Abstract
A subset of differentiated thyroid cancer still lacks effective treatment options, such as locally advanced or metastatic patients. Some of these patients also need to try radiation therapy. The objective of this study is to investigate the potential of the antennapedia-modified SMAC fragment (ANTP-SmacN7) as a radiation sensitizer to enhance the therapeutic efficacy of radiotherapy in papillary carcinoma of thyroid (PTC), ascertain the contribution of X-linked inhibitor of apoptosis protein (XIAP), and explore its associated mechanisms to the apoptotic response in PTC. Methods To determine whether ANTP-SmacN7 enhances radiotherapy sensitivity by promoting apoptosis through XIAP in thyroid cancer, we first performed bioinformatics analysis of XIAP in thyroid cancer (THCA) using TCGA/GTEx THCA datasets. This analysis evaluated XIAP expression levels, its correlation with clinical pathological features and prognosis, functional enrichment, and interactions with apoptosis-related genes of CASP. Subsequently, we treated TPC-1 cells with gamma ray and ANTP-SmacN7 in different groups and assessed cell proliferation, migration, and apoptosis-related protein expression (XIAP, caspase-3, caspase-8, and caspase-9) using CCK-8, colony formation, transwell assays, annexin V/PI double staining, and western blotting. To further elucidate the role of XIAP and the effects of ANTP-SmacN7, we constructed XIAP-overexpressing TPC-1 cells, treated them with gamma ray and ANTP-SmacN7 in different groups, and compared changes of cell function and apoptosis-related protein. XIAP is highly expressed in THCA and demonstrates significant correlations with aggressive clinicopathological features including advanced T stage (p = 0.018) and extrathyroidal extension (p = 0.017) in THCA, while its prognostic stratification potential is evidenced by an AUC of 0.63. It interacts closely with CASP family members involved in apoptosis, which might critically inform therapeutic strategies for radioresistant THCA. The experimental results demonstrate that TPC-1 cells are sensitive to low-dose gamma ray but exhibit radioresistant as the radiation dose increases. While ANTP-SmacN7 significantly enhances gamma ray-induced radiosensitization. When combined with gamma ray, ANTP-SmacN7 markedly reduces cell proliferation, viability, and migration, while simultaneously promoting apoptosis. This effect is mediated by XIAP inhibition, which activates the intrinsic apoptosis pathway through caspase-3 and caspase-9. Additionally, XIAP influences the extrinsic apoptosis pathway by directly or indirectly upregulating caspase-8. ANTP-SmacN7 could be a probable candidate for a pharmaceutical radiosensitizer when combined with gamma ray for the treatment of PTC. These findings may provide a theoretical basis for the development of radiosensitization strategies for locally advanced or metastatic patients.
Similar content being viewed by others
Introduction
Thyroid cancer is diagnosed in about 4.3-143.3 cases per 100,000 person-years in women1,2. PTC constitutes approximately 84% of all thyroid cancer cases and is classified as a well-differentiated type, of which the 5-year survival rate following diagnosis is around 98.5%3. While there is still a subset of PTC that lacks effective treatment options, such as iodine-refractory thyroid cancer and locally advanced/metastatic thyroid cancer which are not amenable to surgery or other therapy, these patients often suffer from symptoms due to tumor invasion of the trachea, esophagus, larynx, and major neck blood vessels4. Typically, they are offered palliative care to alleviate these symptoms5. Although multitargeted tyrosine kinase inhibitors have been approved as first-line therapy, limited life span and dose-limiting toxicities are reported, and stereotactic body irradiation is more favored over systemic treatment in metastatic patients6,7,8. To increase the therapeutic effect, approaches are needed to optimize administered radiation9.
In various cancers, the Inhibitors of Apoptosis Proteins (IAPs) are extensively studied to optimize therapy effects, for they directly participate in the regulation of apoptosis by binding to and inhibiting pro-apoptotic factors10. IAPs include proteins such as Cellular Inhibitor of Apoptosis Protein 1 (cIAP1), Cellular Inhibitor of Apoptosis Protein 2 (cIAP2), Melanoma Inhibitor of Apoptosis Protein (ML-IAP), and X-linked Inhibitor of Apoptosis Protein (XIAP)10,11. In thyroid cancer, the expression of XIAP is found to be related to the lateral neck lymph node metastasis, which leads to the poor prognosis of PTC12. XIAP may influence this process by facilitating the proliferation and progression of thyroid cancer cells through the inhibition of caspase-mediated apoptosis13,14,15,16. In particular, IAP inhibitors have shown potential to increase the sensitivity of thyroid cancer to radiotherapy and chemotherapy17. SMAC/Diablo is a mitochondrial-derived pro-apoptotic protein that can promote apoptosis by binding to IAPs18,19. Although SMAC has emerged as a potential therapeutic agent for cancer treatment, the N-terminal 7-amino acid peptide fragment of SMAC, known as SMAC N7, exhibits limited stability within the cellular milieu and faces challenges in translocating from the extracellular environment into the cell20,21. Consequently, the redesign or mimetic of SMAC has been developed and employed as adjuvant therapy for a variety of solid tumors22,23.
One such promising synthetic molecule is the ANTP-SmacN7, which shows notable adjuvant activity, particularly being used in irradiation radiosensitization24. ANTP, representing the third alpha-helix within the homeodomain of Drosophila antennapedia proteins, is a minimal 16-amino acid peptide (comprising residues 43 to 58) that retains transduction capabilities25,26. This peptide demonstrates the ability to directly interact with the lipid bilayer of biological membranes, thereby facilitating its transmembrane traversal and cellular entry in a manner that is independent of receptors, channels, energy expenditure, and endocytic pathways25,26,27,28. The ANTP-SmacN7 fusion protein is precisely engineered by conjugating the lysine residue at the C-terminus of the SMAC N7molecule to the arginine residue at the N-terminus of ANTP, facilitated by a proline linker29. By employing this method, the ANTP is utilized as a cell-penetrating peptide to facilitate the integration of the SMAC N7 domain into cellular compartments. However, further experimental validation is required to confirm whether ANTP-SmacN7 can enhance radiosensitivity in thyroid cancer cells. Moreover, the precise molecular mechanisms underlying ANTP-SmacN7-mediated radiosensitization through XIAP inhibition remain to be fully elucidated, particularly regarding its potential dual regulation of both intrinsic and extrinsic apoptosis pathways.
This study aims to explore the potential of ANTP-SmacN7 as a radiation sensitizer to enhance the therapeutic efficacy of radiotherapy in PTC. Additionally, the study seeks to elucidate the role of XIAP and its associated mechanisms of apoptotic response in this treatment process. Here, our experiments display that ANTP-SmacN7 induced cell apoptosis in irradiated thyroid cells by inhibiting XIAP and and activating the caspase cascade, including caspase-3, caspase-8, and caspase-9. These results indicate that ANTP-SmacN7 could serve as a probable candidate for a pharmaceutical radiosensitizer when used in conjunction with radiotherapy for PTC. This combined treatment strategy may provide a possible therapeutic option for advanced thyroid cancer, particularly for those targeting IAP inhibitors.
Materials and methods
Data Acquisition and Processing of XIAP
For unpaired pan-cancer analysis, harmonized RNA-seq data integrating TCGA-ALL cohort and GTEx normal tissues were retrieved from the UCSC Xena platform (https://xenabrowser.net/datapages/), processed through the Toil computational reproducibility pipeline to ensure cross-study normalization. For paired pan-cancer analysis, raw RNA-seq BAM files from the TCGA-ALL cohort were acquired (https://portal.gdc.cancer.gov). Alignment and quantification were performed using the STAR aligner, followed by TPM normalization. Only cases with matched tumor-adjacent normal pairs were retained for downstream comparisons. The XIAP-specific analysis in thyroid cancer (THCA) utilized the TCGA-THCA dataset (https://portal.gdc.cancer.gov). Pre-aligned BAM files generated by TCGA’s standardized STAR alignment pipeline were obtained, and samples lacking paired clinical annotations or exhibiting low sequencing depth were excluded after rigorous quality control. Subsequent analyses were performed directly on the retained high-quality data.
Expression analysis of XIAP
Publicly available datasets were analyzed to compare XIAP expression across groups. Statistical analyses were performed using R (v4.2.1) with the stats (https://stat.ethz.ch/R-manual/R-patched/library/stats/html/00Index.html) and car packages (https://CRAN.R-project.org/package=car). Differentially expressed genes were identified using DESeq2 with thresholds of |log2FC| > 1 and FDR-adjusted p < 0.05. Groups with fewer than 3 samples or zero within-group SD were excluded from statistical comparisons but retained for visualization. Normality and homogeneity of variance assumptions were assessed via Shapiro-Wilk and Levene’s tests, respectively. Nonparametric Wilcoxon rank sum tests were applied to evaluate differential XIAP expression between normal and tumor tissues.
Clinicopathological Correlates of XIAP Expression in THCA
The association between XIAP expression and clinicopathological features in THCA was evaluated using R (v4.2.1) with the stats package (https://stat.ethz.ch/R-manual/R-patched/library/stats/html/00Index.html). Clinical variables included age, gender, pathologic T/M stage, pathologic stage, extrathyroidal extension, thyroid gland disorder history, residual tumor, and neoplasm location. Categorical variables were compared between XIAP expression groups using chi-square tests. For sparse data, Yates’ continuity correction was applied. Age was analyzed via Wilcoxon rank sum tests. Pathologic T stage were evaluated using chi-square trend tests. Results were tabulated as counts for categorical variables and medians with interquartile ranges for continuous variables. Statistical measures were included in baseline characteristic tables. Categorical variables were presented as frequency (percentage) in the baseline characteristics table. Percentages were calculated based on the total sample size (N = 512). Categorical variables were presented as frequency (percentage) in the baseline characteristics table. Percentages were calculated based on the total frequency of the sample (N = 512). The calculation formula is as follows:
The percentage data presented in the table are all calculated individually in the above manner to accurately reflect the distribution of each variable across different categories.
The XIAP predictive capacity for survival rates in THCA
XIAP’s prognostic value was assessed via time-dependent ROC analysis. Distribution normality was assessed via Shapiro-Wilk tests, and variance homogeneity via Levene’s tests. Wilcoxon rank-sum tests compared XIAP expression in tumor vs. normal tissues. Time-dependent AUC values quantified XIAP’s predictive capacity at survival endpoints. Analyses were performed using R (v4.2.1) with timeROC (v0.4) (https://CRAN.R-project.org/package=timeROC) and ggplot2 (v3.4.4) (https://CRAN.R-project.org/package=ggplot2packages). Cohorts with < 3 samples or zero variance were excluded from tests but included in plots.
Functional enrichment analysis of co-expressed genes
To delineate XIAP-associated biological networks, we performed integrative enrichment analyses. Functional enrichment analysis of XIAP-associated genes was conducted using KEGG pathways via the R software cluster Profiler package (https://bioconductor.org/packages/clusterProfiler)30. Enriched terms were filtered with a significance threshold of P < 0.05 and false discovery rate (FDR) < 0.25. As a reference, the ‘ALCALA_APOPTOSIS’ gene set was obtained from the Molecular Signatures Database (MSigDB) (http://software.broadinstitute.org/gsea/msigdb)31. We performed GSEA to reveal pathways of apoptosis linked to XIAP expression.
Correlation analysis of XIAP and apoptosis-related CASP family
To investigate the correlation between XIAP and CASP genes in THCA, this study employed Spearman’s rank correlation coefficient to analyze the expression correlation between XIAP and CASP genes using R packages (https://www.R-project.org/). The results were visualized through co-expression heatmaps generated with the ggplot2 package (https://CRAN.R-project.org/package=ggplot2packages). Additionally, a protein-protein interaction (PPI) network was constructed using the STRING database (https://string-db.org/) website for the sake of exploring the potential relationships between XIAP and CASP family. Further analysis focused on the correlation between XIAP and specific CASP genes (CASP3, CASP8, and CASP9), as well as the expression level of CASP3, CASP8, and CASP9 in cancer tissues from the dataset mentioned above.
Cell lines and XIAP-overexpressing lentivirus transfection
We used the human thyroid cancer cell line TPC-1 provided by the Saibaikang Company (Shanghai, China). TPC-1 cells were cultured in DMEM medium containing 10% fetal bovine serum and incubated in a humidiWed (37℃, 5% CO2) incubator. The cells in logarithmic growth phase were then trypsinized to prepare a cell suspension. 1 × 105 cells per well were plated into a 24-well plate and cultured for 24 h. Then 10µL of XIAP overexpression lentivirus was added and infected at 37℃for 20 h. Then the culture medium was replaced with fresh medium and cultured for the next 48 h.
The construction of XIAP-overexpressing lentivirus
A shuttle plasmid (LV-033, YBR, Shanghai, China) was selected as the expression vector for our study. Primers incorporating the restriction enzyme sites Nhe I (5’GCTAGC-3’) and BamHI (5’GGATCC-3’) were designed and synthesized to amplify the target gene XIAP (Gene ID: 331; CDS ID: CCDS14606.1) from genomic DNA via PCR. The amplified gene was then ligated with the expression vector using the ClonExpress II One Step Cloning Kit (Sangon Biotech, Shanghai, China). The recombinant plasmid was introduced into competent E. coli cells, Stable (DL1080, Vigene Biosciences, Rockville, MD, USA), through the heat shock method. Positive clones were selected based on ampicillin resistance and subsequently cultured for expansion. These clones underwent PCR identification and sequencing analysis to confirm the successful insertion of the target gene. For viral production, the pMD2. G vector (Addgene #12259, Didier Trono Lab) and pSPAX2 vector (Addgene #12259, Didier Trono Lab) were co-transfected into 293 T cells (YBR, Shanghai, China). The cell supernatant was harvested 48–72 h post-transfection. The supernatant was concentrated by ultracentrifugation to pellet viral particles, followed by filtration through a 0.45 μm filter to eliminate cellular debris. The viral stock was characterized by its pink, clear appearance and lack of viscosity, indicating a pure preparation. To ensure the absence of bacterial and fungal contamination, the viral stock was added to HEK 293 T cells and incubated for 24 h, followed by microscopic examination. The viral titer was determined using a drug screening method, which involved calculating the number of surviving cells post-infection.
qPCR analysis of XIAP expression
Total RNA was extracted from TPC-1 cells and XIAP-OE TPC-1 cells using TRIzol reagent (Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer’s instructions. The extracted RNA was reverse-transcribed into cDNA using the HiScript QRT SuperMix for qPCR (+ gDNA Wiper) (Vazyme, R123-01). qPCR was performed using the AceQ qPCR SYBR Green Master Mix (Vazyme, Q111-02) on an ABI 7500 Real-Time PCR System. The thermal cycling conditions were: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. The primer sequences for XIAP were as follows: forward, TTTGAGTCCATGCCCAATCC; reverse, TCCATCCTCTGCTGTCACCTCT. The expression level of XIAP was normalized to the endogenous control gene GAPDH using the 2−ΔΔCt method.
ANTP-SmacN7 fusion peptide
The ANTP (A), SmacN7 (S), and ANTP-SmacN7 (AS) recombinant peptides were synthesized by Shanghai Sangon Biotech (Shanghai, China), and the synthesized method as well as amino acid sequences were performed as described previously24,29:
ANTP: Arg-Gln-Ile-Lys-Ile-Trp-Phe-Gln-Asn-Arg-Arg-MetLys-Trp-Lys-Lys-FITC;
SmacN7: Ala-Val-Pro-Ile-Ala-Gln-LysPro-FITC;
ANTP-SmacN7: Ala-Val-Pro-Ile-Ala-Gln-LysPro-Arg-Gln-Ile-Lys-Ile-Trp-Phe-Gln-Asn-Arg-Arg-Met-Lys-TrpLys-Lys-FITC.
Cell irradiation
All irradiation procedures were performed by Co-60 gamma ray device. The PTC cells were exposed to a series of doses of gamma ray (The gradient of total exposure dose is 0, 2, 4, 8 and 10 Gy). Negative control (NC) cells were treated with the vehicle solvent under identical conditions as experimental groups but without radiation or ANTP-SMAC N7. Within 24 h, a CCK-8 assay (Sigma-Aldrich, St. Louis, MO, USA) was performed to assess cell viability and determine the optimal dose and duration of irradiation. At last, we selected the dose of 2 Gy for further experiments. Because at this radiation dose, the cell-killing effect is minimal, which ensured that the physiological functions of the cells would not be too affected when conducting cell function studies, thereby allowing for a more accurate investigation of the effects of sensitizers on the functions of cells after irradiation.
The colony formation assay for cell proliferation
The degree of damage to the proliferative ability of TPC-1 after irradiation is tested by the colony formation. TPC-1 cells in the logarithmic growth phase from each group were digested, resuspended in complete medium (10-013-CVR, Corning, NY, USA). 400 to 1000 cells per well were plated in 6-well culture plates, with triplicate wells for each group. Cells were incubated until most monoclonal colonies contained more than 50 cells. Subsequently, colonies were fixed with 4% paraformaldehyde (Sinopharm Chemical Reagent Co., Ltd) and stained with GIEMSA (AR-0752) (Shanghai Dingguo Biotechnology Co., Ltd). After washing with ddH2O and drying, the colonies were visualized using a digital camera (IX71)(Olympus, Tokyo, Japan), and the number of monoclonal colonies was quantified.
CCK-8 for the level of cell viability
We use the CCK-8 to value the cell viability of cells. TPC-1 cells from each experimental group were seeded in 96-well plates at a concentration of 2000 cells per 100µL per well. Plates were incubated at 37 °C with 5% CO2. Subsequently, 10µL of CCK-8 reagent (96992, Sigma-Aldrich, St. Louis, MO, USA) was added per well, and cells were incubated for 3 h. The optical density at 450 nm was measured using a microplate reader (M2009PR, Tecan Infinite, Switzerland). Cell viability was calculated using the following formula:
An (Experiment): Optical density was measured in wells containing TPC-1 cells, CCK-8 solution, and the experimental group solutions.
A (Blank): Optical density was measured in wells containing only culture medium and CCK-8 solution, without cells.
A (Control): Optical density was measured in wells containing TPC-1 cells, CCK-8 solution, and culture medium only.
Transwell for the level of cell migration
A total of 1 × 105 cells from different groups are suspended in 100 µL of serum-free medium and seeded into the upper chamber of the Transwell insert. The lower chamber is filled with 600 µL of medium containing 30% FBS (VS500T)(Ausbian) as a chemoattractant. After 24 h of incubation at 37℃, non-migrating cells remaining on the upper surface of the membrane are gently removed using a cotton swab to ensure that only the cells that have migrated to the lower surface are counted. Then the migrated cells on the lower surface of the membrane were stained with 400 µl staining solution (3422)(Corning, NY, USA) for 5 minutes, then rinsed with PBS, air-dried, and photographed using a light microscope. Capture images of the stained cells using a microscope for further analysis.
Annexin V and PI double staining method analyze apoptosis induction
Cells were collected 24 h after irradiation, washed with cold PBS, and resuspended in binding buffer (10010-09, Southern Biotech, Birmingham, AL, USA). Let the cell suspension to a final density of 1 × 106 to 1 × 107 cells/ml. Annexin V-FITC (5 µL) and Propidium Iodide (PI) (5 µL) (both from Southern Biotech) were added, and the cells were incubated in the dark for 15 min at room temperature. Apoptotic cells were then analyzed using a MM high-pass flow cytometer (ABC250-22INT, Guava Company, Singapore).
Western blot analysis of the expression of apoptosis-related proteins
Cells from each group were lysed using Cell Lysis Buffer (P0013) and PMSF (ST505, Beyotime Biotechnology, Shanghai, China) at a 100:1 concentration ratio. Equal amounts of protein (20 µg) were subjected to SDS-PAGE (Sangon, Shanghai, China) and then transferred to polyvinylidene difluoride (PVDF) membranes (IPVH00010, Merck Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat dry milk and incubated overnight at 4 °C with primary antibodies against XIAP (ab28151, Abcam, Cambridge, MA, USA), caspase-3 (66470-2-Ig, Proteintech), caspase-8 (13423-1-AP, Proteintech), caspase-9 (ab32539, Proteintech), and GAPDH (60004-1-Ig, Proteintech). After washing with TBST, the membranes were incubated with HRP-conjugated secondary antibodies at room temperature for 3–4 h. (goat anti-rabbit IgG-HRP, A0208; goat anti-mouse IgG-HRP, A0216, both from Beyotime, Shanghai, China). The membranes were washed again to remove any unbound secondary antibodies and developed using the Immobilon Western Chemiluminescent HRP Substrate (Millipore Corporation). Chemiluminescence was captured using the AI600 Chemiluminescence Imaging System (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
Statistical analysis
The statistical methods of bioinformatics analysis are detailed above. The other data analysis was conducted using GraphPad Prism version 9.5.0 for Windows (GraphPad Software, Boston, Massachusetts, USA). Statistical significance was ascertained through pairwise T-test comparisons among experimental groups. The value considered statistically significant is as follows: *P < 0.05; **P < 0.01; ***P < 0.001. All data were depicted as the mean ± standard deviation (SD), based on replicates from three separate experimental trials.
RESULTS
Bioinformatics analysis of XIAP expression in thyroid cancer
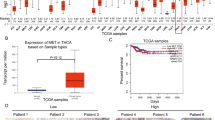
In this study, we conducted a systematic bioinformatics analysis of XIAP in thyroid cancer (Fig. 1). Paired and unpaired pan-cancer analyses revealed that XIAP expression was significantly higher in various malignant tumor tissues, including thyroid cancer, than in normal tissues (Fig. 2A,B). Further validation using the TCGA database confirmed these results: in thyroid cancer, XIAP was not only a significantly upregulated differentially expressed gene (Fig. 2C) but also exhibited significantly elevated expression levels compared to normal tissues (Fig. 2D,E). Functional enrichment analysis implicated XIAP in thyroid cancer development through histone modification, regulation of DNA metabolic processes, and ubiquitination pathways (Fig. 2F). GSEA analysis showed that apoptosis-related gene sets were significantly downregulated in tumor tissues (Fig. 2G), suggesting that XIAP-mediated apoptosis inhibition may play a key role in therapeutic resistance. Notably, despite its high expression in tumors, XIAP exhibited limited predictive efficacy for overall survival in thyroid cancer patients (AUC = 0.62; Fig. 2H). Clinical pathological correlation analysis (Table 1) revealed that XIAP expression was significantly associated with pathologic T stage (p = 0.018) and extrathyroidal extension (p = 0.017) but not with other parameters such as age, gender, or lymph node metastasis. This finding suggests that XIAP may primarily be involved in local tumor invasion rather than distant metastasis regulation.
Correlation analysis of XIAP and CASP family genes
Our bioinformatics analysis of XIAP in thyroid cancer revealed significant insights into its role and interactions within apoptotic pathways. As depicted in the interaction network (Fig. 3A), XIAP is centrally positioned among apoptosis-related genes, including CASP3, CASP7, CASP8, and CASP9, indicating its potential regulatory role in apoptosis. The expression pattern heatmap (Fig. 3B) further illustrated the coordinated expression of XIAP with CASP family genes across high and low expression cohorts. Correlation analysis (Fig. 3C) demonstrated strong positive correlations between XIAP and CASP3 (Spearman R = 0.625, P < 0.001), CASP8 (Spearman R = 0.541, P < 0.001), and CASP9 (Spearman R = 0.451, P < 0.001), underscoring XIAP’s influence on these apoptotic mediators. Notably, tumor tissues exhibited significantly reduced expression of CASP3, CASP8, and CASP9 compared to normal tissues (Fig. 3D), suggesting that XIAP overexpression in thyroid cancer may contribute to tumorigenesis by suppressing apoptosis through the downregulation of these caspases. These findings collectively highlight XIAP’s pivotal role in thyroid cancer and its interplay with key apoptotic regulators.
Impact of gamma ray on TPC-1 viability
Varying doses of gamma ray (0,2,4,8,10 Gy) were performed on TPC-1. Radiation dose and time dependent experiments demonstrated a significant decline in cell viability with increasing radiation doses and prolonged treatment duration (Fig. 4A). The reduction in proliferation at the 2 Gy dose was not statistically significant (P < 0.001). While in comparison with non-irradiated control cells (0 Gy), a statistically significant reduction in cell proliferation was observed following exposure to 4, 8, and 10 Gy doses of gamma ray. And extending the duration to 5 days resulted in cumulative cell death across all dose groups. Notably, 4 Gy resulted in the greatest reduction in cell viability, with further decreases as the dose exceeded 4 Gy. Compared to the non-irradiated control group (NC, 0 Gy), a 4 Gy dose caused the most pronounced decrease in cell viability, establishing 4 Gy as a critical threshold for inducing cell death. Beyond 4 Gy, cell viability continued to diminish, but the rate of decline stabilized, yielding survival rates of 25% at 8 Gy and 15% at 10 Gy.
Synergistic effect of ANTP-SmacN7 and gamma ray on TPC-1 cell function
The TPC-1 cells were categorized into four distinct experimental groups: a control group, a group subjected to gamma ray, a group treated with ANTP-SmacN7, and a combination group receiving both gamma ray and ANTP-SmacN7. The gamma ray group was exposed to a total dose of 2 Gy, while the ANTP-SmacN7 group was incubated with the fusion protein at a concentration of 1 × 10⁻⁵ M for 24 h. The combination group was pre-incubated with ANTP-SmacN7 for 24 h prior to being exposed to 2 Gy of gamma ray. Cell viability, proliferation, and migration were evaluated using the CCK-8 assay, colony formation assay, and transwell assay, respectively. Results showed that, compared to the control group (0 Gy), gamma ray significantly decreased the cell viability, cell proliferation and cell migration of TPC-1 cell lines (P < 0.001). Adding ANTP-SmacN7 further promotes these inhibitory effects (P < 0.001) (Fig. 4B-D). However, it shows that ANTP-SmacN7 alone had no significant effect on cell functions. From the preliminary analysis, the data above showed that ANTP-SmacN7 alone had minimal effect on TPC-1 cells, while it has a significant synergistic effect of gamma ray on cell viability, proliferation, and migration.
ANTP-SmacN7 mediated the apoptosis of TPC-1
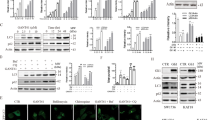
Apoptosis was performed in our experiment. The results displayed that the addition of ANTP-SmacN7 alone did not change the level of apoptosis of TPC-1 cells. However, the apoptotic level was significantly increased in cells receiving gamma ray (P < 0.001). More importantly, compared with gamma ray alone, ANTP-SmacN7 increased the pro-apoptotic effect of gamma ray on thyroid cancer cells (P < 0.001)(Fig. 4E). The participating proteins of apoptosis were also explored in this study. We then detected the expression of XIAP, Caspase-8, Caspase-9, and Caspase-3 by western blot. The results showed that there was a reduction of XIAP expression by the intervention of gamma ray and the gamma ray with ANTP-SmacN7. It also displays the experimental data that gamma ray increases the expressions of caspase-3, caspase-8, and caspase-9. And ANTP-SmacN7 followed by gamma ray was found to further augmented the expressions of active caspase-3 caspase-8 and caspase-9 (Fig. 5A-D).
The role of XIAP on radiation-induced proliferation and apoptosis by ANTP-SmacN7
To further certify the underlying mechanisms of the radiosensitizing effect of ANTP-SmacN7, we employed a lentiviral construct to overexpress XIAP in TPC-1 cells. The overexpression of XIAP was confirmed through western blot analysis (Fig. 6A) and q-PCR (Fig. 6B). Our experimental design encompassed four groups: gamma ray alone, gamma ray combined with ANTP-SmacN7, gamma ray with XIAP overexpression (XIAP-OE), and gamma ray with both ANTP-SmacN7 and XIAP-OE. ANTP-SmacN7 was introduced at a concentration of 1 × 10⁻⁵ M for a duration of 24 h, prior to the application of 2 Gy gamma ray. Subsequently, we evaluated various cellular functions. Cell viability is performed by CCK-8 as shown in Fig. 6C. It had a significant increase when XIAP was overexpressed (P < 0.01). Conversely, the ANTP-SmacN7 weakened the cell viability (P < 0.01). Similarly, the over-expression of XIAP promoted the cell proliferation and cell migration of TPC-1 cells (P < 0.01). While the addition of ANTP-SmacN7 can inhibit these influences effectively (Fig. 6D,E). Annexin V and PI double staining revealed that XIAP overexpression inhibited gamma-ray-induced apoptosis in TPC-1 cells (P < 0.001), while ANTP-SmacN7 increased apoptosis in both gamma-ray-only and gamma-ray plus XIAP-overexpressing groups (P < 0.05 and P < 0.001, respectively) (Fig. 6F). Finally, the expression of apoptotic proteins—Caspase-3, Caspase-8, and Caspase-9—paralleled these findings. XIAP overexpression decreased their expression, and ANTP-SmacN7 reversed this, enhancing the apoptotic effect of gamma rays on TPC-1 cells (Fig. 7).
DISCUSSION
Papillary carcinoma is the most common type of differentiated thyroid cancer7. It has an indolent clinical course when treated by lobectomy at an early stage5. But still, there is a certain number of patients that have poor survival by local progression or failure therapy. Targeted therapeutics, like multikinase inhibitors, can improve the treatment of refractory thyroid cancer but also report high rates of adverse events32. Being another choice, radiotherapy is sometimes considered at straws for these patients, such as patients with disease not amenable to radioactive iodine therapy, patients with local recurrence or metastasis, and patients with local advanced or unresectable gross residual disease in neck2. However, from the perspective of these patients, there is still a hard choice between the therapeutic effect of radiotherapy and the side effects of this treatment33,34,35.
Many studies aim to greatly improve the therapeutic effect of radiotherapy and reduce the side effects. Early, some investigators proposed that the simultaneous addition of chemotherapeutic agents such as doxorubicin with intensity-modulated radiation can improve local control and overall survival36,37. Others have tried that combine radioactive iodine with radiotherapy as another way to improve treatment effectiveness35,38. Also, the attempt of radiotherapy combined with particle therapy is underexplored39. Actually, the resistance or tolerance of radiation is a major factor that can result in failure of radiotherapy40. Our results indirectly reflect this view. In our experiment, the gamma ray can significantly reduce the proliferation of TPC-1 at a dose under 4 Gy and reaches the lowest. But as the dose continues to increase, the proliferation of TPC-1 shows a trend of decline, which means that the TPC-1 develops resistance or is not sensitive to gamma ray as the dose exceeds a certain level. Based on the GSEA analysis results of this study, apoptosis-related gene signatures were significantly downregulated in thyroid tumor tissues compared to normal tissues, suggesting systemic suppression of the apoptotic machinery in the malignant state. We hypothesize that this phenomenon may be associated with apoptosis inhibition as therapeutic radiation doses increase. Radiotherapy typically induces apoptosis by causing DNA damage. However, cancer cells can develop radioresistance by upregulating anti-apoptotic genes and proteins, such as XIAP, thereby inhibiting apoptosis.
Addressing the challenges of radiotherapy tolerance and resistance, a stream of radiotherapy agents and sensitization targets has been found out40. Among these sensitizers, SMAC relevant compounds lower the threshold for cell death induced by cytotoxic stimuli such as radiotherapy23,41. SMAC is a protein released from mitochondria, which promotes apoptosis through antagonizing inhibitors of IAPs23,42. The ANTP-SmacN7 fusion protein is a SMAC relevant engineered protein that guarantees the SMAC N7 domain into the cell24,29. To explore whether this engineered protein could be a radiation sensitizer for thyroid cancer, we investigated the effects of ANTP-SmacN7 on cell survival and cell behaviors in TPC-1 cells. Our current results confirm that the ANTP-SmacN7 can enhance the radiotherapy effects of gamma ray on TPC-1 cells, which is evidenced by a reduction in tumor cell viability, a suppression of their proliferative and migration capabilities. Unlike some small-molecule XIAP inhibitors, the modified SMAC in our study doesn’t alter or interfere with other XIAP functional regions43. Our results show ANTP-SmacN7 has remarkable cell-penetrating ability, effectively entering TPC-1 tumor cells (Supplementary Fig. S2). SmacN7 and other Smac-mimetic peptides may struggle to penetrate cell membranes due to their molecular and charge properties43,44. However, ANTP-SmacN7, fused with Antennapedia’s cell-penetrating peptide, enters cells efficiently, including hard-to-penetrate tumor cells. This advantage in intracellular delivery and distribution also addresses the delivery issues of RNA-based XIAP inhibitors in vivo45. All these results confirm that ANTP-SmacN7 can act as an excellent radiosensitizing agent in thyroid cancer.
Moreover, our experimental data revealed that ANTP-SmacN7 monotherapy exhibited no significant cytotoxicity in TPC-1 cells, contrasting with reports showing Smac mimetics as standalone pro-apoptotic agents in select malignancies23. This divergence suggests that TPC-1 cells are not sensitive to the individual action of ANTP-SmacN7. SMAC relevant compounds work by targeting IAPs, particularly XIAP, to promote apoptosis24,46. If the expression levels of XIAP, are not appropriately high in the TPC-1 cells, the ANTP-SmacN7 monotherapy may not have a pronounced effect15. To verify this possibility, we first analysed the XIAP expression through bioinformatics. The result revealed that XIAP expression was significantly higher in thyroid cancer tissues than in normal tissues and was significantly associated with aggressive clinicopathological characteristics, such as advanced T stage and extrathyroidal extension (Table 1). Similarly, the experiment reveals that the expression of XIAP is detectable in TPC-1 cells, and it is notably reduced when subjected to the gamma ray with ANTP-SmacN7, which is consistent with the results of cellular function experiments. Furthermore, we engineered TPC-1 cells to overexpress XIAP, and the findings indicate that this overexpression leads to the detrimental effects of gamma ray and ANTP-SmacN7 on cellular functions. Thus, the expression level of XIAP is enough to respond to the ANTP-SmacN7 in TPC-1 cells. Other factors such as the dosage of the ANTP-SmacN7, the presence of other growth factors or inhibitors, and the overall genetic makeup of the TPC-1 cells may also contribute to the negative results. Although ANTP-SmacN7 alone does not produce significant antitumor effects, it exhibits marked synergistic anti-apoptotic effects when combined with radiotherapy. Apoptosis is a distinct mode of cell death that is responsible for the deletion of cells in malignant tumors and it can increase tumors responding to different kinds of therapy42. XIAP blocks the activity of the highly conserved family of caspases, thereby averting apoptosis47. Our research demonstrates that ANTP-SmacN7 with gamma ray, enhances the expression of caspase-3 and caspase-9, thereby inducing apoptosis in TPC-1 cells. The overexpression of XIAP has been shown to diminish the expression of caspase-3 and caspase-9, and a suppression of the apoptotic process. In conclusion, whether ANTP-SmacN7 can independently inhibit thyroid cancer cells still requires further experiments for exploration.
Additionally, we have preliminarily explored the apoptosis pathway induced by the treatment of ANTP-SmacN7. The apoptosis is composed of extrinsic cell death pathway and intrinsic cell death pathway48. Caspase-3 and caspase-9 are the primary participants in the intrinsic pathway of apoptosis in tumor cells49. Bioinformatics analysis showed significant positive correlations between XIAP and caspase family genes (Fig. 3), indicating that XIAP may influence thyroid cancer biology by regulating these key apoptotic factors. Our experimental results support that ANTP-SmacN7 can affect XIAP primarily and facilitate the apoptosis of thyroid cancer cells by inhibiting the effector caspase-3 and caspase-9, by intervening in the intrinsic apoptotic pathway. However, one unanticipated finding is that, in addition to caspase-3 and caspase-9, caspase-8 also shows a significant increase as XIAP diminishes. Caspase-8 is the initiator caspase responsible for the extrinsic apoptosis in cells50. The activation of caspase-8 is theoretically inhibited by c-IAP1 and c-IAP247. It is thought that the increase of caspase-8 expression is the activation of tumor cell extrinsic apoptosis by ANTP-SmacN724. We think that besides this case, XIAP may affect caspase-8 activity through an indirect mechanism. XIAP may indirectly affect caspase-8 function by regulating the upstream signaling pathways or downstream effector molecules of caspase-8. There is a cascade of activation relationship between Caspase-8 and caspase-3, and the activation of caspase-8 can lead to further activation of caspase-3 mediated by downstream molecules51. XIAP may subsequently affect the activation and function of caspase-8 by inhibiting the activity of caspase-3. Although further exploration is needed to understand the mechanism by which ANTP-SmacN7 affects caspase-8, it is clear that ANTP-SmacN7 does fully promote apoptosis in thyroid cancer cells.
In conclusion, this study demonstrates the efficacy of ANTP-SmacN7 in enhancing the apoptotic response to radiotherapy and explores the mechanism of action in THCA. It suggests that ANTP-SmacN7 might represent a potential radiation sensitizer for use in combination with radiotherapy for the treatment of PTC, which might be meaningful for the locally advanced or metastasis patients. While future studies should validate these effects in vivo and explore potential crosstalk between XIAP and cIAP1/2. Additionally, the dose selection, the influence of the tumor microenvironment, and the mechanism of the activation pathway, all of which can influence in vivo efficacy, warrant further investigation.
Bioinformatic Analysis of XIAP in Thyroid Cancer. (A) Unpaired pan-cancer analysis of XIAP expression across diverse tissues and cancer types. (B) Paired pan-cancer analysis of XIAP expression in corresponding tissues and cancer types. (C) Volcano plot depicting differentially expressed genes between normal and thyroid cancer tissues. (D) Comparison of XIAP expression levels in thyroid cancer versus normal tissues. (E) Comparison of XIAP expression in paired thyroid cancer and adjacent normal tissues. (F) KEGG pathway enrichment analysis of XIAP-associated biological processes. (G) GSEA enrichment analysis of apoptosis-related pathways linked to XIAP expression. (H) ROC curve analysis evaluating the prognostic impact of XIAP in thyroid cancer. (*p < 0.05, **p < 0.01, ***p < 0.001).
Bioinformatic Analysis of XIAP and Caspases in Thyroid Cancer. (A) Protein-protein interaction network focusing on XIAP and caspase family proteins. (B) Heatmap showing the expression levels of XIAP and caspase family proteins in thyroid cancer. (C) Scatter plot illustrating the positive correlation between XIAP expression and CASP3, CASP8, and CASP9. (D) Differential expression of CASP3, CASP8, and CASP9 in thyroid cancer versus normal tissues. (*p < 0.05, **p < 0.01, ***p < 0.001).
Radiosensitization Effects of ANTP-SmacN7 and Gamma Ray on TPC-1 Cells. (A) OD values measured by CCK8 assay following gamma-ray at different doses. (B) CCK8 assay results across different treatment groups at the same radiation dose. (C) Representative images and statistical graphs of colony formation in TPC-1 cells following different treatments. (D) Representative images and quantitative analysis of TPC-1 cell migration via Transwell assay across different treatment groups. (E) Flow cytometric images and apoptosis graphs of TPC-1 cells in each experimental group. NC indicates the control group.(Error bars represent SD (n = 3). Statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001.).
Western Blot Analysis of XIAP and Apoptosis-Related Protein Expression in TPC-1 Cells Across Experimental Groups. (A) Representative Western blot bands showing XIAP and CASP-related proteins, including their active forms. (B) Quantitative analysis of CASP3 protein expression across different experimental groups. (C) Quantitative analysis of CASP8 protein expression across different experimental groups. (D) Quantitative analysis of CASP9 protein expression across different experimental groups. (Error bars represent SD (n = 3). Statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001.).
Impact of XIAP overexpression on the effects of ANTP-SmacN7 and gamma ray in TPC−1 cells. (A) Expression levels of XIAP in TPC−1 cells and XIAP-overexpressing (XIAP-OE) TPC−1 cells. (B) q-PCR validation of XIAP expression in XIAP-OE TPC−1 cells. (C) Cell viability in TPC−1 cells with varying XIAP expression levels under treatment with ANTP-SmacN7 and gamma ray. (D) Comparative analysis of cell proliferation between different treatment groups, as assessed by colony formation assays. (E) Comparative analysis of cell migration between different treatment groups, as determined by Transwell assays. (F) The role of XIAP-OE and the effect of ANTP-SmacN7 on apoptosis in TPC−1 cells across experimental groups.(Error bars represent SD (n = 3). Statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001.).
Impact of ANTP-SmacN7 and Gamma Ray on CASP-Related Protein Expression in XIAP-Overexpressing TPC−1 Cells. (A) Representative Western blot bands showing the expression of CASP-related proteins and their active forms in XIAP-OE TPC−1 cells following different treatments. (B) Quantitative analysis of CASP3 protein expression across different experimental groups. (C) Quantitative analysis of CASP8 protein expression across different experimental groups. (D) Quantitative analysis of CASP9 protein expression across different experimental groups. (Error bars represent SD (n = 3). Statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001.).
Data availability
Data is provided within the manuscript or supplementary information files.
References
Miranda-Filho, A. et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol. 9, 225–234 (2021).
Durante, C. et al. 2023 European thyroid association clinical practice guidelines for thyroid nodule management. Eur Thyroid J 12(5), e230067 (2023).
Voelker, R. What Is Thyroid Cancer? JAMA (2024).
Filetti, S. et al. ESMO clinical practice guideline update on the use of systemic therapy in advanced thyroid cancer. Ann. Oncol. 33, 674–684 (2022).
Chen, D. W., Lang, B. H. H., McLeod, D. S. A., Newbold, K. & Haymart, M. R. Thyroid cancer. Lancet 401, 1531–1544 (2023).
Gild, M. L., Bullock, M., Robinson, B. G. & Clifton-Bligh, R. Multikinase inhibitors: a new option for the treatment of thyroid cancer. Nat. Rev. Endocrinol. 7, 617–624 (2011).
Boucai, L., Zafereo, M. & Cabanillas, M. E. Thyroid Cancer: Rev. JAMA 331, 425–435 (2024).
Ryu, H. J. et al. Postoperative radiotherapy in the management of advanced or recurrent differentiated thyroid Cancer. Int. J. Radiation Oncology*Biology*Physics. 111, e419 (2021).
Piscopo, L., Zampella, E. & Klain, M. New opportunities for dosimetric approach in patients with differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging. 51, 330–331 (2024).
Hanifeh, M. & Ataei, F. XIAP as a multifaceted molecule in cellular signaling. Apoptosis 27, 441–453 (2022).
Gao, T. et al. Targeting inhibitor of apoptosis proteins (IAPs) enhances susceptibility of oral squamous carcinoma cells to cisplatin. Exp. Cell. Res. 437, 113995 (2024).
Yim, J. H. et al. Expression of X-linked inhibitor of apoptosis protein in neoplastic thyroid disorder. J. Korean Med. Sci. 26, 1191–1195 (2011).
Cheng, Q., Zhang, X., Xu, X. & Lu, X. MiR-618 inhibits anaplastic thyroid cancer by repressing XIAP in one ATC cell line. Ann. Endocrinol. 75, 187–193 (2014).
Kim, S. K. et al. Missense polymorphisms in XIAP-associated factor-1 (XAF1) and risk of papillary thyroid cancer: correlation with clinicopathological features. Anticancer Res. 33, 2205–2210 (2013).
Parvathareddy, S. K. et al. X-linked inhibitor of apoptosis protein (XIAP) predicts disease-free survival in BRAFV600E mutant papillary thyroid carcinoma in middle Eastern patients. Front. Endocrinol. (Lausanne). 13, 1054882 (2022).
Werner, T. A. et al. Survivin and XIAP - two potential biological targets in follicular thyroid carcinoma. Sci. Rep. 7, 11383 (2017).
Chu, S. et al. MON-LB77 inhibitors of XIAP as novel therapeutic agents in thyroid Cancer. J. Endocr. Soc. 4, MON–LB77 (2020).
Santhanam, M. et al. Interaction of SMAC with a survivin-derived peptide alters essential cancer hallmarks: tumor growth, inflammation, and immunosuppression. Mol. Ther. 32, 1934–1955 (2024).
Shoshan-Barmatz, V., Arif, T. & Shteinfer-Kuzmine, A. Apoptotic proteins with non-apoptotic activity: expression and function in cancer. Apoptosis: Int. J. Program. Cell. Death. 28, 730–753 (2023).
Yang, L. et al. Predominant suppression of apoptosome by inhibitor of apoptosis protein in non-small cell lung cancer H460 cells: therapeutic effect of a novel polyarginine-conjugated Smac peptide. Cancer Res. 63, 831–837 (2003).
Khalily, M. P., Gerekçi, S., Güleç, E. A., Özen, C. & Özçubukçu, S. Structure-based design, synthesis and anticancer effect of Cyclic Smac–polyarginine peptides. Amino Acids. 50, 1607–1616 (2018).
Boddu, P., Carter, B. Z., Verstovsek, S. & Pemmaraju N. SMAC mimetics as potential cancer therapeutics in myeloid malignancies. Br. J. Haematol. 185, 219–231 (2019).
Fulda, S. Promises and challenges of Smac mimetics as Cancer therapeutics. Clin. Cancer Res. 21, 5030–5036 (2015).
Xie, Y. et al. ANTP-SMACN7 fusion peptide alone induced high linear energy transfer irradiation radiosensitization in non-small cell lung cancer cell lines. Cancer Biol. Med. 19, 983–994 (2021).
Derossi, D., Joliot, A. H., Chassaing, G. & Prochiantz, A. The third helix of the antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 269, 10444–10450 (1994).
Derossi, D. et al. Cell internalization of the third helix of the antennapedia homeodomain is receptor-independent. J. Biol. Chem. 271, 18188–18193 (1996).
Peng, X. et al. Molecular design, construction and analgesic mechanism insights into the novel transdermal fusion peptide ANTP-BgNPB. Bioorg. Chem. 148, 107482 (2024).
Kravchenko, S. V. et al. Enhancing the Antimicrobial Properties of Peptides through Cell-Penetrating Peptide Conjugation: A Comprehensive Assessment. Int. J. Mol. Sci., 24(23), 16723 (2023).
Du, L. Q. et al. Radiation-sensitising effects of antennapedia proteins (ANTP)-SmacN7 on tumour cells. Int. J. Mol. Sci. 14, 24087–24096 (2013).
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44, D457–D462 (2016).
Zou, R., Zhao, W., Xiao, S. & Lu, Y. A signature of three Apoptosis-Related genes predicts overall survival in breast Cancer. Front Surg 9, 863035 (2022).
Jing, R. et al. Efficacy and safety of multikinase inhibitors for patients with refractory thyroid cancer: systematic review and network meta-analysis. J Clin. Endocrinol. Metab 109(10), 2658–2672 (2024).
Yang, Z., Flores, J., Katz, S., Nathan, C. A. & Mehta, V. Comparison of survival outcomes following postsurgical radioactive iodine versus external beam radiation in stage IV differentiated thyroid carcinoma. Thyroid 27, 944–952 (2017).
Kawamoto, T., Shikama, N., Fukumori, T., Hoshi, M. & Yamada, T. Propensity score matching analysis of adjuvant external-beam radiotherapy for the treatment of papillary thyroid carcinoma with other organ invasions. Endocrine 80, 589–599 (2023).
Adilbay, D. et al. Well-Differentiated thyroid cancer: who should get postoperative radiation?? Ann. Surg. Oncol. 29, 5582–5590 (2022).
Beckham, T. H. et al. Intensity-Modulated radiation therapy with or without concurrent chemotherapy in nonanaplastic thyroid Cancer with unresectable or gross residual disease. Thyroid 28, 1180–1189 (2018).
Romesser, P. B. et al. Intensity-modulated radiation therapy and doxorubicin in thyroid cancer: A prospective phase 2 trial. Cancer 127, 4161–4170 (2021).
Tam, S. et al. Adjuvant external beam radiotherapy in locally advanced differentiated thyroid Cancer. JAMA Otolaryngol. Head Neck Surg. 143, 1244–1251 (2017).
Ciccone, L. P. et al. Charged particle radiotherapy for thyroid cancer. A systematic review. Crit Rev. Oncol. Hematol 202, 104463 (2024).
Wu, Y., Song, Y., Wang, R. & Wang, T. Molecular mechanisms of tumor resistance to radiotherapy. Mol. Cancer. 22, 96 (2023).
Duckett, C. S. IAP proteins: sticking it to Smac. Biochem. J. 385, e1–e2 (2005).
Kerr, J. F., Winterford, C. M. & Harmon, B. V. Apoptosis. Its significance in cancer and cancer therapy. Cancer 73, 2013–2026 (1994).
Fulda, S. & Vucic, D. Targeting IAP proteins for therapeutic intervention in cancer. Nat. Rev. Drug Discov. 11, 109–124 (2012).
Ndubaku, C., Cohen, F., Varfolomeev, E. & Vucic, D. Targeting inhibitor of apoptosis proteins for therapeutic intervention. Future Med. Chem. 1, 1509–1525 (2009).
Jost, P. J. & Vucic, D. Regulation of cell death and immunity by XIAP. Cold Spring Harb Perspect Biol 12(8), a036426 (2020).
Tu, H. & Costa, M. XIAP’s Profile in Human Cancer. Biomolecules 10(11), 1493 (2020).
Pagliari, L. J., Pinkoski, M. J. & Green, D. R. in In Handbook of Cell Signaling. Second edn, 2535–2543 (eds Edition, R. A., Bradshaw & Dennis, E. A.) (Academic, 2010).
Kumar, S., Dorstyn, L. & Lim, Y. The role of caspases as executioners of apoptosis. Biochem. Soc. Trans. 50, 33–45 (2022).
Carneiro, B. A. & El-Deiry, W. S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 17, 395–417 (2020).
Fritsch, M. et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575, 683–687 (2019).
Sahoo, G., Samal, D., Khandayataray, P. & Murthy, M. K. A review on caspases: key regulators of biological activities and apoptosis. Mol. Neurobiol. 60, 5805–5837 (2023).
Acknowledgements
The authors greatly thank Ying Wang for their support.
Funding
This work was partially supported by grants from the Applied Basic Research Plan of Shanxi Province (Grant No.202103021224366). Scientific research project of Shanxi Provincial Health Commission (Grant No.2021147). Fundamental Research Program of Shanxi Province (Grant No.202203021221237).
Author information
Authors and Affiliations
Contributions
Yuhao Zhang, Qiang Liu, Shuxin Wen: conceived and designed the analysis (equal); Li Zhang, Kaixue Wen, Chen Wang: collected the data (equal); Peixia Yu, Zhilin Li, Kaixue Wen: contributed data or analysis tools (equal); Wang Chen, Zhang Yuhao: performed the analysis(equal); Wang Chen, Zhang Yuhao, Li Zhang: writing – review and editing (equal).
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Ethical approval
was granted by the Ethics Committee of Shanxi Betune Hospital.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, C., Zhang, L., Wen, K. et al. ANTP-SmacN7 enhances radiosensitivity in TPC-1 cells through XIAP-mediated activation of apoptotic protein. Sci Rep 15, 25779 (2025). https://doi.org/10.1038/s41598-025-11131-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11131-6