Abstract
Leprosy is a chronic and neglected infectious disease caused by the bacilli Mycobacterium leprae and Mycobacterium lepromatosis, and is considered a public health problem. The genetic basis of the host is a determining factor in the development of the disease, highlighting genetic mutations in the MBL2 gene. The aim of this study was to investigate the associations of the rs1800450 polymorphism in the MBL2 gene in populations from Northeastern Brazil. A retrospective case-control genetic association study was conducted in the state of Alagoas, with replication in a population from Pernambuco/Bahia. The case group included patients diagnosed with leprosy, whereas the control group included healthy individuals. The participants’ DNA was extracted via the salting-out method and genotyped via real-time PCR. The allele and genotypic frequencies of the MBL2 (rs1800450) were obtained and subsequently compared between groups via logistic regression. In the population of Alagoas, 556 individuals were recruited, of which 292 were cases and 264 were healthy controls. A statistically significant association was observed between the SNP rs1800450 at MBL2 gene and protection against leprosy, for both the CT genotype (OR = 0.49, p = 0.001, CI = 0.32–0.75), for the T allele and for T carriers (p = 0.046, OR = 0.51, CI = 0.34–0.78; p = 0.002, OR = 0.51, CI = 0.34–0.78, respectively). However, no association was observed in the replication population. When combining the populations, it continued to show a significant association with protection against leprosy in the CT genotype (p = 0.004, OR = 0.66, CI = 0.51–0.87) and in T carriers (p = 0.009, OR = 0.70, CI = 0.54–0.91) even when adjusted by sex. The SNP rs1800450 in the MBL2 gene was associated with leprosy protection in a population from Northeastern Brazil.
Similar content being viewed by others
Background
Leprosy is a chronic and neglected infectious disease caused by the bacilli Mycobacterium leprae and Mycobacterium lepromatosis, and continues to be a serious public health problem, particularly in countries such as Brazil, India and Indonesia1,2. It is a slowly progressive disease that presents different clinical forms, depending on the level of cellular immune response to the bacteria. It primarily affects peripheral nerves and the skin but can also involve the mucosa of the upper respiratory tract, eyes, lymph nodes, testes and internal organs1,3. The World Health Organization (WHO) classifies leprosy clinically into multibacillary (MB) and paucibacillary (PB) forms on the basis of the number of skin lesions and nerve involvement1,3,4,5.
During the chronic infectious course, acute immune-mediated inflammatory episodes, known as leprosy reactions, can frequently occur, leading to physical disabilities and functional loss, especially when associated with late-diagnosed patients, male sex, and high bacillary loads3,6,7. Research suggests that the incidence of infection in the population is much greater than the incidence of clinical leprosy, as a small proportion of infected individuals develop clinical symptoms, whereas others may have subclinical infections that can persist or heal spontaneously. This discrepancy may be influenced by environmental and socioeconomic factors, such as nutrition and income, as well as bacterial genetic differences and host susceptibility. Therefore, investigating genetic markers related to this susceptibility is essential8.
The complement system is a vital component of the host immune system, and several genetic factors associated with it have been linked to leprosy, one of which is the MBL2, which encodes the MBL protein. MBL is a protein capable of recognizing high-mannose oligosaccharides and N-acetylglucosamine present in a wide variety of bacteria, viruses, fungi, and parasites. This protein plays an important role in protective immunity, as it is responsible for opsonization of pathogens, increased phagocytosis, and activation of the complement cascade via the lectin pathway8,9,10,11. Mutations in the MBL2 gene affect the ability of the MBL protein to perform its functions8,9,10,11.
In recent years, the MBL gene and its protein have been associated as important factors in the development of several diseases. Studies have shown that the presence of polymorphisms in the MBL2 gene predisposes individuals to infections and autoimmune diseases, in addition to increasing susceptibility to the development of bacterial diseases8,10,12,13. On the other hand, SNP haplotypes in the MBL2 gene have been associated with a lower predisposition to diseases, and may modulate clinical severity10,13. Among the diseases associated with this interaction is leprosy and, consequently, studies evaluating polymorphisms in the MBL2 gene have gained prominence8,10,12,13.
Despite the potential role of MBL2 in leprosy, the results of studies conducted in different regions of Brazil have been inconsistent. Some studies reported no associations, whereas others reported significant associations. In other countries, such as China and Colombia, most studies have reported associations indicating either increased risk or protection against leprosy and its operational classifications10,12. Thus, it is crucial to investigate the SNPs of exon 1 of the MBL2 gene in the population from Northeast of Brazil. This study aimed to investigate the rs1800450 polymorphism in the MBL2 gene and its genetic influence on the development of leprosy in the population of Northeastern Brazil. It is worth noting that this is a pioneering study in this region, which contributes to the understanding of the SNPs associated with leprosy.
Methods
Ethical aspects
The study from Alagoas population was submitted and approved by the Ethics Committee of the Federal University of Alagoas (UFAL) under protocol number 4,439,041 (CAAE: 57828716.0.0000.5013) and protocol number 068649/2016. The research included the population of Pernambuco/Bahia was authorized by the Research Ethics Committee of the Hospital das Clinicals of the Federal University of Pernambuco (HC/UFPE) (CAAE: 66179617.7.0000.5196).
Study population and sample collection
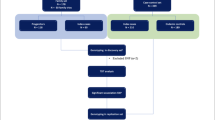
This is a retrospective case-control genetic association study conducted with two populations from Northeastern Brazil: a population sample from the state of Alagoas, in the municipalities of Maceió and Arapiraca, and a replication population from the states of Pernambuco and Bahia, in the municipalities of Petrolina and Juazeiro.
The case group comprised individuals with a confirmed diagnosis of leprosy. Patients with a degree of kinship, pregnant patients, and HIV carriers were excluded from the study. Recruitment were by convenience at reference centers for the diagnosis, monitoring and treatment of patients, and took place in the waiting room, where detailed information about the research was provided. Individuals who agreed to participate signed the Free and Informed Consent Form (FICF) and were referred for collection of biological material.
Subsequently, all patients were operationally classified according to World Health Organization (WHO) criteria, with cases with up to five skin lesions and negative bacilloscopic results being categorized as paucibacillary (PB), and cases with six or more lesions and/or positive bacilloscopic results as multibacillary (MB), thus ensuring the standardization of the clinical stratification of the study participants. This phase of the study was conducted from 2018 to 2023.
Recruitment and sample collection for the control group were carried out at HEMOAL (Alagoas Blood Donation Center) and in the replication population at HEMOBA (Bahia Blood Donation Center). Recruitment was performed in a non-probabilistic manner for convenience, with healthy individuals who voluntarily attended to donate blood, agreed to participate and signed the FICF. The collection took place between 2018 and 2019.
Prior to donating blood, these patients undergo clinical screening, hemoglobin test, and, if necessary, blood typing. In addition, investigation for hepatitis B and C, HIV, HTLV I and II, Chagas disease and syphilis were carried out through chemiluminescence, as determined by the National Health Surveillance Agency and the WHO. The collection took place between 2018 and 2019.
At all collection sites, the tubes were previously identified and the samples were stored properly (-20 °C). Sociodemographic information was also collected and organized in an Excel spreadsheet via Microsoft Office 2016.
DNA extraction and quantification
DNA extraction from case and control samples was performed via the PureLink™ Genomic DNA kit (Thermo Fisher Scientific, Carlsbad, CA, USA) for samples from Alagoas, and the ReliaPrep™ Blood gDNA Miniprep System (Promega, Madison, WI, USA) for samples from Pernambuco/Bahia. The quantity and quality of the extracted DNA were verified verified via spectrophotometry with a NanoDrop™ One (Thermo Fisher Scientific, Carlsbad, CA, USA), and the concentration/absorbency ratios at 280/260 nm and 260/230 nm were measured to assess DNA quality and purity. Extracted DNA samples were diluted to concentrations ranging from 10 to 50 ng/µL.
Genotyping of the MBL2 gene
Genotyping was conducted using the allelic discrimination method by quantitative real-time PCR on a QuantStudio 3 system (Applied Biosystems), employing TaqMan probes and following the manufacturer’s standard protocol (Applied Biosystems, Waltham, MA, USA). Reactions consisted of commercially obtained MBL2 rs1800450 assays (C:2336609_20 and location Chr.10:52771475 in build GRCh38) containing specific primers and probes for amplification of the target sequence [VIC/FAM]: TGGTTCCCCCTTTTCTCCCTTGGTG[C/T]CATCACGCCCATCTTTGCCTGGGAA (Thermo Fisher Scientific, Carlsbad, CA, USA). The reaction consisted of 2.5 µL of MasterMix, 1.375 µL of ultrapure H₂O, 0.125 µL of TaqMan assays of the rs1800450 SNP, and 1 µL of DNA. The cycling program was 10 min at 95 °C and 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The results were analyzed by the StepOne Plus/Applied Biosystems allelic discrimination software, which generated allelic discrimination plots to define the genotypes of each sample.
The SNP rs1800450 in the MBL2 gene, which is known to be involved in the immunopathology of leprosy and was previously associated with risk and protection against the disease in other populations, was investigated. This SNP was selected on the basis of previous genetic studies and functional analyses, as well as frequency criteria from the HapMap database (http://www.1000genomes.org/).
Statistical analysis
Hardy-Weinberg equilibrium (HWE) was assessed using the chi-square test (χ²). The genotyped SNP was compared regarding the frequency of genotypes, alleles, and T carriers between cases and controls. Analyses were initially performed in a homogeneous population from Alagoas, and replicated in an equally homogeneous population from Pernambuco and Bahia, and subsequently in the combined samples of the two populations.
Genetic association estimates were derived by calculating Odds Ratios (OR), p values of 5% and a 95% confidence interval (CI) were considered statistically significant. Sex was included as a covariate in the model to generate adjusted p values, ORs, and CIs. Analyses were performed by genetic logistic regression model through the R environment (The R Foundation for Statistical Computing) , version v.4.5.0, available at https://www.r-project.org/, using the “Genetics” and “SNPassoc” packages. The reliability of the sample power was assessed using the GPower software (v.3.1).
Results
Epidemiological characterization of cases and controls
A total of 556 individuals were recruited in the state of Alagoas, 292 of whom were in the case group and 264 healthy individuals in the control group (Table 1). The most prevalent age group in cases was 30–49 years (42%), and in the control group was 20 to 29 years (45%). Regarding sex, males predominated in both groups. The case group was analyzed according to the operational classification, where 86% of MB and 14% of PB were obtained. The SNP did not deviate from the HWE (χ² = 1.951, p = 0.163). When analyzing the replication population (Pernambuco/Bahia), where 699 individuals (335 cases and 334 controls) were included, they were mainly in the age range of 50 to 69 years (Table 1). The male sex was predominant in cases and controls in this population, as well as the operational classification MB with 93% of cases. HWE analysis was also performed, and no deviation was observed (χ² = 1.707, p = 0.163).
Genotypic and allelic frequencies of SNP rs1800450 in the MBL2 gene
When analyzing the rs1800450 polymorphism in the MBL2 gene, it was observed that this population was represented by the C allele, both in cases (91%) and in controls (86%), while the T allele presented a frequency of 9% in cases and 14% in controls, indicating that this allele is the polymorphic in the studied population (Table 2). Regarding the genotype, the CC was more frequent in both cases (84%) and controls (72%), suggesting a greater distribution of this genotype in the population of Alagoas.
After performing replication in the population of Pernambuco/Bahia, we observed similar results regarding the allele and genotype frequencies found in the population of Alagoas, with emphasis on the C allele in both cases (87%) and controls (86%). The frequency of the T allele was similar in this population, with the lowest proportion of 13% in cases and 14% in controls. Regarding the genotype, this also follows the same characteristics as the population of Alagoas, with CC being the most prevalent, both in cases (76%) and controls (73%), suggesting a greater distribution of this genotype also in the population of Pernambuco/Bahia. The allele and genotype frequencies of Alagoas and Pernambuco/Bahia for the SNP rs1800450 of the MBL2 gene are detailed in Table 2.
Genetic association of the SNP rs1800450 in the MBL2 gene with leprosy per se
In the genotypic association analysis comparing cases and controls in the Alagoas population, there were statistically significant associations for the CT genotype of the rs1800450 SNP with protection against leprosy when compared to healthy controls (p = 0.001, OR = 0.49, CI = 0.32–0.75) maintaining the association after adjusted analysis (p = 0.001, OR = 0.49, CI = 0.32–0.75) (Table 3).
When the alleles were analyzed, also was observed an association of T allele with leprosy protection when compared to healthy controls (p = 0.043, OR = 0.57, CI = 0.33–0.98), which remained significant after adjustment (p = 0.046, OR = 0.51, CI = 0.34–0.78). The frequencies of T carriers were also evaluated, continuing to show a protective effect of the SNP rs1800450 against leprosy in Alagoas, both before (p = 0.001, OR = 0.51, CI = 0.33–0.77) and after adjustment for sex (p = 0.002, OR = 0.51, CI = 0.34–0.78).
In the replication population from Pernambuco/Bahia, no statistically significant associations were detected for the CT genotype (p = 0.306, OR = 0.83, CI = 0.58–1.19), TT genotype (p = 0.244, OR = 0.32, CI = 0.84–1.19), T alleles nor for T allele carriers (p = 0.464, OR = 0.95, CI = 0.61–1.48; p = 0.809, OR = 0.95, CI = 0.61–1.48, respectively), with no difference after adjustment for sex. However, when combining the populations (Alagoas and Pernambuco/Bahia), a statistically significant association of leprosy protection was found in the presence of the CT genotype (p = 0.003, OR = 0.66, CI = 0.50–0.87). The association persisted after adjustment (p = 0.004, OR = 0.66, CI = 0.51–0.87). A protective association was also maintained for T carriers (p = 0.008, OR = 0.70, CI = 0.53–0.91), which remained after adjustment (p = 0.009, OR = 0.70, CI = 0.54–0.91).
The TT genotype in the combined population analysis showed no association with leprosy when compared to healthy controls (p = 0.303, OR = 1.76, CI = 0.60–5.20), even when adjusted for sex (p = 0.303, OR = 1.77, CI = 0.60–5.21). Furthermore, no associations were found for the T allele, both before (p = 0.129, OR = 0.77, CI = 0.55–1.08) or after adjustment (p = 0.138, OR = 0.77, CI = 0.55–1.09). The detailed genotypic and allelic associations for both the original and replicate populations are presented in Table 3.
Genetic association of SNP rs1800450 in the MBL2 gene according to MB status versus controls
No significant difference was observed when analyzing only MB patients versus controls in the Alagoas and replication population, as detailed in Table 4, maintaining the proportion of allele and genotype frequencies between MB patients and controls, as well as the association of protection against leprosy in the presence of the CT genotype (p = 0.001, OR = 0.45, CI = 0.28–0.71), the C allele (p = 0.039, OR = 0.55, CI = 0.31–0.97), and T carriers (p = 0.001, OR = 0.74, CI = 0.30–0.74), maintaining the associations after adjustments for sex. There is also an absence of statistically significant associations for the TT genotype in this comparison.
This analysis still maintains the statistical results of non-association with leprosy in the population of Pernambuco/Bahia in genotypes, alleles and T carriers, even after adjustment for sex. However, in the combined population, when analyzing MB patients with controls, a statistically significant association of the CT genotype with protection was found (p = 0.003, OR = 0.65, CI = 0.49–0.86), which remained significant after adjustment for sex (p = 0.003, OR = 0.65, CI = 0.49–0.86), as well as the T carriers, both before (p = 0.008, OR = 0.69, CI = 0.53–0.91) and after adjustment for sex (p = 0.001, OR = 0.69, CI = 0.53–0.91).
Discussion
One of the specific objectives of this study was to characterize the sociodemographic characteristics of the populations. When analyzing the profile of the group of cases, it was possible to observe a concentration in the age group of 30–49 years in Alagoas, and in the age group of 50–69 years in Pernambuco/Bahia. This trend is mainly linked to the late diagnosis of leprosy, since the disease has a long incubation period and can take years to present noticeable signs and symptoms, which reinforces the need for policies aimed at adults of economically active age. These findings reinforce patterns already identified in previous epidemiological studies and raise important reflections on the factors that influence the age distribution of the disease and its implications for public health14.
Regarding sex, both in cases and controls, males stood out. This may indicate changes in exposure patterns and in the behavior of seeking care between men and women, since men are more resistant and seek health services later, resulting in diagnoses with advanced disease (type MB), as seen in our research in both the population of Alagoas and Pernambuco/Bahia, as well as in several other studies1,10,14,15,16,17. In addition, socioeconomic barriers to access to health are observed, since male individuals may have financial priorities, as well as stigmas related to the disease14.
In addition to socioeconomic factors, immunology also explains why there is this difference between the sex and the prevalence of leprosy. According to studies, women have a more efficient immune response, both Th1 and Th2, than men, which normally depends on the stage of infection or the type of antigen found to mount an efficient immune response. Furthermore, studies suggest that women heal faster and more efficiently than men18. These findings corroborate other published studies conducted with leprosy patients in several regions of Brazil, such as Maranhão and Amazonas, as well as in other countries, such as Mauritania, in northwest Africa, and Oromia, in Ethiopia, showing that males have higher rates than females1,15,16,17.
In the control group, the populations studied showed a prevalence of males and an age range of up to 29 years in Alagoas, while the replication populations showed a higher prevalence, from 30 to 49 years. The predominance of males in the control group may be attributed to the exclusion criteria set by the blood centers involved in the research, which required donors to weigh more than 50 kg and to be free of anemia, potentially leading to lower female participation. Similar trends have been observed in studies conducted in the North and Northwest regions of Paraná and Southern Brazil10.
The progression of the clinical spectrum of leprosy is increasingly recognized as being influenced by genetic factors, particularly the host’s immune response to the bacillus M. leprae10. The MBL2 gene plays a crucial role in recognition and elimination of pathogens, thus significantly impacting the interaction between M. leprae and its human host. Polymorphisms present in exon 1 of MBL2 are known to modify the structure of the MBL protein, which may affect binding avidity, with a possible functional implication of the MBL protein and consequently of the MBL2 gene, which encodes it9,10,11.
Research suggests that the presence of below-normal levels of the MBL protein, associated with SNPs in the MBL2 gene, are responsible for the predisposition to infections and autoimmune diseases. On the other hand, SNPs in the MBL2 gene associated with high levels of MBL protein secretion may modulate the severity of the leprosy9,10,19. Studies that analyzed SNP haplotypes in the MBL2 gene have been related to a protective effect against pathogens that use complement-mediated opsonization to enter phagocytes, such as M. leprae. However, some studies have challenged this hypothesis and suggested that new studies be carried out to confirm these findings10,13. That said, studies that genetically analyze these polymorphisms have received attention recently.
The rs1800450 SNP at exon 1 in the MBL2 gene was the target of our study, which confirmed the hypothesis of an association between this polymorphism and leprosy in the population of Alagoas in the presence of the CT genotype and T carriers, this allele being considered polymorphic in this population, being related to protection against leprosy.
Another important finding of our study was the presence of a protective association when analyzing MB patients versus healthy controls in the presence of the CT genotype and T carriers in Alagoas, remaining a statistically significant association after adjusting for sex. This strengthens the hypothesis that the rs1800450 MBL2 (rs1800450) is associated with the MB operational classification, due to its indirect relationship with the Th2 immune response. These findings suggest that the presence of this SNP in the population of Northeastern Brazil may act as a protective factor, when compared to healthy controls in the development of leprosy and its aggravation. A study in the Nepal region obtained similar results, revealing that dominant genotypes of polymorphisms in the MBL2 gene were associated with protection against lepromatous leprosy, with statistically significant results persisting after adjustments for ethnicity, sex and age13.
An association with protection against leprosy of the CT genotype and T carriers was also found when analyzing the combined population (Alagoas/Pernambuco/Bahia). However, we did not observe association when performing the same analysis in the replication population alone. This observation is in line with a study conducted in Northwestern Brazil, who also reported no association between the MBL2 (rs1800450) and leprosy19.
Although many studies support the association of this SNP with protection against leprosy, some findings diverge. For example, a study conducted in southern Brazil showed an association between leprosy patients and susceptibility to progression to the MB form10. In a study conducted in Malawi, no association was found between the genotypes of the MBL2 polymorphism and susceptibility20. Such discrepancies highlight the variability in results between populations, probably reflecting differences in genetic origins and the weight of social, environmental and economic factors.
Among the limitations faced during the conduct of the present study, we mainly recognize the importance of including genetic ancestry variables in the SNP association analyses. Although the Brazilian population is known to be admixed, recent studies indicate that certain geographic regions, particularly in Northeastern Brazil, present a greater degree of homogeneity in terms of genetic ancestry, especially among geographically close populations such as the states of Alagoas, Pernambuco, and Bahia, as evidenced in the present study21,22,23. Nonetheless, the consistent findings between populations in these states reinforce the validity of our results.
Future studies will be important to further investigate potential variations in genetic backgrounds and MBL protein levels among these populations that may influence the observed associations, and we plan to address these aspects in forthcoming analyses. It is worth noting that there are few genetic association studies involving the MBL2 polymorphism (rs1800450) both in Brazil and internationally, which reinforces the relevance of the present research. This study represents not only a pioneering investigation in the Northeast region of Brazil, but also a significant contribution to the understanding of the role of genetic variants in the development of leprosy.
Conclusions
This study showed that the SNP rs1800450 in the MBL2 gene is associated with protection against leprosy in a population of Northeastern Brazil, suggesting that this genetic variant may lower the susceptibility of the sampled Northeastern Brazilian population to the disease. These findings contribute to the growing list of SNPs associated with leprosy, increasing the understanding of the genetic factors that influence the disease in Northeastern Brazil. These insights inform future research efforts in the region, particularly in identifying populations at risk for leprosy and its progression to more severe clinical forms. Such information may facilitate the development of targeted prophylactic measures aimed at preventing the onset or worsening of leprosy.
Data availability
The datasets used and analyzed, as well as the research data supporting the results of the manuscript are available from the corresponding author upon reasonable request.
Abbreviations
- MBL2:
-
Mannose-Binding Lectin 2
- DNA:
-
Deoxyribonucleic Acid
- PCR:
-
Polymerase Chain Reaction
- SNPs:
-
Single Nucleotide Polymorphisms
- WHO:
-
World Health Organization
- MB:
-
Multibacillary
- PB:
-
Paucibacillary
- PCT:
-
Polychemotherapy
- MBL:
-
Mannose-Binding Lectin
- UFAL:
-
Federal University of Alagoas
- HC:
-
Clinical Hospital
- UFPE:
-
Federal University of Pernambuco
- FICF:
-
Free and Informed Consent Form
- HIV:
-
Human Immunodeficiency Virus
- EDTA:
-
Ethylenediamine tetraacetic acid
- HEMOAL:
-
Alagoas Blood Center
- HEMOBA:
-
Bahia Blood Center
- OR:
-
Odds Ratio
- CI:
-
Confidence Interval
- HWE:
-
Hardy-Weinberg equilibrium
References
Moreira, R. J. et al. Clinical-epidemiological characteristics and temporal trend of new cases of grade 2 disability leprosy in the state of Maranhão, Brazil, 2011–2020. Epidemiology and Health Services [Internet]. Sep 18 [cited 2023 Nov 13], 32, e2022435 (2023). Available from: https://scielosp.org/article/ress/2023.v32n2/e2022435/en/. Accessed 20 Feb 2024.
BRAZIL. Leprosy Epidemiological Bulletin - Special Issue | Jan. 2024 — Ministry of Health [Internet]. www.gov.br. Available from: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/especiais/2024/be_hansen-2024_19jan_final.pdf/view. Accessed 29 Jan 2024.
BRAZIL. Clinical Protocol and Therapeutic Guidelines for Leprosy 2022 — Ministry of Health [Internet]. www.gov.br. Available from: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/h/hanseniase/publicacoes/protocolo-clinico-e-diretrizes-terapeuticas-da-hanseniase-2022/view. Accessed 29 Jan 2024.
de Oliveira, R. A. et al. Spatial distribution and trend of leprosy prevalence in a health region in the Northeast. 2008 [cited 2023 Oct 16]; Available from: https://www.scielo.br/j/ress/a/FGSpJvc7wjXx6FwBDL8HbNS/?format=pdf&lang=pt. Accessed 20 Feb 2024.
de Freitas, B. H. B. M., Silva, B., Jesus, F. & de Alencastro, J. M. F. Leprosy educational practices with adolescents: an integrative literature review. [cited 2021 Jul 22]; Available from: https://www.scielo.br/j/reben/a/48wvrkPD99XKKMprr3knq9L/?format=pdf&lang=pt Accessed 30 Jan 2024.
Mi, Z., Liu, H. & Zhang, F. Advances in the immunology and genetics of leprosy. Front. Immunol. 11, 567. https://doi.org/10.3389/fimmu.2020.00567 (2020). Accessed 29 Jan 2024.
Sugawara-Mikami, M. et al. Pathogenicity and virulence of Mycobacterium leprae. Virulência 13 (1), 1985–2011. https://doi.org/10.1080/21505594.2022.2141987Accessed 20 Feb 2024.
Kalia, N., Singh, J. & Kaur, M. The ambiguous role of mannose-binding lectin (MBL) in human immunity. Open Medicine [Internet]. Feb 17 [cited 2021 Oct 25];16(1):299–310. (2021). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7917369/. Accessed 29 Jan 2024.
de Messias-Reason, I. J. et al. The association between mannan-binding lectin gene polymorphism and clinical leprosy: new insight into an old paradigm. J. Infect. Dis. 196 (9), 1379–1385. https://doi.org/10.1086/521627 (2007). Accessed 05 05 Jun 2024.
Tiyo, B. T. et al. Association of MBL2 Exon 1 Polymorphisms With Multibacillary Leprosy. Frontiers in Immunology. ;11. (2020). https://doi.org/10.3389/fimmu.2020.01927. Accessed 29 Jan 2024.
Zhang, D. F., Huang, X. Q., Wang, D., Li, Y. Y. & Yao, Y. G. Genetic variants of complement genes Ficolin-2, Mannose-binding lectin and Complement factor H are associated with leprosy in Han Chinese from Southwest China. Human Genetics. 132(6), 629–640 (2013). Available from: https://link.springer.com/article/. https://doi.org/10.1007/s00439-013-1273-8. Accessed 29 Jan 2024.
Carmo, R. F. do, Neves, J. R. L., Oliveira, P. R. S., Vasconcelos, L. R. S & Souza, C. D. F. de. The role of Mannose-binding lectin in leprosy: A systematic review. Infect. Genet. Evol. 93, 104945 (2021). https://doi.org/10.1016/j.meegid.2021.104945. Accessed 05 Jun 2024.
Sapkota, B. R. et al. Association of TNF, MBL, and VDR polymorphisms with leprosy phenotypes. Hum. Immunol. 71 (10), 992–998 (2010). Accessed 29 Jan 2024.
Almeida, C. A. et al. Epidemiological profile of leprosy cases in the Central-West region from 2018 to 2022. Sci. Soc. J. 8 (1), 572–586. https://doi.org/10.61411/rsc31879 (2025). Accessed 29 Apr 2025.
Silva, M. N. S. da et al. Association between SNPs in MicroRNAs and MicroRNAs-Machinery genes with susceptibility of leprosy in the Amazon population. Int. J. Mol. Sci. 23 (18), 10628 (2022). https://doi.org/10.3390/ijms231810628. Accessed 05 Jun 2024.
Boushab, B. M. et al. Analyse des données nationales de surveillance de la lèpre en Mauritanie de 2009 à 2019 [Analysis of national leprosy surveillance data in Mauritania from 2009 to 2019]. Med. Trop. Sante Int. ;3(2). doi: https://doi.org/10.48327/mtsi.v3i2.2023.361. (2023). Accessed 05 Jun 2024.
Lema, T. et al. Reaching those at risk: active case detection of leprosy and contact tracing at kokosa, a hot spot district in Ethiopia. PLoS One. 18 (6), e0264100. https://doi.org/10.1371/journal.pone.0264100 (2023). Accessed 29 Jan 2024.
Maciel, L. B. Frequency of gender of leprosy patients in relation to bacilloscopy in municipalities of the State of Espírito Santo [dissertation]. Vitória: Federal University of Espírito Santo, Center of Health Sciences; Accessed 30 Apr 2025 (2013).
Vasconcelos, L. R. et al. Mannose-binding lectin serum levels in patients with leprosy are influenced by age and MBL2 genotypes. Int. J. Infect. Dis. 15(8), e551–e557 (2011). https://doi.org/10.1016/j.ijid.2011.04.008. Accessed 20 Feb 2024.
Wallace, C. et al. Linkage analysis of susceptibility to leprosy type using an IBD regression method. Genes Immun. 5 (3), 221–225 (2004). https://doi.org/10.1038/sj.gene.6364062. Accessed 29 Jan 2024.
Manta, F. S. N., Pereira, R. & Vianna, R. Revisiting the Genetic Ancestry of Brazilians Using Autosomal AIM-Indels. PLoS ONE. 8(9) (2015). https://doi.org/10.1371/journal.pone.0075145. Accessed 30 Apr 2025.
Pena, S. D. J., Santos, F. R. & Tarazona-Santos, E. Genetic admixture in Brazil. American Journal of Medical Genetics. nov 18;184(4):928 – 38. (2020). https://doi.org/10.1002/ajmg.c.31853. Accessed 30 Apr 2025.
Pena, S. D. J., Pietro, G. D. & Fuchshuber-Moraes, M. The Genomic Ancestry of Individuals from Different Geographical Regions of Brazil Is More Uniform Than Expected. PLoS ONE. 2011 feb 16; 6(2): e17063. https://doi.org/10.1371/journal.pone.0017063. Accessed 30 Apr 2025.
Funding
This project was supported by the Research Program for the SUS (PPSUS/Fapeal No. 06/2020), and funded by a CAPES/DS (Coordination for the Improvement of Higher Education Personnel) master’s scholarship, with Financial code 001. The RFC received a research productivity scholarship from the Pernambuco State Science and Technology Support Foundation (FACEPE).
Author information
Authors and Affiliations
Contributions
KRCN, HAF, and CSM contributed to all phases of project development in Alagoas, including recruitment, biological material collection, DNA extraction, dilution, quantification, genotyping, statistical analysis, data interpretation, and manuscript writing. MNVTP, ICN and JLRAS participated in recruitment, biological material collection, DNA extraction, dilution, quantification, and genotyping in Alagoas. ARRS contributed to the manuscript writing and interpretation of the results. RFC and ATPS contributed to recruitment, biological material collection, DNA extraction, dilution, quantification, and genotyping in Pernambuco and Bahia. EOB, JNSF and CACF provided expertise in data evaluation and contributed to the review and editing of the manuscript. All the authors reviewed, translated, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Federal University of Alagoas (UFAL) under opinion number 4,439,041 (CAAE: 57828716.0.0000.5013). Additionally, it received authorization from the Ethics and Research Committee of the Hospital das Clínicas of the Federal University of Pernambuco (HC/UFPE) (CAAE: 66179617.7.0000.5196). The research adhered to the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all participants via the signing of the Free and Informed Consent Form (FICF). Authorization for access to recruitment sites was also granted through the SEIs of the health departments of the states involved.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nogueira, K.R.C., de Almeida Freitas, H., da Paz, M.N.V.T. et al. Association between MBL2 gene polymorphism and protection against leprosy in a population of northeastern brazil: a case-control study. Sci Rep 15, 25578 (2025). https://doi.org/10.1038/s41598-025-11597-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11597-4