Abstract
Dopamine (DA) plays an essential role in regulating γ-aminobutyric acid (GABA) neurons in the brain. This study aimed to investigate the effects of DA receptor agonists on the expression of glutamic acid decarboxylase 67 (GAD67) in the context of 6-hydroxydopamine (6-OHDA)-induced dopaminergic neurodegeneration. To explore the potential involvement of DA receptor agonists in modulating GAD67 expression, these agonists were administered to primary cultured neurons and the substantia nigra in a mouse model with 6-OHDA-induced lesions. The GAD67 expression was subsequently assessed via Western blotting and immunohistochemistry analysis. Results revealed an increased GAD67 expression in in vitro and in vivo models induced by 6-OHDA. Interestingly, treatment with the D1-like receptor agonist SKF38393 led to a decrease in the GAD67 expression. Meanwhile, treatment with the D2-like receptor agonist quinpirole resulted in an increase in the GAD67 expression. Further, the inhibitory effect of SKF38393 on GAD67 was negated when the D1 receptor-selective antagonist LE300 was administered. Conversely, the expression of tyrosine hydroxylase was not affected by the DA receptor agonists SKF38393 or quinpirole. Subsequently, the association between heparanase-1 and the increased expression of GAD67 was examined. The GAD67 and heparanase-1 expression levels increased due to 6-OHDA treatment. However, they decreased when SKF38393 was administered. In contrast, treatment with the heparanase inhibitor suramin led to a reduction in the GAD67 expression. Therefore, the DA D1-like receptor agonist SKF38393 can modulate GAD67 expression under DA degenerative conditions by interacting with heparanase-1.
Similar content being viewed by others
Introduction
Dopamine, a neurotransmitter produced in DAergic neurons in the brain, generates feeling of pleasure and reward1,2. Further, DA is important in regulating memory, mood, sleep, learning, movement, and various bodily functions3,4,5,6. γ-Aminobutyric acid is an inhibitory neurotransmitter7 that plays a pivotal role in mood and cognitive function, similar to DA8,9. To fulfill its function, DA must bind to DA receptors on the surface of DAergic neurons and other non-DAergic neurons like GABAergic neurons, categorized into D1- and D2-like receptors. These receptor groups consist of D1, D5 subtype and D2, D3, and D4 subtype receptors, respectively10. Recent studies have demonstrated that DAergic activity can be regulated by diverse molecular factors such as O-GlcNAcylation, IGF-1, and BDNF, which may influence neuronal survival and synaptic plasticity. These findings highlight the complexity of DAergic modulation and support ongoing efforts to study DA receptor agonists in the context of neurodegeneration11,12. Notably, A-68930 is a selective D1 receptor agonist that can improve cognitive impairment and neuroinflammation. It is commonly associated with mouse Alzheimer’s disease model induced by β-amyloid. Further, it has been promising in mitigating mitochondrial dysfunction and cognitive deficits in streptozotocin-induced mice13,14. Similarly, ML417, a selective D3 receptor agonist, enhances β-arrestin translocation, G-protein activation, and ERK1/2 phosphrylation15. Hence, ongoing research is actively performed to investigate the functional role of DA using various substances and elucidate the regulatory mechanisms via its receptors.
Consequently, the degeneration of DAergic neurons in the substantia nigra (SN) can induce GABAergic neuron alterations. In adulthood, DAergic neurons are intricately intermingled with a substantial population of GABAergic neurons in the midbrain16. In terms of function, an interaction exists between DAergic and GABAergic neurons. Activation of DA D1-like receptors can control the firing rate of GABAergic neurons. DAergic neurons interact with GABAergic neurons and can affect the activity of DAergic neurons at their target sites. On the other hand, activating DA D1-like receptors also regulates the firing rate of GABAergic neurons16,17. D1 receptors are abundant in the nucleus accumbens, caudate–putamen, and olfactory bulb18,19,20. Notably, the substantia nigra pars reticulata (SNr) has a high concentration of D1 receptors21, primarily located on the terminals of GABAergic striatonigral neurons22. Research has shown that D1receptor agonists can elevate GAD65 and GAD67 mRNAs in striatonigral neurons while reducing GABA receptor mRNA levels in the SNr23. In addition, the expression of GABA receptors can be influenced by DA depletion24.
Heparan sulfate proteoglycans are proteins associated with the basement membrane, and they are distributed diffusely within the extracellular matrix25. These proteoglycans comprise linear heparan sulfate glycosaminoglycans. Further, they regulate various cellular functions including neuronal migration, angiogenesis, metastasis, inflammation, neurite outgrowth, and synaptogenesis26,27. The enzyme heparanase can cleave heparan sulfate within the central nervous system, thereby generating heparan sulfate fragment associated with processes such as neuronal cell proliferation and differentiation28. Notably, heparanase is also a hallmark of cancer, and it contributes to the characteristics of cancer cells by sustaining proliferative signaling, evading growth suppressors, resisting cell death, and enabling replicative immortality29. However, the specific impact of heparanase-1 on GABAergic neurons after the denervation of DAergic neurons remains largely unexplored. Given the role of heparanase-1 in modulating neural extracellular matrix and glial signaling, we hypothesized that it may also influence GABAergic neuronal identity in the context of dopaminergic denervation. While no direct link has been established, the convergence of extracellular remodeling, glial activation, and dopaminergic regulation of GAD67 expression provides a rationale for investigating this relationship.
Consequently, the current study aimed to elucidate the effects of DA receptor agonists on GAD67 expression after DA depletion. Moreover, the role of DA receptors associated with heparanase-1 in regulating GAD67 was examined.
Materials and methods
Animals
A colony of 10-week-old ICR mice was procured from Samtaco Bio Korea (South Korea) and maintained in specific pathogen-free conditions. The mice were housed in a room with a 12 h light and dark cycle. For mating purposes, a female mouse was introduced into a cage with a male for 10–12 h in the evening. Subsequently, the female mice were examined for the presence of vaginal plugs at 7:00 AM on the following day. Embryonic day 0 (E0) was defined as the day the vaginal plug was observed. The Animal Ethics Committee of Gyeongsang National University (approval number: GNU-210514-M0045) approved the animal experimental protocols of this study. All research activities were conducted with strict adherence to the European Community guidelines (EEC Directive of 1986;86/609/EEC), US guidelines (National Institute of Health publication #85-23 1985 revision), and ARRIVE guidelines.
Primary cell culture and drug treatments
Primary neuronal cultures were prepared according to previously described methods with minor modifications26. Briefly, mesencephalic region of developing mouse brains were dissected on embryonic day 12 (E12) in Hank’s balanced salt solution (Gibco, Pittsburgh, PA, USA). After enzymatic dissociation with 0.25% trypsin–EDTA at 37 °C for 15 min, cells were separated using a density gradient centrifugation to remove debris and oligodendrocytes. The collected neurons were plated at a density of 1 × 105 cells/cm2 on 0.1 mg/mL poly-L-lysine-coated wells in Neurobasal medium (Gibco) supplemented with B27, basic fibroblast growth factor, L-glutamic acid, and gentamicin. To suppress glial proliferation, 1 μM cytosine β-D-arabinofuranoside (Ara-C) was added on day 2 in vitro. Neurons were cultured at 37 °C in a humidified incubator with 5% CO₂, and all experiments were conducted during 14 days after plating. Next, the cells were simultaneously treated with 6-OHDA (100 μM) and either the D1-like receptor agonist SKF38393 (10 μM, Sigma–Aldrich, St. Louis, MO, USA), the D2-like receptor agonist quinpirole (10 μM, Sigma–Aldrich), the selective D1 receptor antagonist LE300 (10 μM, Tocris Bioscience, Bristol, UK), or suramin sodium salt (10 μM, Sigma–Aldrich) for 24 h. Samples were collected for protein analysis at 14 days after the treatment.
Western blot analysis
To evaluate the changes in GAD67 expression, primary culture cells were treated with 1 µM of 6-OHDA and a DA receptor agonist or antagonist for 7 days. Then, the cultured cells were washed with cold phosphate buffered saline (PBS). Total cell lysates were prepared using cell lysis buffer containing 1 M Tris-HCl (pH 8.0, 5 M NaCl, 1% NaN3, 10% sodium dodecyl sulfate, 10% NP-40, and 0.5% C24H39NaO4). Equal proteins were loaded onto a 10% polyacrylamide gel and then transferred to polyvinylidene membranes. Non-specific binding was blocked with 5% skim milk in 0.1% Tween 20 for 30 min. The membranes were incubated with primary antibodies (1:1000; AB152, Millipore, Billerica, MA, the USA), mouse monoclonal anti-GAD67 (1:1000; MAB5406, Millipore, Billerica, MA, the USA),, and mouse monoclonal anti-beta actin (1:1,000, mAB #3700, Cell Signaling, Danvers, MA, the USA) at a temperature of 4℃ overnight. After washing five times with PBS, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies. Finally, proteins were detected using an enhanced chemiluminescence reagent (Anigen, Seoul, Korea). Densitometry was performed using ImageJ (NIH, Bethesda, MD, the USA).
Stereotaxic surgery and drug treatments
Four groups of female mice were anesthetized with zoletile (10 mg/kg) and xylazine (5 mg/kg) and placed in a stereotaxic frame. To induce Parkinson’s disease, 5 μl of 6-OHDA (2 μg/mouse in saline containing 0.1% ascorbic acid) were unilaterally delivered to the SN (3.0 mm posterior to the bregma, 1.4 mm lateral to the midline, and 4.0 mm ventral to the skull surface) using a mouse brain atlas as a reference27. To assess the neuroprotective effects of DA receptor agonists, the D1-like receptor agonist SKF38393 (10 mg/kg) was administered subcutaneously for 2 weeks with manual injections performed using a Hamilton syringe. The syringe was kept in place for 5 min to prevent reflux. After injection, the scalp incision was sutured. Once the mice had recovered from anesthesia, they were returned to their home cages.
Sample preparation and immunohistochemistry
After 2 weeks of treatment with the DA receptor agonist SKF38393, the mice were deeply anesthetized with zoletile and xylazine. Then, they were sacrificed via intracardiac perfusion of 4% paraformaldehyde (pH of 7.4). Their brains were fixed again in paraformaldehyde for 48 h and embedded in paraffin wax. Tissue samples were then sliced at a thickness of 10 µm using a rotary microtome (Leica, German). Non-specific reactivity was blocked using 3% fetal bovine serum in PBS at room temperature for 1 h. Immunohistochemistry was performed using mouse monoclonal anti-GAD67 (1:500; MAB5406, Millipore, Billerica, MA, the USA), rabbit polyclonal anti-heparanase-1 (1:100; ab232817, Abcam, Milton, Cambridge, the UK), and rabbit polyclonal anti-TH (1:500; AB152, Millipore, Billerica, MA, the USA) antibodies. Streptavidin Alexa Fluor® 594/488-conjugated anti-mouse immunoglobulin G (IgG) (1:400; A30678, Invitrogen, St. Louis, MO, the USA) and streptavidin Alexa Fluor® 488/594-conjugated rabbit monoclonal anti-IgG (1:400; A30629, Invitrogen, St. Louis, MO, the USA) were used as secondary antibodies. The samples were then examined using a fluorescence microscope. Quantification of TH and GAD67 immunofluorescence signals was performed using Fiji (ImageJ, NIH). The region of interest (ROI) for the substantia nigra was manually drawn. Total fluorescent signal area was measured within the ROI and normalized accordingly. For each animal, signal intensity in the lesioned substantia nigra was compared with that in the contralateral side to provide internal control values.
Statistical analysis
Data were presented as mean values ± standard deviations from at least three independent experiments. Analyses of variance were performed to identify statistical differences. The quantified Western blot data were analyzed using one-way analysis of variance (ANOVA). P values of < 0.05 indicated statistically significant differences.
Results
Regulation of GAD67 via DAergic receptors in vitro and in vivo
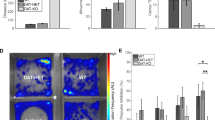
DA interacts with D1 and D2 receptors via direct and indirect pathways28. This study aimed to investigate the effects of 6-hydroxydopamine (6-OHDA) and DA receptors on GAD67 and TH expression in the primary cultured neuronal cells of mice. Western blot analysis revealed that treatment with 6-OHDA significantly increased GAD67 expression compared with the control. However, SKF38393, a D1-like receptor agonist, reduced GAD67 expression to a level similar to that of the control. In addition, when 6-OHDA and the D2-like receptor agonist quinpirole were treated together, the expression of GAD67 increased to more than that when only 6-OHDA was treated. Conversely, 6-OHDA treatment significantly reduced TH expression compared with the control, and there was no significant change after treatment with D1 and D2 receptor agonists (Fig. 1).
Effects of Dopamine Receptor Agonists on Glutamic Acid Decarboxylase 67 and Tyrosine Hydroxylase Expression after 6-Hydroxydopamine Treatment. Western blot analysis was performed to evaluate the expression of glutamic acid decarboxylase 67 (GAD67) and tyrosine hydroxylase (TH) in primary cultured neurons after 6-hydroxydopamine (6-OHDA) treatment. The same analysis was conducted following treatment with dopamine receptor agonists SKF38393 (D1-like receptor agonist) and quinpirole (D2-like receptor agonist). β-actin was used as an internal loading control. Data were expressed as mean ± SD of three independent experiments. ANOVA post hoc was used to calculate statistical significance. *, P < 0.05 compared with the control group; #, P < 0.05 compared with the 6-OHDA group.
The midbrain’s substantia nigra comprises two distinct regions: the SN pars compacta (SNc) and SNr regions29. To corroborate the regulation of TH and GAD67 expression by D1-like receptor agonists observed at the cellular level within DA-deficient conditions in the SN area of mice, 6-OHDA was administered into the SN of the midbrain, followed by 2 weeks of intraperitoneal SKF38393 administration to the mice. The expression levels of GAD67 and TH were subsequently assessed via immunofluorescence. TH-positive cells were found in the ventral tegmental area and SNc. Meanwhile, non-dopaminergic GAD67-positive cells were primarily located in the SNr of the control group (Fig. 2A–C). In the SN of mice treated with 6-OHDA, the number of Th-positive cells decreased due to DA neuron depletion. Meanwhile, the number of GAD67-positive cells in the SNr region increased (Fig. 2D–F). The number of GAD67-positive cells, which had increased due to 6-OHDA, decreased in mice treated with SKF38393 for 2 weeks. Similar to the in vitro results, the number of TH-positive cells was not affected by SKF38393 treatment (Fig. 2G–I). In vitro and vivo experiments confirmed that 6-OHDA and D1-like receptors regulated GAD67 expression. D1-like receptors can be categorized into D1 and D5 subtype receptors. Based on previous findings, to identify which of the two subtype receptors was primarily involved in regulating GAD67 expression, primary cultured neurons were treated with LE300, a D1 receptor-selective antagonist, 6-OHDA, and SKF38393. The expression of GAD67, which had decreased with SKF38393 treatment, increased to the same level as when 6-OHDA alone was administered. Meanwhile, the expression of TH did not change after 6-OHDA treatment (Fig. 3). Taken together, these results showed that the D1-like receptor, particularly the D1 subtype receptor, plays an important role in regulating GAD67 expression.
Impact of D1 Receptor Agonist SKF38393 on GAD67 and Tyrosine Hydroxylase Expression in Mice with 6-Hydroxydopamine-induced Lesions. (A–C) Immunofluorescence images showing the expression of GAD67 and TH in the substantia nigra (SN) of control mice. (D–F) Images from mice treated with 6-hydroxydopamine (6-OHDA), demonstrating altered expression patterns of GAD67 and TH. (G–I) Images showing the effects of SKF38393 treatment on GAD67 and TH expression in 6-OHDA-treated mice. *, P < 0.05 compared with the control group; #, P < 0.05 compared with the 6-OHDA group. Scale bar: 200 μm.
D1-like Receptor Antagonist LE300 Modulates GAD67 Expression in the Presence of SKF38393. Western blot analysis of GAD67 expression in primary cultured neurons treated with 6-OHDA and SKF38393. The effect of co-treatment with the D1 receptor-selective antagonist LE300 on GAD67 expression in the presence of SKF38393 and 6-OHDA. β-actin was used as an internal control. Data were presented as mean ± SD from three independent experiments. Statistical significance was calculated using ANOVA post hoc analysis. *, P < 0.05 compared with the control group; #, P < 0.05 compared with the 6-hydroxydopamine treatment group.
Association between heparanase-1 and GAD67 expression
The role heparanase-1 in the expression of GAD67, a substance that regulates the nervous system, was investigated. To determine whether heparanase-1 plays an essential role in the expression of GAD67, primary cultured neurons were treated with 6-OHDA and suramin, a heparanase-1 inhibitor. The treatment resulted in an increase in the expression of GAD67 due to 6-OHDA and a decrease in the expression due to suramin (as shown in Fig. 4). These findings indicate that suramin reduces the expression of GAD67 by inhibiting heparanase-1. Double IHC was also conducted on cultured neurons to validate the regulation mechanisms. When 6-OHDA was administered, the number of GAD67-positive cells and heparanase-1-positive cells increased. Merging these two positive cells confirmed that GAD67- and heparanase-1-positive cells were mostly identical (Fig. 5A–F). In contrast, the number of GAD67- and heparanase-1-positive cells decreased when SKF38393 was administered (Fig. 5G–I). According to these results, a decrease in the expression of GAD67 by the D1 receptor agonist inhibits the expression of heparanase-1. Finally, the regulation of heparanase-1 and GAD67 was controlled in the same direction while interacting with external stimulation,
Heparanase Inhibitor Suramin Regulates GAD67 Expression after Dopamine Degeneration. Western blot analysis of GAD67 expression in primary cultured neurons treated with 6-OHDA and the heparanase inhibitor suramin. The effect of suramin treatment on GAD67 expression in the absence of SKF38393. β-actin was used as an internal control. Data were presented as mean ± SD from three independent experiments. Statistical significance was determined using ANOVA post hoc analysis. *, P < 0.05 compared with the control group. #, P < 0.05 compared with the 6-hydroxydopamine treatment group.
Co-expression of GAD67 and Heparanase-1 in 6-Hydroxydopamine-Treated Cultured Neurons. (A–C) Double-label immunohistochemistry was performed to assess the co-expression of glutamic acid decarboxylase 67 (GAD67) and heparanase-1 in primary cultured neurons treated with 6-hydroxydopamine (6-OHDA). (D–F) In primary cultured neurons treated with 6-OHDA, the number of GAD67- and heparanase-1-positive cells increased. The enlarged image in panel F shows the merged expression of GAD67 and heparanase-1. (G–I) The number of GAD67- and heparanase-1-positive cells decreased after treatment with SKF38393, a D1 receptor agonist, in 6-OHDA-treated neurons. *, P < 0.05, ***, P < 0.001 compared with the control group; #, P < 0.05, ###, P < 0.001 compared with the 6-OHDA group. Scale bar: 50 μm.
Discussion
Dopamine (DA) and gamma-aminobutyric acid (GABA) are two essential neurotransmitters that maintain neural balance through reciprocal interactions. Their respective receptors not only modulate their own signaling but also exert cross-regulatory effects on each other’s pathways. For instance, a seminal behavioral study demonstrated that DA and GABA interact within the substantia nigra pars reticulata (SNr) to regulate rapid eye movement sleep in rats30. Furthermore, noncanonical interactions between GABAA and D5 dopamine receptors have been implicated in fine-tuning synaptic transmission31. Additional evidence indicates that D3 receptors can potentiate D2 receptor activity, leading to cAMP accumulation and GABA release in the striatopallidal terminals via the CaM–CaMKIIα signaling pathway32. Likewise, activation of D4 receptors has been shown to influence morphine-induced alterations in GAD65/67 and GABAB receptor expression within the basal ganglia. Notably, D4 receptor knockout mice subjected to stress exhibit schizophrenia-like behavioral phenotypes, attributed to disrupted GABAergic transmission33,34. While extensive research has examined both dopaminergic and GABAergic receptors, particular attention has been paid to the interaction between D1 receptors and the GABAergic system. GAD, the enzyme responsible for converting glutamate into GABA, exists in two isoforms—GAD65 and GAD67—classified by their molecular weights35. GAD65 is predominantly localized at nerve terminals and is crucial for activity-dependent GABA release36. In contrast, GAD67 is expressed more broadly in the cytoplasm and is essential for maintaining baseline GABA levels in the brain, thereby playing a key role in the long-term adaptation of GABAergic neurons to dopaminergic changes37,38. Importantly, modulation of GAD65/67 expression has been shown to alleviate neuropathic pain in spinal cord injury models, highlighting their therapeutic relevance39.
Activation of D1 receptors has been shown to modulate GABA release from brain slices encompassing the basal ganglia, including the SNr40,41. This regulatory function suggests that dopaminergic neuron degeneration could lead to compensatory alterations in GAD67 expression. Supporting this notion, previous studies have reported increased expression of GABAA receptors and GAD67 mRNA under conditions of dopamine depletion24. Consistently, in a primate model of dopaminergic neuronal loss, both GAD65 and GAD67 mRNA levels were elevated specifically in striatopallidal neurons42, indicating an adaptive response at the transcriptional level in GABAergic circuits. This study demonstrated that GAD67 expression was significantly increased following dopaminergic neuron depletion induced by 6-OHDA, both in in vitro and in vivo models. Supporting this observation, a previous study showed that a single administration of the D1 receptor agonist SKF-81297 following 6-OHDA lesions led to upregulation of both GAD65 and GAD67 mRNA in the striatum, along with downregulation of GABA receptor mRNA in the SNr23. Interestingly, chronic administration of SKF-38393 selectively increased GAD65 mRNA levels, whereas GAD67 mRNA expression in striatonigral neurons remained unchanged43. These contrasting findings imply that GAD65 and GAD67 isoforms are subject to distinct regulatory mechanisms, especially in the context of D1 receptor signaling in DA-deficient states. Furthermore, the current study extends this understanding by demonstrating that SKF38393 downregulates GAD67 expression under DA-depleted conditions, an effect that was reversed by the D1 antagonist LE300. In contrast, the D2 receptor agonist quinpirole further elevated GAD67 expression. These findings collectively suggest that D1-like receptor signaling exerts an inhibitory effect on GAD67 expression after dopaminergic denervation.
Additionally, our study revealed a novel role of heparanase-1 in mediating the regulatory effects of D1-like receptors on GAD67 expression. Specifically, treatment with 6-OHDA induced upregulation of both GAD67 and heparanase-1, while administration of the D1 receptor agonist resulted in decreased immunoreactivity of both proteins. Notably, suramin—a pharmacological inhibitor of heparanase—also suppressed GAD67 expression in primary cultures. These findings strongly indicate that D1 receptor–mediated regulation of GAD67 may be mechanistically linked to the activity of heparanase-1, positioning it as a potential downstream effector of D1 signaling in the GABAergic system. This adds a new dimension to our understanding of how extracellular matrix remodeling enzymes may participate in neurotransmitter regulation and neural plasticity. Collectively, these results indicate that D1-like receptor signaling negatively regulates GAD67 expression under dopaminergic denervation. Previous studies have reported that both astrocytes and NG2 glia can express GAD67 and heparinase44,45,46.To further explore the molecular mediators involved in this process, we examined the role of heparanase-1, a known regulator of extracellular matrix dynamics and neuronal development.
Heparanase plays a pivotal role in degrading heparan sulfate chains, a process tightly associated with key neurodevelopmental events, such as neurogenesis, axonal growth, and synaptic organization47,48. In the central nervous system, heparan sulfate proteoglycans—including syndecans—serve as co-receptors for multiple growth factors, thereby modulating diverse signaling pathways49. Notably, Syndecan-3 is critical for neural development, and its deficiency has been shown to impair hippocampal structure and function50,51,52. Moreover, heparanase and its cleavage products have been implicated in both physiological development and pathological processes such as glioma progression53. These multifunctional roles underscore the potential of heparanase-1 to modulate neuronal gene expression, including GAD67, in response to upstream DA signaling.
Although TH is commonly used to identify catecholaminergic neurons, it lacks absolute specificity for dopaminergic cells. In our current study, we did not apply DAT-specific immunostaining or pre-treat with desipramine to exclude the contribution of noradrenergic neurons. Nonetheless, previous reports have demonstrated that under standard midbrain primary culture conditions, 6-OHDA exerts preferential toxicity on DA neurons, supporting the validity of our model51,52. Future studies incorporating cell-type–specific labeling and transporter blockade approaches are warranted to precisely dissect the cellular specificity of the observed changes. A further limitation of the present study is the absence of a sham-lesioned group treated with SKF38393, which precludes definitive attribution of GAD67 changes solely to DA depletion. Including such controls in future experiments would help differentiate between baseline D1 receptor effects and those specific to denervation conditions.
Our research showed that suramin, a heparanase inhibitor, can reduce GAD67 expression in primary cultures. Moreover, heparanase-1 had a regulatory effect on GAD67 downregulation. Treatment with 6-OHDA, a neurotoxin, increased the expression levels of both GAD67 and heparanase-1. In contrast, treatment with a DA D1-like receptor agonist decreased the immunoreactivity of both GAD67 and heparanase-1. Further, suramin reduced GAD67 expression in primary cultures. Based on these findings, DA D1-like receptor agonists regulate GAD67 expression via heparanase-1, which could have implications for neurodegeneration (Fig. 6).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Esch, T. & Stefano, G. B. The neurobiology of pleasure, reward processes, addiction and their health implications. Neuroendocrinol. Lett. 25(4), 235–251 (2004).
Speranza, L., di Porzio, U., Viggiano, D., de Donato, A. & Volpicelli, F. Dopamine: The neuromodulator of long-term synaptic plasticity, reward and movement control. Cells 10(4), 735. https://doi.org/10.3390/cells10040735 (2021).
Jenkins, G. & Walton, M. Dopamine: Don’t underestimate the force. Curr. Biol. 30(14), R824–R826 (2020).
Lambrini, K. et al. Sleep and health: Role of dopamine.In Proc Dopamine, Health Disease. 31 (2018).
Lee, J. Y. et al. Dopamine facilitates associative memory encoding in the entorhinal cortex. Nature 598(7880), 321–326 (2021).
Mohebi, A. et al. Dissociable dopamine dynamics for learning and motivation. Nature 570(7759), 65–70 (2019).
Cheng, Y. T. et al. Social deprivation induces astrocytic TRPA1-GABA suppression of hippocampal circuits. Neuron 111(8), 1301–1315 (2023).
Prevot, T. & Sibille, E. Altered GABA-mediated information processing and cognitive dysfunctions in depression and other brain disorders. Mol. Psychiatry 26(1), 151–167 (2021).
Tinok, A. A., Karabay, A., Jong, J., Balta, G. & Akyurek, E. G. Effects of gamma-aminobutyric acid on working memory and attention: A randomized, double-blinded, placebo-controlled, crossover trial. J. Psychopharmacol. 37(6), 554–565 (2023).
Martel, J. C. & Gatti, M. S. Dopamine receptor subtypes, physiology and pharmacology: New ligands and concepts in schizophrenia. Front. Pharmacol. 11, 1003 (2020).
Lee, B. E. et al. O-GlcNAcylation regulates dopamine neuron function, survival and degeneration in Parkinson disease. Brain 143(12), 3699–3716 (2020).
Pristera, A. et al. Dopamine neuron-derived IGF-1 controls dopamine neuron firing, skill learning, and exploration. Proc. Natl. Acad. Sci. u. s. a 116(9), 3817–3826 (2019).
Cheng, Z. Y., Hu, Y. H., Xia, Q. P., Wang, C. & He, L. DRD1 agonist A-68930 improves mitochondrial dysfunction and cognitive deficits in a streptozotocin-induced mouse model. Brain Res. Bull. 175, 136–149 (2021).
Cheng, Z. Y., Xia, Q. P., Hu, Y. H., Wang, C. & He, L. Dopamine D1 receptor agonist A-68930 ameliorates Abeta(1–42)-induced cognitive impairment and neuroinflammation in mice. Int. Immunopharmacol. 88, 106963 (2020).
Moritz, A. E. et al. Discovery, optimization, and characterization of ML417: A novel and highly selective D(3) dopamine receptor agonist. J. Med. Chem. 63(10), 5526–5567 (2020).
Nair-Roberts, R. G. et al. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 152(4), 1024–1031 (2008).
Zhou, F. W., Jin, Y., Matta, S. G., Xu, M. & Zhou, F. M. An ultra-short dopamine pathway regulates basal ganglia output. J. Neurosci. 29(33), 10424–10435 (2009).
Boyson, S. J., McGonigle, P. & Molinoff, P. B. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J. Neurosci. 6(11), 3177–3188 (1986).
Savasta, M., Dubois, A. & Scatton, B. Autoradiographic localization of D1 dopamine receptors in the rat brain with [3H]SCH 23390. Brain Res. 375(2), 291–301 (1986).
Wamsley, J. K., Gehlert, D. R., Filloux, F. M. & Dawson, T. M. Comparison of the distribution of D-1 and D-2 dopamine receptors in the rat brain. J. Chem. Neuroanat. 2(3), 119–137 (1989).
Dawson, T. M., Barone, P., Sidhu, A., Wamsley, J. K. & Chase, T. N. The D1 dopamine receptor in the rat brain: Quantitative autoradiographic localization using an iodinated ligand. Neuroscience 26(1), 83–100 (1988).
Trevitt, T. et al. Interactions between dopamine D1 receptors and gamma-aminobutyric acid mechanisms in substantia nigra pars reticulata of the rat: Neurochemical and behavioral studies. Psychopharmacology 159(3), 229–237 (2002).
Yamamoto, N. & Soghomonian, J. J. Time-course of SKF-81297-induced increase in glutamic acid decarboxylase 65 and 67 mRNA levels in striatonigral neurons and decrease in GABA(A) receptor alpha1 subunit mRNA levels in the substantia nigra, pars reticulata, in adult rats with a unilateral 6-hydroxydopamine lesion. Neuroscience 154(3), 1088–1099 (2008).
Pan, H. S., Penney, J. B. & Young, A. B. Gamma-aminobutyric acid and benzodiazepine receptor changes induced by unilateral 6-hydroxydopamine lesions of the medial forebrain bundle. J. Neurochem. 45(5), 1396–1404 (1985).
Jayatilleke, K. M. & Hulett, M. D. Heparanase and the hallmarks of cancer. J. Transl. Med. 18(1), 453 (2020).
Brewer, G. J. & Torricelli, J. R. Isolation and culture of adult neurons and neurospheres. Nat. Protoc. 2(6), 1490–1498 (2007).
Paxinos G, Franklin KB. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates(Academic Press, 2019).
Radnikow, G. & Misgeld, U. Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata. J. Neurosci. 18(6), 2009–2016 (1998).
Oertel, W. H., Tappaz, M. L., Berod, A. & Mugnaini, E. Two-color immunohistochemistry for dopamine and GABA neurons in rat substantia nigra and zona incerta. Brain Res. Bull. 9(1–6), 463–474 (1982).
Yadav, R. K., Khanday, M. A. & Mallick, B. N. Interplay of dopamine and GABA in substantia nigra for the regulation of rapid eye movement sleep in rats. Behav. Brain Res. 376, 112169 (2019).
Maingret, F. & Groc, L. Characterization of the functional cross-talk between surface GABA(A) and dopamine D5 receptors. Int. J. Mol. Sci. 22(9), 4867. https://doi.org/10.3390/ijms22094867 (2021).
Villalobos-Escobedo, F. S. et al. Dopamine D3 receptor modulates D2 receptor effects on cAMP and GABA release at striatopallidal terminals-Modulation by the Ca2+-Calmodulin-CaMKII system. Eur. J. Neurosci. 59(7), 1441–1459. https://doi.org/10.1111/ejn.16237(2023).
Negrete-Diaz, J. V. et al. Pharmacological activation of dopamine D(4) receptor modulates morphine-induced changes in the expression of GAD(65/67) and GABA(B) receptors in the basal ganglia. Neuropharmacology 152, 22–29 (2019).
Tan, T. et al. Stress exposure in dopamine D4 receptor knockout mice induces schizophrenia-like behaviors via disruption of GABAergic transmission. Schizophr. Bull. 45(5), 1012–1023 (2019).
Erlander, M. G. & Tobin, A. J. The structural and functional heterogeneity of glutamic acid decarboxylase: A review. Neurochem. Res. 16(3), 215–226 (1991).
Pinal, C. S. & Tobin, A. J. Uniqueness and redundancy in GABA production. Perspect. Dev. Neurobiol. 5(2–3), 109–118 (1998).
Chattopadhyaya, B. et al. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron 54(6), 889–903 (2007).
Zhang, K., Chammas, C. & Soghomonian, J. J. Loss of glutamic acid decarboxylase (Gad67) in striatal neurons expressing the Drdr1a dopamine receptor prevents L-DOPA-induced dyskinesia in 6-hydroxydopamine-lesioned mice. Neuroscience 303, 586–594 (2015).
Li, X. et al. Exercise training modulates glutamic acid decarboxylase-65/67 expression through TrkB signaling to ameliorate neuropathic pain in rats with spinal cord injury. Mol. Pain. 16, 1744806920924511 (2020).
Jaber, M., Robinson, S. W., Missale, C. & Caron, M. G. Dopamine receptors and brain function. Neuropharmacology 35(11), 1503–1519 (1996).
Vasudevan, A. et al. Dopaminergic neurons modulate GABA neuron migration in the embryonic midbrain. Development 139(17), 3136–3141 (2012).
Soghomonian, J. J., Gonzales, C. & Chesselet, M. F. Messenger RNAs encoding glutamate-decarboxylases are differentially affected by nigrostriatal lesions in subpopulations of striatal neurons. Brain Res. 576(1), 68–79 (1992).
Laprade, N. & Soghomonian, J. J. Glutamate decarboxylase (GAD65) gene expression is increased by dopamine receptor agonists in a subpopulation of rat striatal neurons. Mol. Brain Res. 48(2), 333–345 (1997).
Lee, M., Schwab, C. & McGeer, P. L. Astrocytes are GABAergic cells that modulate microglial activity. Glia 59(1), 152–165 (2011).
Zhang, X. et al. NG2 glia-derived GABA release tunes inhibitory synapses and contributes to stress-induced anxiety. Nat. Commun. 12(1), 5740 (2021).
Zhang, Y. et al. Mapping heparanase expression in the spinal cord of adult rats. J. Comp. Neurol. 494(2), 345–357 (2006).
Bishop, J. R., Schuksz, M. & Esko, J. D. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446(7139), 1030–1037 (2007).
Qiu, H., Yang, B., Pei, Z. C., Zhang, Z. & Ding, K. WSS25 inhibits growth of xenografted hepatocellular cancer cells in nude mice by disrupting angiogenesis via blocking bone morphogenetic protein (BMP)/Smad/Id1 signaling. J. Biol. Chem. 285(42), 32638–32646 (2010).
Yamaguchi, Y. Heparan sulfate proteoglycans in the nervous system: Their diverse roles in neurogenesis, axon guidance, and synaptogenesis. Semin. Cell Dev. Biol. 12(2), 99–106 (2001).
Kaksonen, M. et al. Syndecan-3-deficient mice exhibit enhanced LTP and impaired hippocampus-dependent memory. Mol. Cell. Neurosci. 21(1), 158–172 (2002).
Nyhus, J. K. & Denburg, J. L. The in vivo regulation of pioneer axon growth by FGF-2 and heparan sulfate proteoglycans in cultured embryos of the cockroach. Mol. Cell. Neurosci. 11(5–6), 305–323 (1998).
Walz, A. et al. Essential role of heparan sulfates in axon navigation and targeting in the developing visual system. Development 124(12), 2421–2430 (1997).
Xiong, A., Spyrou, A. & Forsberg-Nilsson, K. Involvement of heparan sulfate and heparanase in neural development and pathogenesis of brain tumors. Adv. Exp. Med. Biol. 1221, 365–403 (2020).
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (2018R1D1A1B0704925813) funded by the Ministry of Education.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (2018R1D1A1B0704925813) funded by the Ministry of Education.
Author information
Authors and Affiliations
Contributions
S.L.; Conception and design of the study, acquisition of data, drafting the manuscript, revising the manuscript, M.K., S.-E.S.,; Investigation, G.K.;drafting the manuscript and revising the manuscript, C.W.; Conception and design of the study, acquisition of data, drafting the manuscript, revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The Animal Ethics Committee of Gyeongsang National University (approval number: GNU-210514-M0045) approved the animal experimental protocols of this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, S., Kim, M., Sung, SE. et al. SKF-38393 regulates the expression of glutamic acid decarboxylase 67 via heparanase-1 in 6-hydroxydopamine-induced neurodegeneration. Sci Rep 15, 26306 (2025). https://doi.org/10.1038/s41598-025-11672-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11672-w