Project of Hunan Provincial Health Commission (20233462), The Research Foundation of Education Bureau of Hunan Province (23A0347), and Natural Science Foundation of Gansu province (22JR5RA889, 22JR11RA031).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: Yuan Yang, Xinyuan Li, Fan Huang. (II) Administrative support: Guangsheng Hu, Bin Zeng, Weiwei Zhou, Yuan Yang. (III) Provision of study materials: Fuchao Cai, Qilin Jiang. (IV) Collection and assembly of data: Xinyuan Li, Fan Huang, Fuchao Cai, Xufeng Ning. (V) Data analysis and interpretation: Lingshan Zhou, Yuan Yang. (VI) Manuscript writing: All authors. (VII) Final approval of manuscript: All author.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, Y., Li, X., Huang, F. et al. Comprehensive multiomics profiling reveals the protective function of gypenosides against dextran sulfate sodium-induced colitis. Sci Rep 15, 31803 (2025). https://doi.org/10.1038/s41598-025-11010-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11010-0
Keywords
This article is cited by
-
Comprehensive investigation of synthesis, properties and biomedical utilization of multifunctional dextran-based polymeric nanoparticles
Colloid and Polymer Science (2025)