Abstract
Purpose In children with Autism Spectrum Disorder (ASD), motor disorders (MD) are a common occurrence, and developing effective interventions continues to be challenging. This study aims to investigate the effects of a 12-week ball combination training program (BCTP) combined with continuous theta burst stimulation (cTBS) on MD in children with ASD. Methods: The study employed a 4 (cTBS, BCTP, cTBS*BCTP, control group) × 2 (pre-test, post-test) mixed design methodology. 50 participants were allocated to three experimental groups(38) and one control group(12), receiving interventions for a period of 12 weeks. MD were measured using the Movement Assessment Battery for Children - Second Edition (MABC-2). Results: Results indicated that the cTBS*BCTP group significantly improved the overall MABC-2 scores (P < 0.05) and Manual dexterity scores (P < 0.05) in children with ASD, and the BCTP group also significantly improved overall MABC-2 scores. Compared to the control group, the effect size for the cTBS*BCTP group was 1.03 (95% CI: 0.13 to 1.94), showing the best intervention effect, outperforming the BCTP group (effect size: 0.82, 95% CI: -0.09 to 1.72) and the cTBS group (effect size: 0.43, 95% CI: -0.38 to 1.23). Conclusion: Overall, the BCTP*cTBS group showed the most significant intervention effects across various dimensions, demonstrating the efficacy of combined interventions in improving MD in children with ASD, confirming that combined interventions are superior to single interventions.
Similar content being viewed by others
Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder that typically appears in early childhood and lasts throughout life1,2. The core symptoms of ASD include difficulties in social communication, restricted or repetitive behaviors, and a narrow range of interests or activities, which significantly limit or impair the individual’s daily functioning3,4. ASD characteristics differ among individuals, with severities ranging from mild to severe5. Additionally, Motor Disorders (MD) are also a significant behavioral characteristic in children with ASD. MD, a behavioral abnormality, typically involves impaired muscle control and coordination, resulting in reduced or abnormal motor abilities. These disorders affect various movement aspects, such as Manual dexteritys, coordination of large muscle groups, balance, and posture control6. The significance of MD in ASD should not be overlooked. Despite not being included in the ASD diagnostic criteria, the high prevalence of MD has attracted widespread attention7. MD were observed as a characteristic of developmental disorders as early as ASD was first described, with cases reporting abnormal gait and motor anomalies, suggesting that MD in children with ASD may precede the onset of atypical social behaviors and language impairments8. Researchers speculate that MD may be both a comorbid symptom of ASD and a key factor in its pathology’s development. Therefore, it is crucial to understand and manage MD effectively in children with ASD, this can enhances their quality of life and daily functioning.
Given the impact of MD, physical exercise interventions offer a cost-effective, easily implementable, and safe non-pharmacological treatment option. Previous research demonstrates that physical exercise positively affects MD in children with ASD9,10,11. In the development of movement interventions for ASD, ball sports interventions have increasingly attracted researchers’ interest due to their many positive effects. Mini basketball, as a flexible game with adaptable rules, has the potential to improve communication among peers and positively impact behavior and physical health12,13. A study on preschool children with ASD found that a 12-week mini basketball training program (MBTP) could enhance motor skills14. Furthermore, participating in other types of ball sports, such as soccer, can significantly improve physical health and motor skills for individuals with ASD, enhancing their enjoyment and engagement in sports15. It is worth noting that focusing on a single ball sport may differ in its impact on physical interventions for children with ASD. Basketball mainly targets upper body training, whereas soccer focuses on the lower body. Thus, A single ball sport varies in the focus of exercise intervention for children with ASD. Additionally, ball sports are entertaining and provide a variety of activities and rich scenarios, offering children with autism diverse stimuli and experiences. Thus, integrating basketball and soccer could lead to a comprehensive improvement in motor abilities for children with ASD. Other research suggests that comprehensive and integrated intervention programs have a positive effect on children with ASD7underlining the potential benefits of combined sports interventions. However, it remains to be determined whether a combined basketball and soccer intervention program (BCTP) effectively improves MD in children with ASD, necessitating further research.
In addition to physical exercise, transcranial magnetic stimulation (TMS) has become increasingly popular for rehabilitating children with ASD, showing positive outcomes16. Continuous Theta Burst Stimulation (cTBS) is a specific pattern that stimulates the cortex directly via magnetic and electric pulses17. This technique effectively modifies brain plasticity18. As a neuromodulation method, cTBS shows potential to enhance brain function and behavior in children with ASD19. The left dorsolateral prefrontal cortex (DLPFC), associated with speech and cognitive control, and the right DLPFC, linked to emotional regulation and social cognition, serve different functions in the brain. Consequently, some studies have started exploring how cTBS interventions in these areas might alleviate motor impairments in children with ASD. For example, researchers stimulated the left DLPFC of children with ASD using cTBS in one study and observed improvements in motor function20. Another study found that stimulating the right DLPFC enhanced both emotional and social cognition, as well as motor skills21. Although studies on the application of cTBS in children with ASD remain limited, broader research on neurostimulation across various populations provides valuable insights. such as depression22Alzheimer’s disease23and Parkinson’s disease24. In addition, repetitive TMS (rTMS) and tDCS have been found to modulate motor and cognitive functions in children with Attention Deficit Hyperactivity Disorder (ADHD) and developmental coordination disorder (DCD), showing improvements in motor planning, postural control, and executive functioning25,26. These findings support the rationale for exploring brain stimulation methods like cTBS to enhance motor control by modulating neuroplasticity in relevant brain regions.
The rationale for combining cTBS and ball skill training lies in their complementary mechanisms. cTBS modulates cortical excitability and neural connectivity, potentially priming the brain for improved motor learning. On the other hand, ball-based physical training (BCTP) provides task-specific sensorimotor input that reinforces functional motor pathways through repetitive, structured activity. Combining neuromodulation with behavioral training has been shown to produce synergistic effects in other populations. For example, paired neurostimulation with physiotherapy has enhanced gait and motor recovery in children with cerebral palsy and adults post-stroke. These findings suggest that simultaneous physiological (neural plasticity) and behavioral (motor practice) stimulation can yield greater benefits than either approach alone. In our study, we combined BCTP with cTBS interventions for the first time, utilizing the advantages of both interventions to maximize effects and explore their impact on MD in children with ASD. Initially, cTBS, as an intervention affecting neuronal activity in the brain, can regulate neural plasticity and improve brain functional connectivity. Meanwhile, BCTP improves motor disorders in children with ASD through extensive ball-sport training. By combining these two interventions, we can conduct a comprehensive and effective intervention on the motor impairments of children with ASD at both physiological and behavioral levels. However, further research is needed to fully understand the effects of combined BCTP and cTBS interventions on MD in children with ASD.
Children with ASD display a variety of symptoms and behaviors, and single interventions often fail to meet their complex needs. However, combined interventions can simultaneously target different neural and behavioral mechanisms, potentially having a more positive impact on the multifaceted issues of children with ASD. But research on combined interventions in ASD is limited, and evidence supporting their impact on MD is scarce. This study BCTP and cTBS combined program may yield synergistic effects on behavioral and physiological aspects, more effectively addressing MD in children with ASD. Therefore, we employed three intervention methods: BCTP intervention, cTBS intervention, and combined BCTP and cTBS interventions, and determine if combined intervention effectively improves MD in children with ASD.
In summary, MD serious impairs daily life quality for children with ASD, causing substantial family stress. This study aims to explore effective intervention strategies:
-
(1)
Explore the effectiveness of BCTP intervention on MD in children with ASD.
-
(2)
Explore the effectiveness of cTBS intervention on MD in children with ASD.
-
(3)
Explore the effectiveness of combined BCTP and cTBS intervention on MD in children with ASD.
-
(4)
Compare the outcomes of the three interventions on MD in children with ASD.
This study results can confirm the effects of three interventions on MD in children with ASD, and whether combined intervention is superior to single intervention, aiming to provide more effective interventions for children with ASD. By gaining a deeper understanding of these intervention strategies may enable more effective support for children with ASD.
Methods
Study design
This study is a 4*2 mixed experimental design, where the groups (cTBS, cTBS*BCTP, BCTP, CG) are between-subject factors, and testing times (pre-test, post-test) are within-subject factors. Participants were screened at the Yangzhou Chuying Children Development Center, with diagnoses made by professional doctors at the Yangzhou Maternal and Child Health Hospital. Meanwhile, we detailed the study’s purpose and specifics to the participants parents or guardians, who subsequently signed informed consent forms. Participants underwent assessments before and after a 12-week training intervention, which included scores from the Movement Assessment Battery for Children - Second Edition (MABC-2) and physical fitness evaluations. Ethical approval for the study was granted by the Ethics Committee of Yangzhou University School of Medicine, approval number YXYLL-2023-147. The trial is registered on the Chinese Clinical Trial Registry number ChiCTR2400087262 (23/07/2024). The study complies with the Helsinki Declaration’s ethical guidelines.
Participants
A priori sample size calculation was conducted to identify the recommended sample for the experiment. Given an effect size of 0.25,set the significance level (α) at 0.05, and considering four groups, statistical power of 0.80, the software G*power (version 3.1.9.6, Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany) determined an advisable participant size of 48 individuals.
Eligibility criteria for participants included: (i) Chinese Han children aged 3–10 diagnosed with ASD; (ii) those diagnosed with moderate to severe autism spectrum disorder per DSM-V criteria; (iii) candidates suitable for transcranial magnetic stimulation and physical therapy.Exclusion criteria included: (i) individuals with significant head trauma and metal objects in the skull; (ii) neurological and psychiatric disorders, such as epilepsy or tics; (iii) carriers of pacemakers, implantable defibrillators, nerve stimulators, and other internal devices; (iv) individuals taking medications affecting the central nervous system; (v) those who had briefly participated in ball sports training in the past 12 months; (vi) those with physical disabilities preventing participation in physical interventions; (vii) visual and auditory impairments; and (viii) those who had received transcranial magnetic stimulation in the past 12 months. After initial screening, 75 participants were found eligible and assigned to four groups: cTBS (18 participants), cTBS*BCTP (16 participants), BCTP (19 participants), and Control Group (22 participants). All participating children received uniform standard rehabilitation treatments at institutions, with no participation in additional physical interventions allowed during the study period. The control group received a series of rehabilitation sessions provided by the agency based on Applied Behavior Analysis (ABA) theory, including Early Intensive Behavioral Intervention (EIBI) and Discrete Trial Training (DTT), among others, with each type of intervention session being 45 min long and occurring five times per week. Meanwhile, the other groups underwent cTBS, cTBS*BCTP, and BCTP interventions over a 12-week period. Thirteen participants withdrew from the study due to personal or injury-related reasons. Ultimately, 62 participants completed the intervention, but the data from 12 participants assessments that were not completed were not included in the final statistical analysis. Finally, a total of 50 participants were included in the analysis across four groups(Fig. 1): cTBS (n = 15; 11 boys, 4 girls), cTBS × BCTP (n = 12; 7 boys, 5 girls), BCTP (n = 11; 9 boys, 2 girls), and the Control Group (n = 12; 11 boys, 1 girl), with a mean age of 7.02 ± 2.07 years (range: 6–10 years).
Methodological proceduresa
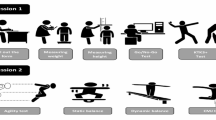
Participants were measured at the Yangzhou Chuying Children Development Center before and after the intervention, including anthropometric measurements, MABC-2 testing, and physical fitness tests, conducted in a room maintained at 24ºC and 55% relative humidity. Prior to the intervention, assessments included the Childhood Autism Rating Scale (CARS)27,28the Children’s Sleep Habits Questionnaire (CSHQ)29and the Children’s Eating Behaviour Questionnaire (CEBQ)30. A professional physician conducted the Childhood Autism Rating Scale (CARS) assessments, and experienced researchers carried out the other tests.
Movement Assessment Battery for Children (MABC-2) Tool Test
Currently, no specific motor ability assessment tools are designed exclusively for the individuals with ASD. However, several scales and tools have been successfully applied across typically developing and clinical populations, including individuals with ASD, demonstrating substantial applicability. The MABC-2 is a standardized diagnostic tool for Developmental Coordination Disorder that assesses manual dexterity, aiming & catching, and balance. It provides strict and clear demonstrations with detailed instructional requirements. Recognized for its broad applicability across age ranges31it is highly regarded for its practical utility in assessments. This tool is also effective in evaluating motor impairments in children with ASD32,33.
In this study, the MABC-2 tool was employed to assess motor skills in children with ASD. The MABC-2 testing encompasses three areas: (1) manual dexterity, including tasks like placing coins, threading beads, and drawing; (2) aiming & Catching, such as throwing and catching small bags; (3) balance, demonstrated through tiptoe walking, single-leg balancing, and carpet jumping. Formal tests were conducted by MABC-2 certified personnel, who followed stringent guidelines for tool arrangement and procedures. Children with ASD were encouraged to perform optimally throughout these tests. Examiners recorded test scores and completed subjective evaluations based on the children’s performance. Currently, numerous scholars widely employ the MABC tool to research motor abilities in children with autism.
Training intervention
The exercise intervention spanned 12 weeks, consisting of five 45-minute sessions each week. The exercise program progressively escalated in difficulty, beginning with simple exercises and advancing to more challenging workouts. The program was divided into three phases: adaptation, foundational, and advanced (Phases I, II, and III) (refer to Fig. 2). Appendix 1 offers a detailed breakdown of the training types during the intervention period.
This training frequency and intensity are consistent with the standard rehabilitation schedules currently implemented in local Chinese institutions and hospitals for children with developmental disorders. While the frequency may appear intensive compared to practices in some other countries, it reflects typical therapeutic routines in our regional context. Moreover, this mode of intervention has been previously validated in children with ASD and has demonstrated feasibility and effectiveness in real-world rehabilitation settings34.
Continuous Theta Burst Stimulation(cTBS) intervention
The therapeutic device employed was the Rapid2 Transcranial Magnetic Stimulation device from Magstim, UK, equipped with a figure-eight coil and set to the cTBS stimulation mode.A defining feature of the TBS mode is its delivery of three burst pulses every 200 milliseconds (5 Hz) at a 50 Hz frequency.The cTBS mode continuously delivers a total of 600 pulses35. Appendix 2 provides detailed information on the intensity, targets, and procedures of the cTBS intervention.
Individuals with autism exhibit an imbalance in the excitatory/inhibitory ratio within cortical layers, notably in the DLPFC36. Research indicates that low-frequency rTMS effectively reduces excessive gamma oscillatory brain activity in the frontal lobe, increases inhibitory neurotransmitters significantly, and restores cortical excitatory/inhibitory balance37. The modality-specific regulatory effects of TBS were first demonstrated in the motor cortex38 and later explored in the frontal cortex areas. In the motor area39continuous TBS (cTBS) mainly reduces excitability in targeted cortical neurons40whereas intermittent TBS (iTBS) enhances it. Consequently, given the functional differences in cortical responses between the left and right DLPFC, stimulation of each area is conducted over a six-week period37,41,42.
Two professionals administered the cTBS intervention in a one-on-one format. Given the clinical symptoms of autism that hinder children’s cooperation with cTBS stimulation, at least one guardian must be present during the sessions to facilitate the smooth completion of the intervention. Should an experimental session be paused, stimulation will resume following the conclusion of the next child session. Attendance for each session is recorded by a professional instructor, and any absences should be reported to the instructor at least one day in advance. Continuous absences should not exceed three days, and total absences should not surpass seven days to maintain the stimulation progress.
cTBS*BCTP Intervention
The specific intervention parameters of the BCTP and cTBS were consistent with those described in the previous section. To prevent the intensity of the cTBS stimulation treatment and the exercise intervention, the cTBS stimulation was scheduled to be implemented at 9:00–11:00 a.m. every Monday through Friday; the BCTP was scheduled to be implemented at 16:00–17:00 p.m. every Monday through Friday. The overall intervention process is shown in Fig. 3.
Statistical procedures
Variance and normality were assessed using Levene’s test and the Shapiro-Wilk test, respectively. If the data met the assumptions of normal distribution and variance homogeneity, One-Way ANOVA was employed for analysis; otherwise, non-parametric tests were applied. One-way ANOVA and chi-square tests assessed the homogeneity of demographic characteristics (age, gender, BMI), diagnostic reports (CARS, CSHQ, CEBQ), and the MABC-2, among 50 participants. Repeated measures ANOVA determined if significant changes in motor disorders occurred among four groups of children with ASD pre- and post-intervention, significant time-group interactions prompted further simple effects analysis. Data were presented as mean ± standard deviation (M ± SD). This study evaluated the importance of intervention outcomes by calculating effect sizes, defined as the difference between post-test and pre-test scores. Effect sizes were compared with the control group using Cohen’s d, calculated as the ratio of the mean difference to the pooled standard deviation, and categorized as small (0.2), medium (0.5), or large (0.8). Data analysis was conducted with SPSS version 27.0.1. Statistical analyses were two-tailed, with a significance threshold of 0.05.
Results
Baseline
To maintain the integrity of the research, we testing potential influencers of MD in children with ASD: demographic characteristics (age, gender, BMI), diagnostic reports (CARS, CSHQ, CEBQ), and the MABC-2, prior to starting the experiment. Consequently, controlling these factors and conducting differential testing on all participants were deemed essential throughout the study to safeguard the experimental results.
The chi-square test results show no significant differences in gender among the four groups of participants (χ2 = 3.931, p > 0.05). Similarly, the ANOVA for age [F(3,46) = 2.326, p > 0.05], BMI [F(3,46) = 2.591, p > 0.05], CARS [F(3,46) = 0.266, p > 0.05], CSHQ [F(3,46) = 1.892, p > 0.05], CEBQ [F(3,46) = 0.956, p > 0.05], manual dexterity [F(3,46) = 1.068, p > 0.05], aiming & catching [F(3,46) = 0.898, p > 0.05], balance [F(3,46) = 2.078, p > 0.05], and MABC-2 total points [F(3,46) = 1.024, p > 0.05] also showed no significant differences. These findings demonstrate the homogeneity in demographic characteristics, diagnostic reports, and MABC-2 scores across the four groups. Descriptive statistics can be found in Table 1.
Between and within-group analysis
We performed a repeated measures ANOVA to assess motor function across four participant groups. The results indicated that the differences in Aiming & Catching scores [F(3,46) = 12.607, p > 0.05, ηp2 = 0.090] and Balance scores [F(3,46) = 13.039, p > 0.05, ηp2 = 0.036] assessed by the MABC-2 tool were not statistically significant in the time×group interaction. The differences in Manual dexterity scores [F(3,46) = 2.935, p < 0.05, ηp2 = 0.176] and Total scores [F(3,46) = 1.024, p < 0.05, ηp2 = 0.207] were statistically significant in the time-by-group interaction. Subsequent simple effects analysis indicated a significant uptrend in Manual dexterity scores for the cTBS*BCTP group (p < 0.05), with no significant changes observed in the cTBS, BCTP, and control groups (p > 0.05). Regarding Total scores, significant increases were noted in the cTBS*BCTP and BCTP groups (p < 0.05), however, the cTBS and control groups showed no significant changes(p > 0.05). Descriptive statistics of the participants motor disorder performance scores before and after the experiment are shown in Table 2, and Fig. 4 illustrates the differences between pre- and post-tests across the groups.
MABC−2: Movement Assessment Battery for Children Second edition.
Effect sizes compared between the three experimental groups and the control group
Relative to the control group, the effect sizes were 0.43 (95% CI: −0.38 to 1.23) for the cTBS group, 1.03 (95% CI: 0.13 to 1.94) for the cTBS*BCTP group, and 0.82 (95% CI: −0.09 to 1.72) for the BCTP group on the MABC-2 total score.This indicates that the intervention effectiveness relative to the control group is greatest in the cTBS*BCTP group, followed by the BCTP group, and then the cTBS group.
In the MABC-2 sub-dimension for Manual dexterity, effect sizes were as follows: 0.64 (95% CI: −0.18 to 1.46) for the cTBS group, 0.97 (95% CI: 0.07 to 1.86) for the cTBS*BCTP group, and 0.66 (95% CI: −0.24 to 1.55) for the BCTP group.This suggests that the intervention effects were most pronounced in the cTBS*BCTP group, relative to the control group, with both the cTBS and BCTP groups demonstrating a improvement trend.
For Aiming & Catching scores, the effect size was − 0.32 (95% CI: −1.12 to 0.48) for the cTBS group, 0.38 (95% CI: −0.47 to 1.24) for the cTBS*BCTP group, and 0.48 (95% CI: −0.40 to 1.37) for the BCTP group. This indicates that the cTBS group scored lower than the control group in Aiming & Catching, cTBS might have influenced neural circuits in a way that temporarily disrupted Aiming & Catching, particularly if the stimulation was not precisely aligned with the neural pathways that directly support Aiming & Catching. while the cTBS*BCTP and BCTP groups showed trends of improvement.
For Balance scores, effect sizes were 0.41 (95% CI: −0.39 to 1.22) for the cTBS group, 0.31 (95% CI: −0.54 to 1.17) for the cTBS*BCTP group, Both cTBS and BCTP may enhance balance through similar neural pathways, leading to overlapping benefits rather than additive effects, 0.51 (95% CI: −0.37 to 1.39) for the BCTP group. This indicates an improvement trend in the intervention outcomes across all three groups.
Overall, the intervention effects of the cTBS*BCTP group were the most significant across multiple dimensions, while the cTBS and BCTP groups also showed trends of improvement, albeit with some uncertainty. In general, while observing the effect sizes, we noted that some results had confidence intervals that crossed zero, suggesting these effects might not be statistically significant. This likely reflects the small sample size of the study and the uncertainty inherent in studying children with ASD, particularly those with high-functioning disorders, who may exhibit significant pre- and post-intervention differences. In this study, even though some effect sizes were not statistically significant, this does not affect the overall trend of the results. These findings still hold important implications for improving motor disorder in children with ASD in practical applications. The effect sizes for the three experimental groups compared to the control group are shown in Table 3.
Discussion
This study investigated the effects of BCTP combined with cTBS intervention on MD in children with ASD, identifying four key findings. Initially, after 12 weeks of combined BCTP and cTBS intervention, children with ASD demonstrated significant improvements in total MABC-2 scores and the manual dexterity subdimension. While improvements in other subdimensions were not statistically significant, a trend towards improvement was evident. Subsequently, with 12 weeks of BCTP intervention alone, children with ASD also showed significant improvements in total MABC-2 scores, and while other subdimensions did not show statistical significance, improvements were consistently observed. Following the 12-week cTBS intervention, there were no significant improvements in the total MABC-2 scores or subdimensions for children with ASD. In a comparison of three intervention schemes and a control group, the BCTP*cTBS group demonstrated the most significant effects across multiple dimensions, achieving the best overall outcomes.
Effectiveness of BCTP on MD (Objective 1)
In our study, BCTP intervention alone for 12 weeks significantly improved total MABC-2 scores, with visible, though statistically non-significant, trends in other subdimensions. These findings suggest that BCTP alone can effectively enhance motor skills in children with ASD. The structure and progression of the BCTP program, including its focus on foundational ball skills, likely contributed to neuromuscular improvements and motor planning. Substantial evidence from previous research aligns with our findings that physical interventions positively affect motor functions in children with ASD43. For instance, an 8-week study involving daily four-hour sessions of exercises intervention, while this intervention targeted larger muscle groups than ours, it included similar activities such as balance beams and one-foot stands, the motor functions of these children improved significantly, with notable gains in fine object control and gross motor skills44. Research shows that various physical interventions impact motor function improvements in children with ASD differently. Unlike previous findings, in one study, 18 children with ASD were divided into experimental and control groups. The experimental group engaged in a 6-week Tai Chi program, meeting three times weekly for 60 min. This regimen led to significant improvements in balance and motor coordination in these children45. Another study involved 10 children with ASD in an 8-week structured exercise program, with three 60-minute sessions weekly, resulting in significant enhancements in their gross motor skills46. This study did not find significant improvements in Manual dexterity, Aiming & Catching, or Balance in children with ASD, although improvements were apparent. Our intervention sessions lasted 45 min, shorter than the 60 min or more common in other studies, potentially influencing our results. Moreover, our sample primarily included children with moderate to severe ASD, who often exhibited lower motor coordination and required assistance during physical interventions. To ensure successful participation, trained therapists and caregivers provided step-by-step guidance, verbal prompts, and hands-on assistance when needed. Additionally, the ball-training program was adapted to match the children’s abilities, using smaller groups and modified tasks to reduce coordination demands. This ensured that even children with severe ASD could engage in the intervention while gradually improving their motor skills. Overall, this study affirms the positive impact of physical intervention on motor functions in children with ASD and underscores the need for more targeted intervention strategies.
Effectiveness of cTBS on MD (Objective 2)
In the realm of neuromodulation, cTBS may modulate neuronal activity by regulating levels of neurotransmitters such as glutamate and γ-aminobutyric acid (GABA)47. This modulation of neuroplasticity in the brains of patients with ASD could enhance motor skills by normalizing brain connectivity and neuronal activity48. This study focused on the dorsolateral prefrontal cortex, a key brain region involved in motor execution49. However, this study revealed that a 12-week cTBS intervention did not significantly enhance overall motor functions or specific subdimensions in children with ASD. Although no significant improvements were found in Aiming & Catching and Balance dimensions, a trend toward improvement was observed. The effect size showed lower scores in Aiming & Catching compared to the control group, which might be due to the large individual variability in children with moderate to severe ASD7. Differences in ASD severity, cognitive abilities, language proficiency, and motor skills may have influenced their responses to the intervention, leading to variability in outcomes. Given the broad age range of 3–10 years, developmental differences across cognitive, language, and motor domains could further affect intervention effectiveness. While the sample size was sufficient, the inherent heterogeneity of ASD symptoms and responses to intervention may have played a role in these findings. Extending the intervention duration may be necessary to achieve significant effects across different developmental stages47.
Effectiveness of combined BCTP and cTBS intervention(Objective 3)
This study revealed that a 12-week combined intervention significantly improved overall motor disorder and manual dexterity scores in children with ASD. While the other two subdimensions did not show significant improvements, improvement trends were noted. The combined intervention demonstrated a greater efficacy than single interventions in improving motor disorders in children with ASD, providing partial support for our fourth hypothesis. The combined intervention may have synergistic effects, maximizing the therapeutic benefits of each component. The enhanced effects observed in the combined intervention group may be attributed to the complementary mechanisms of BCTP and cTBS. cTBS has been shown to regulate synaptic plasticity by modulating the balance between excitatory (glutamatergic) and inhibitory (GABAergic) neurotransmission, potentially normalizing aberrant neural connectivity in ASD48. This neurophysiological modulation may create a more receptive neural environment for motor learning. Meanwhile, BCTP directly engages motor pathways through structured and repetitive physical training, reinforcing neuromuscular control and motor coordination. The integration of neuromodulation and task-specific motor practice likely amplifies intervention efficacy, aligning with previous findings in neurorehabilitation.
While our findings suggest a synergistic effect of combining BCTP and cTBS, it is important to interpret these results with caution. The absence of a sham combined intervention group (i.e., sham neurostimulation + BCTP) limits our ability to fully isolate the specific contribution of cTBS beyond the psychological or motivational effects of receiving two concurrent interventions. Although we have acknowledged this as a limitation, future studies should incorporate a placebo-controlled combined group to better distinguish between the specific and additive effects of each component. Such a design would strengthen the causal inference regarding the efficacy of combined interventions and avoid potential overestimation of treatment benefits due to expectancy or increased attention effects.
Comparison of the three intervention outcomes (Objective 4)
Research supports this hypothesis, demonstrating that combining high-frequency repetitive transcranial magnetic stimulation with task-oriented mirror therapy significantly enhances hand function in acute stroke patients50. Similarly, transcranial magnetic stimulation combined with Action Observation (AO) has been shown to improve hand motor and upper limb functions51. Increasingly, studies highlight the effectiveness of multimodal interventions in ASD, with evidence that integrating exercise training and cognitive-behavioral therapy improves both motor and cognitive functions52. Furthermore, neuromodulatory approaches, such as transcranial direct current stimulation coupled with treadmill training, have been found to enhance balance and motor performance in children with cerebral palsy53. These findings underscore the potential of combining neuromodulation and physical training to optimize motor function improvements in children with ASD. This study found that combining BCTP with cTBS yielded the most significant improvements in motor development in children with ASD, surpassing the effects of either intervention alone. The cTBS intervention may help mitigate the limitations of BCTP by enhancing cortical excitability, while BCTP may compensate for the limited direct motor effects of cTBS by reinforcing motor pathway activation. Together, these internal (neuromodulatory) and external (task-based) stimulations may enhance the therapeutic impact on motor function development in children with ASD. These results support our hypothesis that combining BCTP and cTBS produces greater improvements in motor development than either intervention alone.
This study highlights the need for further exploration of personalized intervention strategies tailored to autism severity, optimization of target selection, and timing. Given the complexity of ASD, future research should investigate extended intervention durations and conduct long-term follow-up studies to assess the persistence and potential delayed effects of interventions54. Additionally, integrating functional neuroimaging tools such as fMRI or fNIRS could provide deeper insights into cTBS mechanisms. Moreover, employing biomarkers like BDNF level changes, along with robust methodologies such as randomization, double-blinding, and placebo controls, could enhance the reliability of findings. These aspects should be explored in future research and are not within the scope of the present study.
Conclusions
This study revealed the impact of cTBS*BCTP interventions on motor disorders in children with ASD. Practically, this research offers valuable insights for designing more effective intervention programs. We observed that combined interventions could produce synergistic effects, suggesting that integrating different intervention methods may maximize therapeutic outcomes. Additionally, our study underscores the need for personalized intervention plans due to varying motor disorders and individual differences in children with ASD. Future studies should extend intervention durations and conduct long-term follow-ups to assess the persistence of effects, explore synergies between methods, and identify optimal intervention combinations to improve motor disorder and quality of life in children with ASD.
Data availability
“The anonymized dataset used for analysis will be made available from the corresponding author upon reasonable request”.
References
Guha, M. Diagnostic and Statistical Manual of Mental Disorders: DSM-5 (5th edition). Reference Reviews, 28, 36–37 (2014).
Mughal, S., Faizy, R. M. & Saadabadi, A. Autism Spectrum Disorder. In StatPearls. StatPearls Publishing (2022).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th edn (American Psychiatric Association, 2013).
Lord, C., Elsabbagh, M., Baird, G. & Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 392 (10146), 508–520. https://doi.org/10.1016/S0140-6736(18)31129-2 (2018).
Lai, M. C., Lombardo, M. V. & Baron-Cohen, S. Autism Lancet, 383(9920), 896–910 (2014).
Bhatia, K. P. & Marsden, C. D. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain: J. Neurol. 117 (Pt 4), 859–876. https://doi.org/10.1093/brain/117.4.859 (1994).
Kaur, M., Srinivasan, S. & Bhat, A. N. Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without autism spectrum disorder (ASD). Res. Dev. Disabil. 72, 79–95. https://doi.org/10.1016/j.ridd.2017.10.025 (2018).
Kanner, L. Autistic disturbances of affective contact. Acta Paedopsychiatr. 35 (4), 100–136 (1968).
Mortimer, R., Privopoulos, M. & Kumar, S. The effectiveness of hydrotherapy in the treatment of social and behavioral aspects of children with autism spectrum disorders: A systematic review. J. Multidisciplinary Healthc. 7, 93–104. https://doi.org/10.2147/JMDH.S55345 (2014).
Borgi, M. et al. Effectiveness of a standardized equine-assisted therapy program for children with autism spectrum disorder. J. Autism Dev. Disord. 46 (1), 1–9. https://doi.org/10.1007/s10803-015-2530-6 (2016).
Ansari, S., Hosseinkhanzadeh, A. A., AdibSaber, F., Shojaei, M. & Daneshfar, A. The effects of aquatic versus kata techniques training on static and dynamic balance in children with autism spectrum disorder. J. Autism Dev. Disord. 51 (9), 3180–3186. https://doi.org/10.1007/s10803-020-04785-w (2021).
Zhou, D. et al. Decreased functional and structural connectivity is associated with core symptom improvement in children with autism spectrum disorder after mini-basketball training program. Journal of Autism and Developmental Disorders. Advance online publication. (2023). https://doi.org/10.1007/s10803-023-06160-x
Shao, Z., Bezmylov, M. M. & Shynkaruk, O. A. Individual characteristics of physical and mental development and their connection with regular physical exercises when playing basketball. Current Psychology (New Brunswick, N.J.), 1–10. Advance online publication. (2022). https://doi.org/10.1007/s12144-022-03692-w
Cai, K. L. et al. Mini-basketball training program improves physical fitness and social communication in preschool children with autism spectrum disorders. J. Hum. Kinetics. 73, 267–278. https://doi.org/10.2478/hukin-2020-0007 (2020).
Barak, S., Oz, M., Dagan, N. & Hutzler, Y. The game of life soccer program: effect on skills, physical fitness, and mobility in persons with intellectual disability and autism spectrum disorder. J. Appl. Res. Intellect. Disabilities: JARID. 32 (6), 1401–1411. https://doi.org/10.1111/jar.12620 (2019).
Ni, H. C. et al. Y. A lack of efficacy of continuous theta burst stimulation over the left dorsolateral prefrontal cortex in autism: A double-blind randomized sham-controlled trial. Autism Research: Official J. Int. Soc. Autism Res. 16 (6), 1247–1262. https://doi.org/10.1002/aur.2954 (2023).
Jannati, A. et al. Continuous theta-burst stimulation in children with high-functioning autism spectrum disorder and typically developing children. Front. Integr. Nuerosci. 14, 13. https://doi.org/10.3389/fnint.2020.00013 (2020).
Jannati, A. et al. Modulation of motor cortical excitability by continuous theta-burst stimulation in adults with autism spectrum disorder. Clin. Neurophysiology: Official J. Int. Federation Clin. Neurophysiol. 132 (7), 1647–1662. https://doi.org/10.1016/j.clinph.2021.03.021 (2021).
Sokhadze, E. M., El-Baz, A. S., Sears, L. L., Opris, I. & Casanova, M. F. rTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism. Front. Syst. Neurosci. 8, 134. https://doi.org/10.3389/fnsys.2014.00134 (2014).
Smith, A. B. & Jones, C. D. Effects of cTBS stimulation on motor function in children with autism spectrum disorder. J. Autism Dev. Disord. 49 (8), 3191–3200 (2019).
Brown, E. F. & White, L. K. Improvement of motor skills in children with autism spectrum disorder following cTBS stimulation of the right DLPFC. J. Autism Dev. Disord. 50 (6), 2201–2210 (2020).
Kochanowski, B., Kageki-Bonnert, K., Pinkerton, E. A., Dougherty, D. D. & Chou, T. A review of transcranial magnetic stimulation and transcranial direct current stimulation combined with medication and psychotherapy for depression. Harv. Rev. Psychiatry. 32 (3), 77–95. https://doi.org/10.1097/HRP.0000000000000396 (2024).
Brem, A. K. et al. Corticomotor plasticity predicts clinical efficacy of combined neuromodulation and cognitive training in alzheimer’s disease. Front. Aging Neurosci. 12, 200. https://doi.org/10.3389/fnagi.2020.00200 (2020).
Jin, Z. H. et al. Intermittent theta-burst stimulation combined with physical therapy as an optimal rehabilitation in parkinson’s disease: study protocol for a randomised double-blind controlled trial. Trials 24 (1), 410. https://doi.org/10.1186/s13063-023-07425-7 (2023).
Westwood, S. J., Radua, J. & Rubia, K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J. Psychiatry Neurosci. 46 (1), E14–E33. https://doi.org/10.1503/jpn.190179 (2021).
Akremi, H. et al. Cerebellar transcranial direct current stimulation in children with developmental coordination disorder: a randomized, double-blind, sham-controlled pilot study. J. Autism Dev. Disord. 52 (7), 3202–3213. https://doi.org/10.1007/s10803-021-05202-6 (2022).
Sarabzadeh, M., Azari, B. B. & Helalizadeh, M. The effect of six weeks of Tai Chi Chuan training on the motor skills of children with autism spectrum disorder. J. Bodyw. Mov. Ther. 23 (2), 284–290. https://doi.org/10.1016/j.jbmt.2019.01.007 (2019).
Castaño, P. R. L. et al. Effects of physical exercise on gross motor skills in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. Advance online publication. (2023). https://doi.org/10.1007/s10803-023-06031-5
Turner, S., Upham, J. W. & Editorial Asthma in children and adults - What are the differences and what can they tell Us about asthma? Front. Pead. 8, 141. https://doi.org/10.3389/fped.2020.00141 (2020).
Blumberger, D. M. et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. Lancet (London England). 391 (10131), 1683–1692. https://doi.org/10.1016/S0140-6736(18)30295-2 (2018).
Pascual-Leone, A., Amedi, A., Fregni, F. & Merabet, L. B. The plastic human brain cortex. Annu. Rev. Neurosci. 28, 377–401. https://doi.org/10.1146/annurev.neuro.27.070203.144216 (2005).
Oberman, L. M., Enticott, P. G., Casanova, M. F., Rotenberg, A. & Pascual-Leone, A. Transcranial magnetic stimulation in autism spectrum disorder: challenges, promise, and roadmap for future research. Autism Res. 9 (2), 184–203. https://doi.org/10.1002/aur.1527 (2016).
Kim, J. & Yim, J. Effects of high-frequency repetitive transcranial magnetic stimulation combined with task-oriented mirror therapy training on hand rehabilitation of acute stroke patients. Med. Sci. Monitor: Int. Med. J. Experimental Clin. Res. 24, 743–750. https://doi.org/10.12659/msm.905636 (2018).
Xu, D. et al. Effects of ball combination exercise combined with cTBS intervention on sleep problems in children with autism. J. Autism Dev. Disord. https://doi.org/10.1007/s10803-024-06555-4 (2024). Advance online publication.
Noh, J. S., Lim, J. H., Choi, T. W., Jang, S. G. & Pyun, S. B. Effects and safety of combined rTMS and action observation for recovery of function in the upper extremities in stroke patients: A randomized controlled trial. Restor. Neurol. Neurosci. 37 (3), 219–230. https://doi.org/10.3233/RNN-180883 (2019).
Smith, A. L., Ault, M. J. & Parish-Morris, J. Combined motor and cognitive behavior therapy improves motor skills and cognition in children with autism spectrum disorder. J. Autism Dev. Disord. 1–14. https://doi.org/10.1007/s10803-022-05334-5 (2022).
Duarte, N., deA., Grecco, L. A., Galli, M., Fregni, F. & Oliveira, C. S. Effect of transcranial direct-current stimulation combined with treadmill training on balance and functional performance in children with cerebral palsy: A double-blind randomized controlled trial. PloS One. 9 (8), e105777. https://doi.org/10.1371/journal.pone.0105777 (2014).
Schopler, E., Reichler, R. J., DeVellis, R. F. & Daly, K. Toward objective classification of childhood autism: childhood autism rating scale (CARS). J. Autism Dev. Disord. 10 (1), 91–103. https://doi.org/10.1007/BF02408436 (1980).
Chu, J. H. et al. Comparison of diagnostic validity of two autism rating scales for suspected autism in a large Chinese sample. World J. Clin. Cases. 10 (4), 1206–1217. https://doi.org/10.12998/wjcc.v10.i4.1206 (2022).
Owens, J. A., Spirito, A. & McGuinn, M. The children’s sleep habits questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep 23 (8), 1043–1051 (2000).
Goodlin-Jones, B. L., Sitnick, S. L., Tang, K., Liu, J. & Anders, T. F. The children’s sleep habits questionnaire in toddlers and preschool children. J. Dev. Behav. Pediatrics: JDBP. 29 (2), 82–88. https://doi.org/10.1097/dbp.0b013e318163c39a (2008).
Henderson, S., Sugden, D. & Barnett, A. L. The Movement Assessment Battery for Children 2nd edn (The Psychological Corporation, 2007).
Landa, R. Early communication development and intervention for children with autism. Ment. Retard. Dev. Disabil. Res. Rev. 13 (1), 16–25. https://doi.org/10.1002/mrdd.20134 (2007).
Whyatt, C. P. & Craig, C. M. Motor skills in children aged 7–10 years diagnosed with autism spectrum disorder. J. Autism Dev. Disord. 42 (9), 1799–1809. https://doi.org/10.1007/s10803-011-1421-8 (2012).
Gutiérrez-Muto, A. M., Castilla, J., Freire, M., Oliviero, A. & Tornero, J. Theta burst stimulation: technical aspects about TMS devices. Brain Stimul. 13 (3), 562–564. https://doi.org/10.1016/j.brs.2020.01.002 (2020).
Chan, M. M. Y. et al. Effects of multisession cathodal transcranial direct current stimulation with cognitive training on sociocognitive functioning and brain dynamics in autism: A double-blind sham-controlled randomized EEG study. Brain Stimul. 16 (6), 1604–1616. https://doi.org/10.1016/j.brs.2023.10.012 (2023).
Abujadi, C., Croarkin, P. E., Bellini, B. B., Brentani, H. & Marcolin, M. A. Intermittent theta-burst transcranial magnetic stimulation for autism spectrum disorder: an open-label pilot study. Revista Brasileira De Psiquiatria (Sao Paulo Brazil: 1999). 40 (3), 309–311. https://doi.org/10.1590/1516-4446-2017-2279 (2018).
Huang, Y. Z., Edwards, M. J., Rounis, E., Bhatia, K. P. & Rothwell, J. C. Theta burst stimulation of the human motor cortex. Neuron 45 (2), 201–206 (2005).
Grossheinrich, N. et al. Theta burst stimulation of the prefrontal cortex: safety and impact on cognition, mood, and resting electroencephalogram. Biol. Psychiatry. 65 (9), 778–784 (2009).
Enticott, P. G. et al. Repetitive transcranial magnetic stimulation (rTMS) improves movement-related cortical potentials in autism spectrum disorders. Brain Stimul. 6 (4), 579–586 (2013).
Kang, J. N., Song, J. J., Casanova, M. F., Sokhadze, E. M. & Li, X. L. Effects of repetitive transcranial magnetic stimulation on children with low-function autism. CNS Neurosci. Ther. 25 (11), 1254–1261. https://doi.org/10.1111/cns.13150 (2019).
Jin, Y. R. et al. Efficacy of motor interventions on functional performance among preschool children with autism spectrum disorder: A pilot randomized controlled trial. Am. J. Occup. Therapy: Official Publication Am. Occup. Therapy Association. 77 (6), 7706205020. https://doi.org/10.5014/ajot.2023.050283 (2023).
Ketcheson, L., Hauck, J. & Ulrich, D. The effects of an early motor skill intervention on motor skills, levels of physical activity, and socialization in young children with autism spectrum disorder: A pilot study. Autism: Int. J. Res. Pract. 21 (4), 481–492. https://doi.org/10.1177/1362361316650611 (2017).
Wischnewski, M. & Schutter, D. J. Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimul. 8 (4), 685–692 (2015).
Acknowledgements
The authors would like to express their gratitude to all the children who participated in this study, teachers and parents of children for their support, and research assistants who helped with data collection and other contributions. In addition, special thanks to Professor Chen Aiguo of Nanjing Institute of Physical Education and Professor Marcin Białas the Gdansk University of Physical Education and Sport for their support.
Funding
This research was supported by grants from the National Natural Science Foundation of China (31771243), the National Social Science Foundation of China (23ATY008).
Author information
Authors and Affiliations
Contributions
Kai Qi, Zhiyuan Sun, Yifan Shi, Xuan Xiong, Yufei Liu, Kelong Cai, Aiguo Chen and Marcin Bialas conceived the study, participated in data collection and treatment and written, revised and approved the article. Marcin Bialas provided funding support. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Ethical approval for the study was granted by the Ethics Committee of Yangzhou University School of Medicine, approval number YXYLL-2023-147. The trial is registered on the Chinese Clinical Trial Registry number ChiCTR2400087262 (23/07/2024). Informed consent was obtained from the parents or guardians of all participants.
Consent to participate
Informed consent was obtained from the parents or guardians of all participants.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qi, K., Sun, Z., Shi, Y. et al. Effects of ball combination training program combined with cTBS intervention on motor disorder in children with autism spectrum disorder. Sci Rep 15, 26418 (2025). https://doi.org/10.1038/s41598-025-11540-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11540-7