Abstract
This study sought to evaluate the feasibility and effectiveness of the LEO or LEO baby within enterprise (EP) overlapping stents for treating ruptured vertebrobasilar artery dissecting aneurysms (VBDAs). A retrospective analysis was conducted on 20 patients with a total of 20 ruptured VBDAs, who received reconstructive treatment utilizing the LEO/LEO baby within enterprise overlapping stents combined with coil embolization, between December 2018 and June 2023. Patient characteristics, clinical outcomes, and radiological results were thoroughly reviewed. Functional data were evaluated using the modified Rankin scale (mRS). The outcomes for patients treated with the overlapping stent technique were compared to those of the single-stent treatment group which included 14 cases with LEO/LEO Baby stent and 12 cases with Enterprise stent. Among the 20 ruptured VBDAs identified, 13 were vertebral artery aneurysms (65.00%), and 7 were basilar artery aneurysms (35.00%). All cases were effectively treated during the acute phase of onset. Three patients (15.00%) experienced significant in-hospital adverse events, with one patient (5.00%) succumbing to rebleeding of the arterial aneurysm during the perioperative period. A follow-up period of at least six months was achieved for 19 patients. The rates of mortality or dependency (mRS scores of 3 to 6) were 15.00% at discharge and 10.00% at the final follow-up. Ischemic events occurred in two patients (10.00%); one during the perioperative period and one during follow-up. One patient (5.00%) required ventriculoperitoneal shunt placement. Immediate post-operation angiography revealed Raymond grade I, II, and III occlusions in 55.00%, 30.00%, and 15.00% of cases, respectively. At a mean follow-up of 14.84 ± 7.51 months, 17 patients (89.47%) had mRS scores of 0–2, indicating favorable outcomes, while two patients (10.53%) had scores between 3 and 6. The initial complete or near-complete obliteration rates of aneurysms were higher in the overlapping-stent group compared to the single-stent group (85.00% vs. 65.38%, P = 0.18). Follow-up angiography indicated superior rates of complete or near-complete aneurysm obliteration in the double-stent group (94.74% vs. 73.08%, P = 0.11). The overlapping stents technique, utilizing the LEO/LEO baby within EP stent configuration in conjunction with coiling, demonstrated both efficacy and safety in the treatment of ruptured VBDAs. Follow-up evaluations revealed a high rate of aneurysm occlusion, suggesting that this approach may offer a dependable intervention strategy for managing these complex cases.
Similar content being viewed by others
Introduction
Dissecting aneurysms of the vertebrobasilar artery present a considerable risk, particularly as they can lead to acute ischemic stroke or subarachnoid hemorrhage (SAH), most frequently affecting the middle-aged population1,2. The prognosis for patients with aneurysmal SAH in the posterior circulation is generally poor3,4. In cases of untreated ruptured VBDAs, Mizutani and colleagues5 observed that approximately 70% of patients experienced subsequent hemorrhages, typically within the first 24 h, resulting in a mortality rate approaching 50%. While the literature discusses various endovascular approaches, including deconstructive treatment and stent-assisted coiling, either single or multiple, for ruptured VBDAs6 such cases are relatively rare, leading to a scarcity of comprehensive data on treatment efficacy and the risk-benefit profile of different endovascular strategies. Therefore, further research is necessary to evaluate the safety and effectiveness of these endovascular treatments for ruptured VBDAs.
In this study, we employed the LEO/LEO baby within enterprise (EP) overlapping stent for treating ruptured VBDAs. The initial laser-cut stent featured appropriately sized mesh holes and robust radial support, facilitating rapid embolization—especially critical when premature withdrawal of the microcatheter occurred, thereby preventing further aneurysm rupture through the microcatheter’s through-strut technology. Subsequently, the deployment of a second braided stent enhanced metal coverage, which is vital for successful vascular reconstruction. The objective of our research was to evaluate the feasibility and effectiveness of this stent combination therapy for ruptured VBDAs.
Materials and methods
Ethics statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Tongji Hospital, affiliated with Huazhong University of Science and Technology (IRB ID: TJ20230114). Before undergoing endovascular treatment, all participants signed a written informed consent form.
Patient population
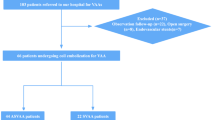
Our single-center database was retrospectively analyzed, reviewing a consecutive series of cases with ruptured VBDAs treated using the LEO/LEO baby within EP overlapping stent endovascular therapy from December 2018 to June 2023. Our study included 20 patients with 20 ruptured VBDAs who underwent received endovascular treatment. Additionally, we included patients treated with a single stent—either the LEO/LEO Baby stent (n = 14) or the Enterprise stent (n = 12) in conjunction with coiling—for comparative analysis.
The baseline characteristics of both the dual-stent and single-stent groups were thoroughly documented. Key demographic information encompassed age, sex, and medical history, with particular attention to the presence of hypertension, diabetes mellitus, and hyperlipidemia. Aneurysm characteristics were also recorded, including the location (vertebral artery or basilar artery) and the severity of preoperative hemorrhage, evaluated using the Hunt-Hess and modified Fischer grading scales. Additionally, the distribution of National Institutes of Health Stroke Scale (NIHSS) and modified Rankin Scale (mRS) scores prior to treatment was noted, reflecting the patients’ functional status.
The inclusion criteria were as follows: (A) solitary dissecting aneurysms originating from the main trunk of the vertebrobasilar artery; (B) confirmation of SAH by computed tomography (CT), and identification of the target aneurysm by digital subtraction angiography (DSA)7including fusiform dilation, an intimal flap, and the pearl-and-string sign, which are hallmark features of dissecting aneurysms8; (C) treatment with the specified overlapping stent technique; (D) endovascular therapy administered within 72 h post-SAH; and (E) stent selection for rVBDAs: single-stent deployment was preferred for certain patients based on the aneurysm’s morphology and the neuro-interventionalist’s assessment. This approach was suitable for cases with a short dissecting segment (typically ≤ 5 mm in diameter), located in a less tortuous artery, with well-defined true and false lumens, and without significant arterial dilation. Conversely, for larger aneurysms (typically > 5 mm), a dual-stent overlapping technique was favored.
Exclusion criteria included: (A) aneurysms such as those located at superior cerebellar artery (SCA), anterior inferior cerebellar artery (AICA), posterior inferior cerebellar artery (PICA) or basilar tip; (B) aneurysms involving extracranial segments of the vertebral artery; (C)vertebrobasilar dolichoectasia, intracranial aneurysms with a saccular morphology, or those resulting from trauma or iatrogenic causes; (D) multiple intracranial dissecting aneurysms; and (E) complications arising from other treated intracranial aneurysms.
Antiplatelet therapy during perioperative period
Patients need to receive a loading dose of aspirin (300 mg) and clopidogrel (300 mg), administered either orally or via a nasogastric tube, at least 60 min prior to the operation. Subsequently, patients were placed on a maintenance regimen of dual antiplatelet treatment, which entailed daily administration of aspirin (100 mg) and clopidogrel (75 mg) no less than 6 weeks. Clopidogrel was discontinued six weeks postoperatively. Following this period, patients transitioned to monotherapy with aspirin (100 mg) for an additional 180 days. Tirofiban was administered at a dose of 0.1 µg/kg/min and maintained for 24 to 48 h post-procedure. Approximately 2 h prior to discontinuing tirofiban, aspirin (100 mg) and clopidogrel (75 mg) were administered, followed by the continuation of the prescribed daily dual antiplatelet regimen. Thromboelastography (TEG) was employed at least 72 h after the initiation of dual antiplatelet treatment to monitor platelet function in all patients. In cases where patients exhibited hypo-responsiveness or non-responsiveness to clopidogrel, the treatment was switched to ticagrelor (90 mg twice daily).
Endovascular treatment procedures
An appropriate guiding catheter was positioned within the distal vertebral artery (VA) after placing a sheath. Three-dimensional reconstructive angiography was utilized to establish an optimal working projection for the precise execution of coil embolization and to guide the selection of the appropriate stent size. Generally, the chosen stent corresponded to the proximal diameter of the parent artery. Aneurysm coiling commenced under the support of an EP stent (Codman Neurovascular, Massachusetts, USA), utilizing semi-jailing technique. Following the deployment of the first stent, a minimum anchoring distance of 5 mm was maintained at both ends of the aneurysm. Subsequently, a second LEO or LEO Baby stent (Balt, rue de la Croix Vigneron, Montmorency, France) was deployed within the initial stent. The LEO baby stent was selected for cases involving small-caliber arteries (typically less than 2.5 mm in diameter) or highly tortuous vascular segments where the deployment of a standard LEO stent might be challenging9. Coiling continued until the aneurysm was adequately packed or further packing was deemed unfeasible.
Evaluation and Follow-Up
We collected baseline variables including patient demographics, clinical data, and aneurysmal characteristics. Immediate and follow-up angiographic outcomes were evaluated using the Raymond classification. Occlusion degrees were categorized as complete (Raymond class I), neck residual (Raymond class II), and sac residual (Raymond class III)7. Technical success was defined by effective deployment, stable positioning, and optimal adherence of the stent, full coverage of the dissecting aneurysm neck, and unobstructed patency of the parent artery.
For post-embolization monitoring, follow-up angiography was scheduled between 90 and 180 days, with subsequent CTA recommended at one year and biennially thereafter. The NIHSS serves as a clinically validated assessment tool for quantifying neurological impairment severity10,11. Current clinical stratification delineates mild stroke as an NIHSS score of ≤ 5, while moderate-to-severe stroke classifications correspond to scores exceeding this threshold12,13. Patient outcomes were assessed at discharge and during follow-up using the mRS, with scores obtained through physical exams or telephone interviews7. Scores of 3–5 indicated dependence, while a score of 6 denoted mortality. We documented all postprocedural serious adverse events (SAEs), such as cerebral infarction, aneurysm rebleeding, and shunt-dependent hydrocephalus, that required hospitalization or extended hospital stays. Two senior neuro-interventionists reviewed all clinical and radiological follow-up data independently.
Statistical analysis
Continuous variables were presented as the mean ± standard deviation and analyzed using the t-test for normally distributed data. Categorical data were reported as percentage and assessed using the chi-squared test. A p-value of less than 0.05 was considered statistically significant. Statistical analyses were conducted using SPSS version 22.0 (IBM, USA).
Results
Baseline characteristics and patient demographic
This study included 20 patients with 20 ruptured VBDAs, with a mean age of 58.25 ± 6.65 years. The cohort consisted of 11 male patients (55.00%) and 9 female patients (45.00%). A history of hypertension was present in more than half of the patients, with 13 out of 20 (65.00%) affected. Clinically, 14 patients (70.00%) were assessed with a Hunt–Hess grade of 1–2, while 6 patients (30.00%) presented with a grade of 3–5. The modified Fischer grade indicated that 14 patients (70.00%) were in the grade 1–2 category, and 6 patients (30.00%) were in the grade 3–4 category. Prior to treatment, the distribution of modified Rankin Scale (mRS) scores was as follows: 8 patients (40.00%) exhibited mild symptoms (mRS 1), 5 patients (25.00%) had a slight disability (mRS 2), and 7 patients (35.00%) were within the mRS 3–6 range. Among the 20 aneurysms, 15 (75.00%) originated from the vertebral artery, while 5 (25.00%) arose from the basilar artery. 13 (65.00%) patients exhibited NIHSS scores ≤ 5, consistent with mild stroke classifications, while seven individuals (35.00%) manifested scores surpassing this threshold. In the single-stent cohort, 26 patients were included, with a mean age of 57.46 ± 7.56 years and a male representation of 46.15%. The prevalent comorbidities included hypertension (76.92%), diabetes mellitus (65.38%), and hyperlipidemia (73.08%). The severity of preoperative hemorrhage was categorized as follows: Hunt-Hess grade 1–2 in 61.54% of patients and grade 3–5 in 38.46%. According to the modified Fischer scale, 57.69% were classified as grade 1–2, while 42.31% were in grade 3–4. Regarding aneurysm location, 69.23% were found in the vertebral artery and 30.77% in the basilar artery. Pre-treatment functional status, as assessed by mRS, indicated that 57.69% scored between 0 and 2, while 42.31% scored between 3 and 6. NIHSS scores showed that 57.69% of patients had scores of ≤ 5, while 42.31% had scores exceeding 5. Detailed patient characteristics are presented in Table 1.
In-hospital SAEs
Three patients (15.00%) experienced SAEs during hospitalization, including one fatality. One patient (5.00%), who was admitted with a Fisher grade of 3, suffered rebleeding the day following the procedure and subsequently passed away. One patient (5.00%) experienced brainstem infarctions with decreased consciousness one day post-procedure, resulting in an mRS score of 3 at the time of hospital discharge. Additionally, another patient (5.00%) with a Fisher grade of 3 developed hydrocephalus and required ventriculoperitoneal shunt placement a month post-embolization, also with an mRS score of 3 at discharge.
Clinical outcomes
At discharge, the rate of dependency or death (mRS score of 3 to 6) was 15.00% (3 out of 20 patients). Follow-up was conducted on 19 patients for periods ranging from 6 to 30 months, with an average duration of 14.84 ± 7.51 months. The vast majority, 17 patients (89.47%), regained functional independence and were able to return to work and their daily routines. Detailed outcomes are presented in Table 2. Regarding the rate of successful aneurysm obliteration, the group treated with overlapping stents demonstrated a higher success rate compared to the single stent group (94.74% vs. 73.08%, p = 0.11) as shown in Table 3.
Case 1
A 64-year-old female patient exhibited a ruptured dissecting aneurysm in the basilar artery, accompanied by severe headaches, nausea, and vomiting. (Fig. 1B, C). Upon admission, her condition was assessed as Hunt-Hess grade II, with a modified Fisher score of 3, a NIHSS score of 7 and a mRS score of 3 (Fig. 1A). Following comprehensive preoperative preparation, the ruptured dissecting aneurysm was successfully treated using the double stent overlap technique. Initially, an enterprise 2 stent (4 × 23 mm) was deployed, followed by a LEO stent (3.5 × 25 mm), in conjunction with coil embolization to effectively occlude the false lumen and reconstruct the parent artery (Fig. 1D). The patient did not develop any new neurological deficits post-operation and was discharged seven days later. Nine months following the endovascular treatment, angiographic imaging confirmed the absence of aneurysm recurrence (Fig. 1E).
Case 2
A 57-year-old female manifesting acute severe headache secondary to a ruptured left V4 segment vertebral artery dissecting aneurysm underwent comprehensive preoperative evaluation, revealing Hunt-Hess grade II, modified Fisher grade III, NIHSS 6, and mRS 3 (Fig. 1F-H). The lesion was effectively treatment via overlapping dual-stent deployment strategy with an enterprise 2 stent (4 × 16 mm) and a LEO baby stent (2.5 × 25 mm) (Fig. 1I). Six-month post-endovascular intervention, angiographic surveillance demonstrated Raymond-Roy Occlusion Classification Grade I aneurysm occlusion (Fig. 1J).
Case 3
A 74-year-old patient with a ruptured left vertebrobasilar artery dissecting aneurysm (VBDA) underwent preoperative evaluation, revealing Hunt-Hess grade II, revised Fisher grade III, NIHSS score of 6, and mRS score of 3 (Fig. 1K-M). The dissecting aneurysm was successfully embolized using an overlapping dual-stent deployment strategy combining an enterprise 2 stent (4 × 23 mm) and a LEO stent (3.5 × 25 mm) (Fig. 1N). Six-month angiographic follow-up confirmed successful occlusion without evidence of recurrence (Fig. 1O).
Exemplar cases of ruptured vertebrobasilar dissecting aneurysms (VBDA) managed via overlapping dual-stent strategies. (A–D) A ruptured basilar artery (BA) dissection underwent endovascular coiling with adjunctive Enterprise 2 and LEO stent deployment. Nine-month follow-up angiography (E) confirmed complete aneurysm obliteration. (F–I) A left V4 vertebral artery (VA) ruptured dissection was treated with coiling supported by Enterprise 2 and LEO Baby stents. Six-month surveillance (J) demonstrated stable occlusion (Raymond-Roy Occlusion Classification [RROC] Grade I). (K–N) A vertebrobasilar dissection received coiling augmented by an Enterprise 2-LEO stent construct. Six-month imaging (O) revealed parent vessel patency with successful aneurysm exclusion.
Discussion
Our study’s analysis of 20 patients with 20 ruptured VBDAs presents significant findings consistent with existing literature that underscores the importance of timely intervention to reduce mortality and enhance clinical outcomes5,14. The technical success rate of 100% achieved using Leo and Enterprise stents underscores the effectiveness of current endovascular techniques in managing these complex cases.
The 100% technical success rate in our study mirrors the high success rates reported in similar studies7. However, despite this procedural success, the occurrence of serious adverse events (SAEs) in 15.00% of patients, including rebleeding and neurological deficits leading to one fatality, underscores the significant risks associated with these treatments. Specifically, our study documented one fatality (5.00%) due15 to rebleeding, and moderate-to-severe hydrocephalus occurred in 10.00% of patients. These complications highlight the need for careful consideration of treatment strategies, particularly given the unique pathological characteristics of the vertebrobasilar artery and its connections to perforating branches, which may limit the applicability of certain techniques in patients with sufficient collateral vascularization.
VBDAs are frequently implicated in SAH or posterior ischemic strokes and pose significant treatment challenges. The risk of rebleeding following VBDAs rupture is considerable, with a mortality rate of 46.7% after rebleeding, underscoring the critical need for prompt intervention16,17. Historically, surgical approaches such as trapping, reconstruction clipping, and open bypass procedures were utilized, with the choice of treatment largely depending on the aneurysm’s morphology and its anatomical relationship with key collateral vessels18. However, these methods often resulted in high complication rates due to the fragility of the parent vessel wall, particularly when the PICA was involved19. Consequently, endovascular treatment may be the preferred option for patients with ruptured VBDAs, especially in cases involving complex anatomical dissections7,20,21. Endovascular treatment strategies include both deconstructive and reconstructive techniques. Proximal occlusion for ruptured VBDAs can achieve high occlusion rates22,23 and effectively prevent re-rupture. However, due to technological limitations, this method is suitable only for patients with robust collateral vascularization and lesions that do not involve the dominant blood supply of the vertebral or basilar arteries, as it otherwise poses a high risk of ischemic stroke19,24,25. Reconstructive endovascular therapy, utilizing single stent-assisted coil embolization and flow-diverting (FD) devices, preserves the integrity of the parent vessel and is more commonly employed in the treatment of VBDAs6,14,26. However, previous studies have indicated that traditional single stent-assisted coiling may be associated with a higher risk of aneurysm recurrence6,14. FD devices can reduce recurrence rates, particularly in large or complex aneurysms, offering advantages over traditional stents27. Nonetheless, the use of FD devices in SAH cases remains controversial due to their potential delay in aneurysm occlusion28. Additionally, concerns regarding metal coverage and antiplatelet regimens pose significant risks of arterial branch occlusion and rupture in posterior circulation aneurysms7,27,29,30,31. Existing meta-analyses suggest that posterior circulation intracranial aneurysms may constitute an independent risk factor for complications associated with flow-diverting (FD) devices, with pooled data revealing a statistically significant elevation in both morbidity and mortality outcomes27,32,33. Therefore, the safety and efficacy of using FD devices off-label for treating ruptured VBDAs still requires further verification.
Recently, the double-stent technique combined with coil embolization has proven effective in maintaining parent artery patency, enhancing thrombosis, and preventing aneurysm rupture, making it a preferred approach over parent artery occlusion7,20,21,28,34. In previous studies, the methods for overlapping stents have varied significantly, with combinations of enterprise (EP), Neuroform, Atlas, LVIS/LVIS Jr. (Low-profile Visualized Intraluminal Support), or LEO/LEO baby stents being used, and a standardized strategy has yet to be established7,20,21. Our study indicates that the successful occlusion rate of 94.74% in the group treated with overlapping stents, compared to 73.08% in the single stent group. In our research, the Enterprise stent, a laser-cut, closed-cell stent, is deployed first. The enterprise stent features appropriately sized mesh holes, allowing easy selection of the microcatheter into the aneurysm sac, which facilitates rapid embolization of the rupture site and prevents secondary aneurysm rupture. When addressing large dissection lesions, the enterprise stent’s strong radial support acts as a “skeleton,” and, together with the coil filled in the false lumen, it reduces the risk of stent trapping within the aneurysm neck and limits the outward expansion of the second stent20,21. The subsequent deployment of a second braided stent (LEO/LEO baby) increases metal coverage, enhancing the flow-diverting effect, promoting vascular endothelial growth, and significantly contributing to aneurysm neck reconstruction7,20,21,35,36. Additionally, the braided stent provides internal support to the enterprise stent, improving the compaction of the local coil within the aneurysm sac and reducing the risk of aneurysm recurrence.
LEO/LEO Baby stents are self-expandable devices made of braided nitinol mesh wires, incorporating 2 platinum wires that facilitate continuous radiographic visualization during the entire stent deployment process. This feature allows the surgeon to adjust the stent’s positioning in real-time, ensuring better opening and adhesion. Compared to laser-cut stents, LEO stents offer higher metal coverage (18%)36 and pore density (0.979 pores/mm²)37 similar to LVIS stents. The LEO/LEO Baby stents offer the notable advantage of being re-sheathable or re-positionable up to approximately 95% of their length38. This feature significantly surpasses that of non-retrievable open-cell stents and EP stents, which permit retrieval up to only 70% of their length, as well as LVIS/LVIS Jr. stents, which can be withdrawn until 80% deployed39,40. Additionally, the length range of LEO stents is more extensive than that of LVIS stents, spanning from 12 to 75 mm, allowing for the direct treatment of long-segment dissecting lesions without the need for bridging stents. Furthermore, the LEO Baby stent, comprised of sixteen wires, offers increased radial force compared to the LVIS Jr., which contains twelve wires38,41. The LEO Baby stent was employed in cases with small-caliber arteries (< 2.5 mm diameter) or highly tortuous anatomy where standard LEO stent deployment proves challenging. Its reduced profile and enhanced flexibility facilitate improved navigability and vessel wall apposition in complex vasculature, mitigating malposition risks while optimizing stent positioning. This aligns with prior research demonstrating superior performance of low-profile flexible stents in anatomically constrained vessels9.
Research has shown that two overlapping LVIS stents can achieve higher metal coverage and produce a more effective flow-diverting effect compared to a single pipeline embolization device (PED)42,43. However, this increased metal coverage also raises the risk of ischemic complications20,21. The relatively low wall adhesion of braided stents increases the risk of stent displacement during the placement of the second stent, complicating the process of positioning it in the ideal location20. Therefore, combining a laser-cut stent with a braided stent offers more balanced metal coverage and enhances the overall performance and interaction between the two stents.
For ruptured posterior circulation dissecting aneurysms, the paramount therapeutic intervention involves prompt coil embolization of the aneurysmal rupture site to prevent rebleeding, rendering solitary flow diverters (FD) suboptimal as a primary strategy7. Initial deployment of laser-cut stents with optimized mesh dimensions and robust radial force facilitates expedited embolization- a critical advantage particularly in scenarios of inadvertent microcatheter disengagement7,20,21. This structural integrity permits subsequent microcatheter navigation through stent interstices using the strut traversal technique to achieve complementary embolization of the rupture zone. In contrast, primary utilization of flow diverters (FD) or braided stents with adjunctive coiling presents substantial technical limitations; should premature catheter kickback occur, the dense metallic architecture precludes effective trans-mesh microcatheter recanalization, thereby compromising definitive rupture site occlusion7. In summary, the procedural superiority of laser-cut stent platforms over flow diverters (FD) in ruptured aneurysm embolization lies in their balanced modulation of porosity profiles and augmented radial resistive forces. This equilibrium ensures stable stent maintenance during complex mirocatheter manipulations while maintaining through-strut technique microcatheter re-access capacity for adjunctive embolization—a critical determinant of therapeutic success in hemorrhage-prone neurovascular scenarios where procedural adaptability dictates clinical outcomes.
Recently, Liu et al.20 reported favorable angiographic outcomes at an average follow-up of 5.7 months, achieving 100% complete or near-complete obliteration of VBDAs in 15 patients using the LVIS stent combined with Neuroform EZ stent. Similarly, Wu et al. reported a 93.3% successful occlusion rate in ruptured VBDAs patients treated with the combination of LVIS and EP stents32. In our study, we utilized the LEO/LEO baby within EP stent-assisted coiling therapy for ruptured VBDAs, achieving a 94.74% complete occlusion rate. This suggests that overlapping the LEO/LEO baby within EP stents is both safe and effective for patients with ruptured VBDAs. Previous studies have identified ischemic complications as the primary concern following endovascular treatment of vertebrobasilar aneurysms28,44,45. Our research shows that the rates of ischemic complications is 5.00% during the perioperative period and 10.00% overall postoperatively, aligning with the data from prior research21,44,45. Factors contributing to these ischemic events may include insufficient stent expansion, non-response or hypo-response to antiplatelet medications, and thrombus detachment during the embolization. Wang et al. reported that during the perioperative period of treating intracranial aneurysms with dual LEO stents, the implementation of a judicious antiplatelet strategy can significantly reduce the incidence of thromboembolic events46.
Study limitations
This study has several limitations, including a small sample size, its retrospective design, and a relatively short follow-up period. These factors underscore the necessity for further research. Future prospective, multicenter studies with larger cohorts and extended follow-up periods are crucial to validate these findings and draw more definitive conclusions regarding the long-term benefits and potential risks associated with the overlapping-stent technique.
Conclusion
Our study suggest that the LEO/LEO baby combined with the EP stent in an overlapping technique, along with coil embolization can be used to successfully treat ruptured VBDAs. The technical and clinical outcomes were encouraging, providing partial confirmation of the feasibility and effectiveness of this strategy.
Data availability
The data generated and analyzed in this study are available from the corresponding author on reasonable request.
References
Ansari, S. A. et al. Emergent endovascular management of Long-Segment and Flow-Limiting carotid artery dissections in acute ischemic stroke intervention with multiple tandem stents. AJNR Am. J. Neuroradiol. 38, 97–104. https://doi.org/10.3174/ajnr.A4965 (2017).
Zhao, K. J. et al. The interaction between stent(s) implantation, PICA involvement, and immediate occlusion degree affect symptomatic intracranial spontaneous vertebral artery dissection aneurysm (sis-VADA) recurrence after reconstructive treatment with stent(s)-assisted coiling. Eur. Radiol. 24, 2088–2096. https://doi.org/10.1007/s00330-014-3225-7 (2014).
Dharia, A., Lacci, J. V., Mascitelli, J. & Seifi, A. Impact of ruptured aneurysm circulation on mortality: A nationwide inpatient sample analysis. J. Stroke Cerebrovasc. Dis. 29, 105124. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105124 (2020).
Schievink, W. I. et al. The poor prognosis of ruptured intracranial aneurysms of the posterior circulation. J. Neurosurg. 82, 791–795. https://doi.org/10.3171/jns.1995.82.5.0791 (1995).
Mizutani, T. et al. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery 36, 905–911. https://doi.org/10.1227/00006123-199505000-00003 (1995). discussion 912 – 903.
Jeon, J. P. et al. Stent-Assisted coil embolization of vertebrobasilar dissecting aneurysms: procedural outcomes and factors for recanalization. Korean J. Radiol. 17, 801–810. https://doi.org/10.3348/kjr.2016.17.5.801 (2016).
Zhou, M. et al. Overlapping Stent Treatment for Ruptured Dissecting Aneurysms in Posterior Circulation. Brain Sci. https://doi.org/10.3390/brainsci13111507 (2023).
Hosoya, T. et al. Clinical and neuroradiological features of intracranial vertebrobasilar artery dissection. Stroke 30, 1083–1090. https://doi.org/10.1161/01.str.30.5.1083 (1999).
Yan, Y. et al. Predictors of perioperative complications during Leo baby stent treatment for acutely ruptured intracranial aneurysms: A retrospective multicenter study. Neurosurgery https://doi.org/10.1227/neu.0000000000002780 (2023).
Muir, K. W., Weir, C. J., Murray, G. D., Povey, C. & Lees, K. R. Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke 27, 1817–1820. https://doi.org/10.1161/01.str.27.10.1817 (1996).
Adams, H. P. Jr. et al. Baseline NIH stroke scale score strongly predicts outcome after stroke: A report of the trial of org 10172 in acute stroke treatment (TOAST). Neurology 53, 126–131. https://doi.org/10.1212/wnl.53.1.126 (1999).
McCarthy, D. J. et al. More expansive horizons: a review of endovascular therapy for patients with low NIHSS scores. J. Neurointerv Surg. 13, 146–151. https://doi.org/10.1136/neurintsurg-2020-016583 (2021).
Toth, G. et al. The safety and feasibility of mechanical thrombectomy for mild acute ischemic stroke with large vessel occlusion. Neurosurgery 86, 802–807. https://doi.org/10.1093/neuros/nyz354 (2020).
Kim, J. H., Ko, Y. S., Kwon, S. M., Kim, C. H. & Lee, C. Y. Predictive factors of recurrence after endovascular treatment of unruptured vertebrobasilar fusiform aneurysms. Clin. Neuroradiol. 33, 73–86. https://doi.org/10.1007/s00062-022-01184-9 (2023).
Zhu, D. et al. Overlapped stenting combined with coiling for blood Blister-Like aneurysms: comparison of Low-Profile visualized intraluminal support (LVIS) stent and Non-LVIS stent. World Neurosurg. 104, 729–735. https://doi.org/10.1016/j.wneu.2017.03.092 (2017).
Chan, R. S., Mak, C. H., Wong, A. K., Chan, K. Y. & Leung, K. M. Use of the pipeline embolization device to treat recently ruptured dissecting cerebral aneurysms. Interv Neuroradiol. 20, 436–441. https://doi.org/10.15274/INR-2014-10042 (2014).
Jin, S. C., Kwon, D. H., Choi, C. G., Ahn, J. S. & Kwun, B. D. Endovascular strategies for vertebrobasilar dissecting aneurysms. AJNR Am. J. Neuroradiol. 30, 1518–1523. https://doi.org/10.3174/ajnr.A1621 (2009).
Corley, J. A., Zomorodi, A. & Gonzalez, L. F. Treatment of dissecting distal vertebral artery (V4) aneurysms with flow diverters. Oper. Neurosurg. (Hagerstown). 15, 1–9. https://doi.org/10.1093/ons/opx180 (2018).
Santos-Franco, J. A., Zenteno, M. & Lee, A. Dissecting aneurysms of the vertebrobasilar system. A comprehensive review on natural history and treatment options. Neurosurg. Rev. 31, 131–140. https://doi.org/10.1007/s10143-008-0124-x (2008). discussion 140.
Liu, X. L. et al. Overlapping stents-Assisted coiling for vertebral artery dissecting aneurysm: LVIS stent within neuroform EZ stent. J. Korean Neurosurg. Soc. 65, 523–530. https://doi.org/10.3340/jkns.2021.0275 (2022).
Wu, Q. et al. LVIS-within-enterprise double-stent technique with coil embolization in the treatment of patients with acutely ruptured intracranial vertebrobasilar artery-dissecting aneurysms. Front. Neurol. 14, 1069380. https://doi.org/10.3389/fneur.2023.1069380 (2023).
Schob, S. et al. Segment occlusion vs. Reconstruction-A single center experience with endovascular strategies for ruptured vertebrobasilar dissecting aneurysms. Front. Neurol. 10, 207. https://doi.org/10.3389/fneur.2019.00207 (2019).
Sonmez, O., Brinjikji, W., Murad, M. H. & Lanzino, G. Deconstructive and reconstructive techniques in treatment of vertebrobasilar dissecting aneurysms: A systematic review and Meta-Analysis. AJNR Am. J. Neuroradiol. 36, 1293–1298. https://doi.org/10.3174/ajnr.A4360 (2015).
Carlson, A. P., Alaraj, A., Dashti, R. & Aletich, V. A. The bihemispheric posterior interior cerebellar artery: anatomic variations and clinical relevance in 11 cases. J. Neurointerv Surg. 5, 601–604. https://doi.org/10.1136/neurintsurg-2012-010527 (2013).
Madaelil, T. P. et al. Endovascular parent vessel sacrifice in ruptured dissecting vertebral and posterior inferior cerebellar artery aneurysms: clinical outcomes and review of the literature. J. Neurointerv Surg. 8, 796–801. https://doi.org/10.1136/neurintsurg-2015-011732 (2016).
Griessenauer, C. J. et al. Experience with the pipeline embolization device for posterior circulations aneurysms: A multicenter cohort study. Neurosurgery 87, 1252–1261. https://doi.org/10.1093/neuros/nyaa277 (2020).
Brinjikji, W., Murad, M. H., Lanzino, G., Cloft, H. J. & Kallmes, D. F. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 44, 442–447. https://doi.org/10.1161/STROKEAHA.112.678151 (2013).
Kiyofuji, S. et al. Meta-analysis of treatment outcomes of posterior circulation non-saccular aneurysms by flow diverters. J. Neurointerv Surg. 10, 493–499. https://doi.org/10.1136/neurintsurg-2017-013312 (2018).
Boogaarts, H. D. et al. Aneurysm diameter as a risk factor for pretreatment rebleeding: a meta-analysis. J. Neurosurg. 122, 921–928. https://doi.org/10.3171/2014.12.JNS14931 (2015).
Kulcsar, Z. et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am. J. Neuroradiol. 32, 20–25. https://doi.org/10.3174/ajnr.A2370 (2011).
Shi, G. et al. Overlapping stent-assisted coil embolization for vertebrobasilar dissecting aneurysms: a single-center study. Neurol. Res. 43, 701–707. https://doi.org/10.1080/01616412.2021.1922172 (2021).
Lv, X., Yang, H., Liu, P. & Li, Y. Flow-diverter devices in the treatment of intracranial aneurysms: A meta-analysis and systematic review. Neuroradiol. J. 29, 66–71. https://doi.org/10.1177/1971400915621321 (2016).
Wang, C. B., Shi, W. W., Zhang, G. X., Lu, H. C. & Ma, J. Flow diverter treatment of posterior circulation aneurysms. A meta-analysis. Neuroradiology 58, 391–400. https://doi.org/10.1007/s00234-016-1649-2 (2016).
Yeung, T. W. et al. Long-term outcome of endovascular reconstruction with the pipeline embolization device in the management of unruptured dissecting aneurysms of the intracranial vertebral artery. J. Neurosurg. 116, 882–887. https://doi.org/10.3171/2011.12.JNS111514 (2012).
Lebeaupin, F. et al. Short- and Long-Term Safety and Efficacy of Self-Expandable Leo Stents Used Alone or with Coiling for Ruptured and Unruptured Intracranial Aneurysms: A Retrospective Observational Study. J. Clin. Med. https://doi.org/10.3390/jcm10194541 (2021).
Shen, Y. et al. Periprocedural thromboembolic complications of LEO baby stent in endovascular treatment of intracranial aneurysms: experience in 149 patients. SAGE Publ. https://doi.org/10.1177/15910199231217547 (2025).
Cho, S. H. et al. Bench-top comparison of physical properties of 4 Commercially-Available Self-Expanding intracranial stents. Neurointervention 12, 31–39. https://doi.org/10.5469/neuroint.2017.12.1.31 (2017).
Cohen, J. E., Melamed, I. & Itshayek, E. X-microstenting and transmesh coiling in the management of wide-necked tent-like anterior communicating artery aneurysms. J. Clin. Neurosci. 21, 664–667. https://doi.org/10.1016/j.jocn.2013.09.003 (2014).
Mohlenbruch, M. et al. The LVIS jr. microstent to assist coil embolization of wide-neck intracranial aneurysms: clinical study to assess safety and efficacy. Neuroradiology 56, 389–395. https://doi.org/10.1007/s00234-014-1345-z (2014).
Poncyljusz, W. et al. The LVIS/LVIS jr. stents in the treatment of wide-neck intracranial aneurysms: multicentre registry. J. Neurointerv Surg. 7, 524–529. https://doi.org/10.1136/neurintsurg-2014-011229 (2015).
Akmangit, I. et al. Dual stenting using low-profile LEO baby stents for the endovascular management of challenging intracranial aneurysms. AJNR Am. J. Neuroradiol. 36, 323–329. https://doi.org/10.3174/ajnr.A4106 (2015).
Wang, C. et al. Flow diverter effect of LVIS stent on cerebral aneurysm hemodynamics: a comparison with enterprise stents and the pipeline device. J. Transl Med. 14, 199. https://doi.org/10.1186/s12967-016-0959-9 (2016).
Ge, H. et al. LVIS stent versus enterprise stent for the treatment of unruptured intracranial aneurysms. World Neurosurg. 91, 365–370. https://doi.org/10.1016/j.wneu.2016.04.057 (2016).
Church, E. W. et al. Treatment of posterior circulation fusiform aneurysms. J. Neurosurg. 134, 1894–1900. https://doi.org/10.3171/2020.4.JNS192838 (2021).
Peng, Q. et al. Reconstructive endovascular treatment of Basilar trunk and vertebrobasilar junction aneurysms: A review of 77 consecutive cases. Front. Neurol. 13, 885776. https://doi.org/10.3389/fneur.2022.885776 (2022).
Wang, K. et al. Safety and effectiveness of LEO stents for dual stent-assisted embolization combined with IA and IV intra-procedural infusion of Tirofiban in the treatment of wide-necked intracranial bifurcation aneurysms. Front. Neurol. 15, 1393310. https://doi.org/10.3389/fneur.2024.1393310 (2024).
Author information
Authors and Affiliations
Contributions
Z.W. conceptualized the clinical trial and drafted the main manuscript. M.H.Z. contributed to writing the manuscript. A.M. and Y.H. managed clinical follow-up, data collection, and statistical analysis. X.G. prepared the tables, while C.C. created Figure 1. T.L. supervised the entire retrospective clinical trial. M.X.Z. and K.S. developed the core concept of the trial, guided its execution, and reviewed and edited the manuscript. All authors have read and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, Z., Zhou, M., Maalim, A.A. et al. LEO or LEO baby within enterprise overlapping stents combined with coil embolization treatment for ruptured vertebrobasilar artery dissecting aneurysms. Sci Rep 15, 25153 (2025). https://doi.org/10.1038/s41598-025-11035-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11035-5