Abstract
Marine ecosystems are healthy with a high degree of biodiversity. Assessing how factors affect spatial and temporal patterns of biodiversity is an essential task for the Ecosystem Based Management approach. In this work, we investigated the effect of fisheries disturbance and ocean variables in determining α- and β-diversity of Mediterranean demersal assemblages. Generalized additive mixed models were used to explain the spatio-temporal variability of diversity indices from 2014 to 2020 in three Mediterranean subregions as a function of covariates. An in-depth analysis also made it possible to decouple the effects of bottom trawling from the other covariates. The results show that several fishing activities and environmental variables influence biodiversity, but the direction of change depends on the subregion considered. Bottom trawling instead has a quasi-linear erosion effect on α- and β-diversity in all areas. Valuable commercial species and threatened rays and sharks importantly characterized the sites with low fishing impact. Results are a step towards the development of conservation and management strategies, particularly in the context of the Marine Strategy.
Similar content being viewed by others
Introduction
Marine biodiversity traditionally refers to all the different kinds of living organisms within a given area. It includes organisms from large animals such as mammals to tiny, single-cell algae and underpins species interconnection, stability of ecosystem and evolutive complexity1,2,3,4. With less biodiversity, these connections weaken and sometimes even break down, harming species and impacting ecosystem functioning5. Ecosystems with high biodiversity are generally stronger and more resilient to regime shifts than those with fewer species. Higher biodiversity increases the capacity of an ecosystem to absorb disturbances and reorganize while maintaining essential functions and services6,7.
Marine biodiversity is important to humans in many ways. Phytoplankton, algae, and plants, for example, are essential to life by largely contributing to oxygen production and the carbon cycle8,9. Marine species also provide food, bioproducts, medicines, and aesthetic benefits10. If biodiversity declines, these services could decrease, and with them human health and income10,11,12. Although the Earth’s biodiversity is so rich that many species have yet to be discovered, many marine species are threatened with local extinction due to human impacts such as habitat destruction, overexploitation, effects of climate change, introduction and spread of invasive species, and pollution13. Assessing the role of human impact and environmental factors in determining the spatial and temporal effects on biodiversity is an essential task for the Ecosystem Based Management (EBM) approach14which allows for understanding the complex patterns and processes in the ecosystem and potentially protecting the largest number of species that are simultaneously under multiple threats15. A better understanding of this complexity and the multidimensionality of diversity operating at different spatial and temporal scales is, therefore, an important step towards an integrative ecosystem assessment16.
Overfishing is one of the most worrisome disturbances on marine ecosystems across short temporal scales14,17,18. It affects fish populations and benthic assemblages by leading to changes in their size, abundance, and composition19,20,21. In intensely fished areas, especially where bottom trawling is practiced, the loss of demersal predatory biomass19,22,23 and a severe decrease of long-lived species abundance24 determines a reshuffle of trophic processes through direct and indirect effects25,26,27,28 with a consequent alteration of biodiversity19. Despite the importance of biodiversity, its quantification through classical indices such as α- and β-diversity and direction of changes in relation to human impacts, especially at large scales are quite limited all around the world19,29. This kind of assessment could be particularly useful for the Mediterranean Sea, where hotspots of biodiversity30 could be loomed with high fishing pressure and climate changes31,32,33.
Previous works on Mediterranean biodiversity generally focused on single habitats or local communities close to the coast. They were mainly based on abundance ranked counts by visual census34,35. At a large scale, the diversity of the Mediterranean has been investigated by using presence–absence maps of fish built on expert-based knowledge36,37,38reviewed through online databases of taxa diversity39 or modelled by considering trophic relationships of functional groups and anthropogenic stressors40.
An assessment based on standardized scientific monitoring at large temporal and spatial scales was rarely carried out due to the associated logistical difficulties and costs related to the collection of data at the species level. In one case, the biodiversity of demersal communities of continental shelf and slope has been explored by using datasets coming from the International Bottom Trawl Survey in the Mediterranean (MEDITS) program41 until to the year 201242. More recently, a similar evaluation, but at a smaller spatial scale, has been proposed in the Ionian Sea43 and in the Balearic Sea44 by examining β-diversity across the years 1994–2012 and 1994–2015, respectively. This kind of biodiversity evaluation at a regional scale along environmental gradients and impacting human activities, for the Mediterranean Sea, is lacking.
Studying how multiple factors may influence biodiversity can provide insights into marine conservation and spatial management and, particularly, can contribute to the implementation of the EU Marine Strategy Framework Directive (Directive 2008/56/EC, MSFD hereafter) process. The quantification of biodiversity changes in relation to covariates can help explain potential causes of declining biodiversity. Disentangling the effects of fishing from other sources of biodiversity variability, such as environmental variables, might support future management actions.
This work examined the spatial patterns of demersal assemblage biodiversity over seven years (from 2014 to 2020) in large areas of three Mediterranean subregions defined by the MSFD. Generalized additive mixed models were used to explain the spatio-temporal variability of biodiversity in relation to environmental covariates and fishing pressure. Based on data collected by the MEDITS program, we calculated α-diversity, measured as the Effective Number of Species45 and the non-directional β-diversity following the approach by Legendre and De Cáceres46. Β-diversity is in direct correspondence with the variance of community structure47,48and its decomposition also allows for expressing the degree of uniqueness of a site (haul) with respect to a subregional average community. Moreover, to disentangle the effect of bottom trawling at the community and species level connected with changes in biodiversity, we focused on differences between two groups of hauls markedly different in terms of the fishing pressure.
Materials and methods
Area of study
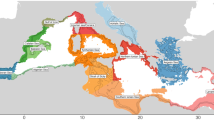
In this study, we focused on the three Mediterranean subregions bordering Italy as identified by the MSFD: the Adriatic Sea (MAD), the Ionian Sea and the Central Mediterranean Sea (MIC), and the Western Mediterranean Sea (MWE) (Fig. 1).
MAD is very shallow and under the influence of intense lateral (river runoff and southward transport) and surface (wind and air temperature) stresses, and the northern basin represents the largest shelf area in the Mediterranean Sea49. The shelf floor is of sedimentary origin, and further from the shore, the mud gradually takes over, favoring the establishment of a community of muddy sands. From a fishery perspective, the Adriatic Sea is one of the areas in the Mediterranean with the highest number of operating trawlers and landings of demersal fish50.
MIC subregion is an oligotrophic basin deeper than the Adriatic Sea and acts as an intersection for major Mediterranean currents51. The Ionian basin shows a complex geomorphology and biocenotic structure, especially along the Calabrian-Sicilian coasts, crossed by active canyons that transport materials from the break of the platform to the bathyal depth52. The Sicilian Channel is characterized by a two-layer exchange current system with an east-west gradient of inflows and outflows53. The shallowest offshore seabed, the Adventure Plateau, which is considered a biodiversity hotspot54is in the north-western sector and is stippled by several rocky banks55. The bottom trawling fleet is one of the most commercially important in the Mediterranean Sea and targets mixed assemblages of fish and cephalopods on the outer shelf and deep-water shrimps on the upper and middle slope54.
MWE subregion is characterized by the presence of three main water masses and circulation structures56. The Tyrrhenian shores are characterized by the presence of a dense network of submarine canyons57while in the open sea submerged marine terraces and seamounts58 contribute to the structural complexity of the regional marine system. The relatively small extension of the shelf makes this area denser in terms of trawlers among the Italian seas50; in addition to common target species (fish, cephalopods and crustaceans), the main target of bottom trawling is the red shrimp localized in canyons and accessible bathyal plains59.
Spatial domain of large portions of the three MSFD subregions. Data from GSAs 17 and 18 were used to represent the Adriatic Sea subregion (MAD). Data from GSAs 15, 16, and 19 were used to represent the Ionian Sea and Central Mediterranean Sea subregion (MIC). Data from GSAs 9, 10, and 11 were used to represent the Western Mediterranean Sea subregion (MWE). GSAs are the geographical subareas defined by the GFCM (General Fisheries Commission for the Mediterranean Sea).
Data and analysis
Exploring patterns of diversity
To explore the pattern of diversity, we first calculated α and β-diversity indices from the data collected by the MEDITS program41 across Geographical Sub-Areas (GSAs, Fig. 1). For each subregion, the analysis was performed on the biomass (bij) (Kg Km−2) of the demersal species j per haul i (that is a single trawl operation, where a fishing net (trawl) is towed along the seafloor to collect samples of marine life), over the years 2014–2020 (Fig. 2). Species were retained based on the list used by Granger et al. (2015) and if they were present in more than 1% of hauls (cut-off) in each subregion.
For each haul, \(\:i\) the composition (proportion p) of the \(\:j\) species was calculated using the biomass data following Eq. 1:
The choice to work with biomass rather than abundance has been guided by the fact that the MEDITS samples cover a wide range of sizes. Because of the power-law distribution of marine organisms60we expected to find a great number of small organisms. Thus, using biomass the community would have appeared more balanced in proportion between large (top predators) and small species (small pelagic fish).
Alpha diversity index
For each haul, we calculated the Hill number of order 1 (also known as Shannon entropy, H’) on community composition as an index of α-diversity. Later, we transformed H’ into the Effective Number of Species (ENS) as a more intuitive measure of diversity. Particularly, ENS is the number of species with even biomass that generate the same H’ calculated (Jost et al. 2006). H’ and the ENS α- diversity index were calculated following Eq. 2a and 2b using the < diversity > function of the R package < vegan.R>:
Beta diversity index
To compute the total β-diversity (BD) of each subregion, we followed the method proposed by Legendre and De Cáceres (2013) that isthe biomass data were transformed using the square root of their proportions (Hellinger transformation). Then, the BD contributions were obtained by calculating the Euclidean distance from the average community profile \(\:\stackrel{-}{\sqrt{{p}_{j}}}\) (Eq. 3a) and divided by the total variance\(\:\:{\sum\:}_{i}^{n}{\sum\:}_{j}^{m}{bd}_{ij}\:\).
For every haul \(\:i\), the Local Contribution to β-diversity \(\:\left({LCBD}_{i}\right)\) can be calculated by summing the contribution of all species m for each haul, following Eq. 3b
.
Generalized additive mixed model (GAMM)
To analyze the spatial and temporal patterns of diversity at the local scale (individual haul, ENS values, Supplementary Fig. 1, 2, 3) and understand why specific sites (hauls) exhibit greater uniqueness in species composition (LCBD values, Supplementary Fig. 4, 5, 6), we gathered data on habitats, environmental variables, and fishing activities (Fig. 2a).
Habitats
The benthic habitat data was obtained by a marine biotope classification map (EUSeaMap 2023 Broad-Scale Predictive Habitat Map for Europe) provided by EMODnet service (https://emodnet.ec.europa.eu/en; Montefalcone et al.61). The table of benthic habitats used in this study is reported in Supplementary Tables 1 and showed in Supplementary Fig. 7.
Ocean variables
Additionally, a series of relevant ocean variables for the marine environment were used (OVs - Fig. 2a Data panel and Supplementary Fig. 7). The OVs considered in this work are bottom temperature (tmp_bot in °C), bottom dissolved oxygen (oxy in mmol m−3), bottom salinity (sal_bottom in PSU), integrated chlorophyll-a 0–200 m (chl in mg m−2), bottom particulate organic carbon (poc_bot in mg m−3), and integrated net primary production in the water column 0–200 m (npp in mg m−2). OVs were extracted from the Copernicus Marine Service (CMS, https://data.marine.copernicus.eu/products) as estimated by the 3D coupled physical and biogeochemical model for the Mediterranean Sea with a horizontal resolution of 1/24°62.
Fishing effort
Finally, several fishing effort activities (Supplementary Fig. 7) were added as covariates because of their well-recognized impact on demersal assemblages. Yearly fishing effort was obtained from the Global Fishing Watch service (GFW, https://globalfishingwatch.org/) in the form of hours of apparent fishing effort calculated from the automatic identification system (AIS) with a horizontal resolution of 1/100°. The fishing activities considered were: trawling, seiners, fixed gears, and others (squid jaggers, dredges, trollers, drifting longlines, and pole and line). Each MEDITS haul point (black point Fig. 2b Indices panel) was then associated with the corresponding benthic habitats (dark gray shape Fig. 2b Indices panel) and the closest OV detection point (light gray point Fig. 2b Indices panel). Additionally, local fishing effort was associated with each MEDITS haul point. Since the two datasets did not match spatially, a square of 6/100° (cell = 36 km2) centered on the OV detection point (dark gray square Fig. 2b Indices panel) was used to sum fishing effort.
The spatial and temporal distribution of diversity indices and their relationship with the variables gathered was studied through the application of a Generalized Additive Mixed Model (GAMM)63 per subregion, considering a total of 1947, 1501 and 1997 hauls for MAD, MIC and MWE, respectively. Hauls covered a depth range between 10 and 800 m in all subregions. After preliminary analyses (Supplementary Fig. 8), the full model included a temporal factor (year), a spatial factor (benthic habitats), a set of covariates (depth, OVs, and the log effort of four fishing activities). Moreover, where the condition of homogeneity of variance (homoscedasticity - LeveneTest) of fixed factor was not respected, we added a random spatial factor (such as in the case of benthic habitats).
For each subregional model, the covariates (OVs + fishing effort) were checked for collinearity using their variance inflation factor (VIF) and definitively eliminated if no increase in the Akaike Information Criterion (AIC) was detected (Fig. 2c). Once any further simplification was possible, we determined the adequate number of nodes per covariate with the help of the gam.check function of the < mgcv.R > package. Finally, we checked for possible residuals’ autocorrelation patterns by adding to the model a spatially defined Gaussian autocorrelation term and by visual inspection of the semi-variograms (Supplementary Final models section with Supplementary Figs. 9–11).
Given the continuous and strictly positive nature of the α- and β-diversity values, the distribution family used in the model was Gamma. Assuming a multiplicative effect of the covariates on the response variable, the link function chosen was the logarithm.
Effects of bottom trawling on biodiversity
To assess the effects of bottom trawling we calculated biodiversity indices at the community and species level. We selected two groups of hauls that are largely different in the fishing pressure for each subregion and analyzed for differences in the biodiversity indices. The two groups were: low, the hauls impacted by less than 10 h of trawling during the year (effort < 10 h×cell−1×year−1) which is also corresponded approximately to the 10° percentile in the distribution of trawling effort and high, the hauls being trawled for more than 1000 h in a year (effort > 1000 h×cell−1×year−1), corresponding to about the 90° percentile.
Community level
Differences between the two groups were first investigated at the community level by performing the analysis on the residuals of the GAMM model once the trawling was removed from the covariates pool. Pairwise tests on residual ENS (α index) and LCBD (β index), respectively, were conducted to test median differences between the two groups by the application of the non-parametric quantile test (QT)64. The QT was carried out using the quantileTest function of < EnvStats > package.
Species level
Differences between the two groups at species level were evaluated by using the IndVal index65which was calculated for each species using the untransformed biomass table (Fig. 2a). The index is given by the multiplication of two independent components: the fidelity and the specificity of species t in the group of hauls and has a range between 0 and 1 (package < indicspecies.R>). Finally, the statistical significance of the relationship between the species and each site group is tested using a permutation test with p < 0.05 for significance. In addition, we checked if among indicative species there were vulnerable and threatened species (https://www.iucnredlist.org/) and their consistent difference between the two groups. Handling of data and all the statistical analyses were performed with R computing environment66 and R Studio67.
Results
Explaining patterns of biodiversity
After applying the cut-off, more than 100 species for each of the three sub-regions were retained. The complete list of species used in this study is presented in Supplementary Tables 2, and the position of the hauls is presented in Supplementary Figs. 1–6.
The MAD subregion was characterized by the lowest BD (0.67), while the MIC and MWE subregions had a higher degree of diversity (MIC = 0.79, MWE = 0.78). When considering the temporal distribution, both diversity indices did not show any clear trends in the three subregions, but some years were characterized by higher-than-average values (Supplementary Fig. 12).
The proportion of deviance explained depended on the subregion. For α-diversity, the model explained 39.8% of the total deviance for MAD, 21.3% for MIC, and 7.36% for MWE. When considering β-diversity, the models explained a greater portion of deviance. For the MAD, the proportion of deviance explained was the greatest (62.1%), followed by the MIC (53.3%) and the MWE (43.5%). The relations between ENS and LCBD and the covariates depended on which subregion is taken into consideration and a summary of the unique contributions of each covariate in explaining the total deviance is shown in Table 1.
For ENS, depth, trawling effort, and benthic habitats uniquely explain more deviance than other covariates. The effect of trawling on ENS was negative, while the effect on depth and benthic habitats was nonlinear. Among the OVs, only temperature and oxygen were always significant in explaining the α-diversity patterns. However, among the three subregions, there was no common pattern in the shape of the smoothing curves (Figs. 3a, 4a and 5a). Similarly, when considering LCBD, depth, benthic habitats, and trawling effort showed the most important and consistent pattern across the three systems, and again, the impact of trawling effort was mainly negative (Figs. 3b, 4b and 5b). The effect of depth on ENS and LCBD was always significant, but when compared inside the subregion, it appeared to show opposite trends between the two. This is not immediately evident from Table 1 since the patterns were non-linear. Yet observing the splines we can see for MAD that ENS peaked when LCBD was the lowest between 100 and 200 m (Fig. 3a and b). Similarly, in MWE the ENS was the lowest when the LCBD was the highest in the shallowest hauls (Fig. 5a and b). Among the OVs, four covariates were always significant in their contribution to the LCBD model (Chlorophyll-a, POC, NPP, and oxygen); also in this case, it was not possible to identify a common pattern for the OVs across the three subregions (Figs. 3b, 4b and 5b).
Bottom trawling effects on biodiversity
Considering hauls grouped by effort (high and low), quantile test shows that in each subregion, the median of both ENS and LCBD diversity at community level was always significantly higher in the low group, except for residual ENS in MWE (Supplementary Fig. 13).
In all the three subregions, the high group had a lower number of indicative species resulting from the INDVAL analysis with the permutation test (15 in MAD, 10 in MIC, and 21 in MWE, Fig. 6 and Supplementary Table 3) than the low group (44 in MAD, 39 in MIC, and 40 in MWE, Fig. 6 and Supplementary Table 3) and was generally composed of small fish and common cephalopods. Indicative species of the low group included several commercial species, species of higher body mass and rare species as well. Although vulnerable or endangered rays and sharks contributed little in biomass to the total community, they were almost exclusively and significantly higher in INDVAL, in the low group (Supplementary Table 3).
Discussion
Our results show that bottom trawling and environmental variables significantly affect biodiversity patterns in each of the three subregions studied. These effects are in addition to the expected effects of factors such as benthic habitat and natural variability that commonly characterize ecosystems over time (e.g., Pérès and Picard68, Montefalcone et al.61).
Exploring patterns of biodiversity
We found that several environmental variables, specific for each subregion considered, play a role in explaining the variance of the diversity indices. Depth and oxygen always affect the variance of α-diversity (ENS) and β-diversity (LCBD) in all subregions, with depth making the largest contribution in terms of deviance. The role of depth as a structuring factor of abundance, biomass, and diversity has been demonstrated for many different taxa43,69,70. However, the relationship of biodiversity with depth does not always meet expectations70and it can appear in opposite directions depending on the taxonomic group studied and the diversity index used. For example, species richness derived from the total count of many taxonomic groups was overall higher in shallow waters than in deep waters in a previous work71. On the contrary, the β-diversity of fish and invertebrates in the Balearic was higher in deeper waters than in coastal waters44.
Although we confirm that depth is a major factor for diversity, a smoothed, hump-shaped curve was observed, especially in the Adriatic subregion (MAD). In addition, the ENS and LCBD functions in each subregion showed an opposite concavity with a maximum near 150–200 m depth when viewed visually. Higher values of α-diversity (ENS) corresponding to hauls at the end of the continental shelf can be explained by a concentration of fish biomass of several species in this zone. A similar pattern of fish biomass was found by Katsanevakis et al. (2009) in a study on the distribution of demersal fish species in the Mediterranean Sea as a function of depth. The same ecological phenomenon may also explain why we found lower β-diversity values (LCBD) at higher ENS. Indeed, at 150–200 m depth, the hauls were more similar to the typical community taken as reference (i.e. common species with an average biomass), making the species composition in the hauls more homogeneous (lower LCBD values).
The relationships between all the other environmental variables and biodiversity indices are mainly described by nonlinear functions with unclear directions between indices and specific patterns for each subregion. The variability of these environmental variables is generally high, but even if species can respond quickly, their specific behavior may not be reflected in the α- and β community indicators or may be masked in some way by the main variation at different scales, which is determined by more structuring environmental factors such as depth and benthic habitats or a combination of several variables. Further studies focusing on areas with different prevailing environmental conditions may help in assessing the effect of environmental variables on diversity indices.
Although fishing activities, such as seiners and fixed gear, significantly explain the variance in ENS and LCBD, only bottom trawling consistently has a negative quasi-linear impact on biodiversity indices in all subregions. For this reason, we focused further analysis on the relationships between bottom trawling and biodiversity.
Bottom trawling effects on biodiversity
Although the importance of ecosystem-based management is increasingly recognized15,33,72,73there is little scientific evidence of the actual impact of fisheries on biodiversity indices for large oceanic systems and the Mediterranean Sea in particular. This is likely due to the impossibility of controlled experimental ecosystem manipulations and the lack of suitable time series or spatial replicates that allow experimental approaches74. However, some indirect evidence comes from visual fish counts in coastal marine protected areas24,75.
By focusing on two groups of hauls that differ greatly in terms of fishing pressure and separating the effects of bottom trawling from the other variables, we were able to show that this fishing activity has a consistent negative effect at the level of individual hauls (ENS) and across hauls (LCBD) in each subregion. Although not always statistically significant, median α and β index values were lower in more heavily trawled hauls than in less heavily fished hauls. Similar results have been reported from the North Sea, where the direct and indirect effects of fishing were negative on biomass, species richness76,77and Rényi entropy78. In addition, K dominance curves used in other studies have shown that intensively fished areas are dominated by fewer species79 and suggest analogy with our findings.
To date, the behavior of β-diversity indices in relation to anthropogenic stressors such as fishing and at the scales studied here has been little investigated. Only recently have authors demonstrated higher values of a directional β-diversity index between groups associated with site heterogeneity, particularly invertebrate composition, due to the high cumulative human impacts near the Spanish coast44.
We found that hauls with a lower influence of bottom trawling have higher values of β-diversity (higher LCBD), i.e., these hauls are more unique and heterogeneous. Although high heterogeneity has been linked to low diversity in other systems44in our case, the heterogeneity characterizing the group of hauls less affected by bottom trawling is not ecologically negative. In fact, the indicative species (INDVAL) analysis shows a higher biomass of important commercial species such as hake, pandora, and red mullet in less impacted haul group.
It is widely recognized that bottom trawling reduces the biomass of demersal species by negatively affecting the abundance and size of species19,33,77 and could promote a prey release80. Small pelagic fish and cephalopods may have surged in abundance due to a reduction in predation pressure by resulting as indicative species in the higher trawled hauls.
Our results indicate that sensitive species such as rays and sharks, although with low absolute biomass, are more likely to occur in less trawled hauls. In the North Sea, the impact of fishing was limited to inducing decreases in the abundance of species that have slow life histories and are particularly vulnerable to exploitation81. This suggests that also changes in the biomass of vulnerable indicator species may also provide a measure of the impact of fishing.
Several studies carried out in the Mediterranean Sea have investigated the distribution of α-diversity measures to determine spatial variation of biodiversity82. In this study we found that α-diversity is significantly affected by environmental variables and fisheries although it was difficult to define common relationships between α-diversity and OVs across subregions. In particular, the ENS as a measure of α-diversity has shown relationship with temperature and oxygen in all subregions analyzed. However, the explained deviance of models relating ENS to OVs and effort was very low in some cases thus suggesting very low predictive capacities.
Conversely, models relating β-diversity and covariates were performing better in terms of explained deviance (around 50%). The β-diversity index here used reflects community composition relative to a sub-regional average and resulted very useful in identifying how unique places are related to covariates especially to bottom trawling.
Overall, both α- and β-diversity resulted higher in less disturbed areas, possibly indicating where communities are more stable and resilient83. Our findings evidence the prominent negative role of bottom trawling with respect to other fishing activities in driving biodiversity of demersal communities. Pollution84nutrient enrichment85 and invasive species86 have been shown to have detrimental effects on species richness and biodiversity, especially near the coasts. Although further studies need to explore the potential role of these factors and other stressors once large dataset became available and more investigations must be done to develop a clear reference value, the characterization of α- and β-diversity in relation to covariates at the large scales here used represents a step towards the development of conservation and management strategies, particularly in the context of the Marine Strategy.
Data availability
The datasets supporting this article are within the paper or have been uploaded as part of the Supplementary Material. Raw MEDITS data can be found at the Joint Research Centre Data Catalogue (https://data.jrc.ec.europa.eu/dataset/f25092c4-3f0f-449f-ba60-5fbfe385defc).
References
Cardinale, B. J. et al. Biodiversity loss and its impact on humanity. Nature 2012 486:7401 486, 59–67 (2012).
Thrush, S. F., Ellingsen, K. E. & Davis, K. Implications of fisheries impacts to seabed biodiversity and ecosystem-based management. ICES J. Mar. Sci. 73, i44–i50 (2016).
Ulanowicz, R. E. Biodiversity, functional redundancy and system stability: subtle connections. J. R Soc. Interface. 15, 20180367 (2018).
Moreno-Mateos, D. et al. The long-term restoration of ecosystem complexity. Nature Ecology & Evolution 2020 4:5 4, 676–685 (2020).
Jackson, J., Kirby, M., Berger, W., Bjorndal, K. & Botsford, L. Historical overfishing and the recent collapse of coastal ecosystems. Sci. (1979). 293, 626–638 (2001).
Yachi, S. & Loreau, M. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl. Acad. Sci. U S A. 96, 1463–1468 (1999).
McCann, K. S. The diversity–stability debate. Nature 2000 405:6783 405, 228–233 (2000).
Partensky, F., Hess, W. R. & Vaulot, D. Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol. Mol. Biol. Rev. 63, 106–127 (1999).
Lyons, T. W., Reinhard, C. T. & Planavsky, N. J. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 2014 506:7488 506, 307–315 (2014).
Pepin, K. M. et al. Steps towards operationalizing one health approaches. One Health. 18, 100740 (2024).
Bayraktarov, E. et al. The cost and feasibility of marine coastal restoration. Ecol. Appl. 26, 1055–1074 (2016).
Costanza, R. Valuing natural capital and ecosystem services toward the goals of efficiency, fairness, and sustainability. Ecosyst. Serv. 43, 101096 (2020).
Halpern, B. S. et al. Recent Pace of change in human impact on the world’s ocean. Sci. Rep. 9, 1–8 (2019).
Micheli, F. et al. Cumulative human impacts on mediterranean and black sea marine ecosystems: assessing current pressures and opportunities. PLoS One. 8, e79889 (2013).
O’Hara, C. C. et al. Cumulative human impacts on global marine fauna highlight risk to biological and functional diversity. PLoS One. 19, e0309788 (2024).
Vargas-Fonseca, O. A., Frazier, M., Lombard, A. T. & Halpern, B. S. Knowns and Unknowns in Future Human Pressures on the Ocean. Earths Future 12, e2024EF004559 (2024).
Puig, P. et al. Ploughing the deep sea floor. Nature 2012 489:7415 489, 286–289 (2012).
Bianchi, D., Carozza, D. A., Galbraith, E. D., Guiet, J. & Devries, T. Estimating global biomass and biogeochemical cycling of marine fish with and without fishing. Sci. Adv. 7 (2021). https://www.science.org
Bianchi, G. et al. Academic Press,. Impact of fishing on size composition and diversity of demersal fish communities. in ICES Journal of Marine Science vol. 57 558–571 (2000).
De Juan, S., Thrush, S. F. & Demestre, M. Functional changes as indicators of trawling disturbance on a benthic community located in a fishing ground (NW mediterranean Sea). Mar. Ecol. Prog Ser. 334, 117–129 (2007).
McCauley, D. J. et al. Marine defaunation: animal loss in the global ocean. Sci. (1979). 347, 247–254 (2015).
Fortibuoni, T., Libralato, S., Raicevich, S., Giovanardi, O. & Solidoro, C. Coding early naturalists’ accounts into Long-Term fish community changes in the Adriatic sea (1800–2000). PLoS One. 5, e15502 (2010).
Agnetta, D. et al. Sizing up the role of predators on Mullus barbatus populations in mediterranean trawl and no-trawl areas. Fish. Res. 213, 196–203 (2019).
Edgar, G. J. et al. Global conservation outcomes depend on marine protected areas with five key features. Nature 2014 506:7487 506, 216–220 (2014).
Pauly, D., Christensen, V., Dalsgaard, J., Froese, R. & Jr, F. T. Fishing down marine food webs. Sci. (1979). 279, 860–863 (1998).
Sala, E. The past and present topology and structure of Mediterranean subtidal rocky-shore food webs. Ecosystems vol. 7 333–340 Preprint at (2004). https://doi.org/10.1007/s10021-003-0241-x
Estes, J. A. et al. Trophic downgrading of planet Earth. Sci. (1979). 333, 301–306 (2011).
Agnetta, D. et al. Erosion of fish trophic position: an indirect effect of fishing on food webs elucidated by stable isotopes. Phil Trans. R Soc. 379, 20230167 (2024).
Ellingsen, K. E. et al. The role of a dominant predator in shaping biodiversity over space and time in a marine ecosystem. J. Anim. Ecol. 84, 1242–1252 (2015).
Bianchi, C. N. & Morri, C. Marine biodiversity of the mediterranean sea: situation, problems and prospects for future research. Mar. Pollut Bull. 40, 367–376 (2000).
Giorgi, F. Climate change hot-spots. Geophys. Res. Lett. 33, 8707 (2006).
Piroddi, C., Coll, M., Steenbeek, J., Moy, D. M. & Christensen, V. Modelling the mediterranean marine ecosystem as a whole: addressing the challenge of complexity. Mar. Ecol. Prog Ser. 533, 47–65 (2015).
Agnetta, D. et al. Interactive effects of fishing effort reduction and climate change in a central mediterranean fishing area: insights from bio-economic indices derived from a dynamic food-web model. Front. Mar. Sci. 9, 1–16 (2022).
Bevilacqua, S., Plicanti, A., Sandulli, R. & Terlizzi, A. Measuring more of β-diversity: quantifying patterns of variation in assemblage heterogeneity. An insight from marine benthic assemblages. Ecol. Indic. 18, 140–148 (2012).
Guidetti, P. et al. Large-Scale assessment of mediterranean marine protected areas effects on fish assemblages. PLoS One. 9, e91841 (2014).
Ben Rais Lasram, F., Guilhaumon, F. & Mouillot, D. Fish diversity patterns in the mediterranean sea: deviations from a mid-domain model. Mar. Ecol. Prog Ser. 376, 253–267 (2009).
Mouillot, D. et al. Protected and threatened components of fish biodiversity in the mediterranean sea. Curr. Biol. 21, 1044–1050 (2011).
Coll, M. et al. The mediterranean sea under siege: Spatial overlap between marine biodiversity, cumulative threats and marine reserves. Glob. Ecol. Biogeogr. 21, 465–480 (2012).
Coll, M. et al. The biodiversity of the mediterranean sea: estimates, patterns, and threats. PLoS ONE. 5 https://doi.org/10.1371/journal.pone.0011842 (2010). e11842 Preprint at.
Piroddi, C. et al. Modelling the Mediterranean Sea ecosystem at high spatial resolution to inform the ecosystem-based management in the region. Scientific Reports 2022 12:1 12, 1–12 (2022).
Spedicato, M. T. et al. The MEDITS trawl survey specifications in an ecosystem approach to fishery management. Sci. Mar. 83, 9–20 (2019).
Granger, V. et al. Large-scale spatio-temporal monitoring highlights hotspots of demersal fish diversity in the mediterranean sea. Prog Oceanogr. 130, 65–74 (2015).
Carlucci, R. et al. Exploring spatio-temporal changes in the demersal and benthopelagic assemblages of the Northwestern ionian sea (central mediterranean sea). Mar. Ecol. Prog Ser. 598, 1–19 (2018).
Pennino, M. G. et al. Spatial patterns of β-diversity under cumulative pressures in the Western mediterranean sea. Mar. Environ. Res. 195, 106347 (2024).
Jost, L. Entropy and diversity. Oikos 113, 363–375 (2006).
Legendre, P. & De Cáceres, M. Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecol. Lett. 16, 951–963 (2013).
Legendre, P. Studying beta diversity: ecological variation partitioning by multiple regression and canonical analysis. J. Plant. Ecol. 1, 3–8 (2008).
Anderson, M. J. et al. Navigating the multiple meanings of β diversity: A roadmap for the practicing ecologist. Ecol. Lett. 14, 19–28 (2011).
Gačić, M., Lascaratos, A., Manca, B. B. & Mantziafou, A. Adriatic deep water and interaction with the Eastern mediterranean sea. Phys. Oceanogr. Adriatic Sea. 111–142. https://doi.org/10.1007/978-94-015-9819-4_4 (2001).
Colloca, F., Scarcella, G. & Libralato, S. Recent trends and impacts of fisheries exploitation on mediterranean stocks and ecosystems. Front. Mar. Sci. 4, 267643 (2017).
Malanotte-Rizzoli, P. et al. A synthesis of the ionian sea hydrography, circulation and water mass pathways during POEM-Phase I. Prog Oceanogr. 39, 153–204 (1997).
Capezzuto, F. et al. The bathyal benthopelagic fauna in the north-western ionian sea: structure, patterns and interactions. Chem. Ecol. 26, 199–217 (2010).
Béranger, K. et al. The dynamics of the Sicily strait: a comprehensive study from observations and models. Deep Sea Res. Part II. 51, 411–440 (2004).
Di Lorenzo, M., Sinerchia, M. & Colloca, F. The North sector of the Strait of sicily: a priority area for conservation in the mediterranean sea. Hydrobiologia 821, 235–253 (2018).
Civile, D. et al. The lost adventure Archipelago (Sicilian channel, mediterranean Sea): Morpho-bathymetry and Late Quaternary palaeogeographic evolution. Glob Planet. Change. 125, 36–47 (2015).
Millot, C. Circulation in the Western mediterranean sea. J. Mar. Syst. 20, 423–442 (1999).
Budillon, F. et al. The Bulgheria canyon-fan: A small-scale proximal system in the Eastern tyrrhenian sea (Italy). Mar. Geophys. Res. 32, 83–97 (2011).
Bo, M. et al. Characteristics of the mesophotic megabenthic assemblages of the Vercelli seamount (North tyrrhenian Sea). PLoS One. 6, e16357 (2011).
Carpi, P., Scarcella, G. & Cardinale, M. The saga of the management of fisheries in the Adriatic sea: history, flaws, difficulties, and successes toward the application of the common fisheries policy in the mediterranean. Front. Mar. Sci. 4, 267604 (2017).
Hatton, I. A., Heneghan, R. F., Bar-On, Y. M. & Galbraith, E. D. The global ocean size spectrum from bacteria to whales. Sci. Adv. 7, 3732 (2021).
Montefalcone, M., Tunesi, L. & Ouerghi, A. A review of the classification systems for marine benthic habitats and the new updated Barcelona convention classification for the mediterranean. Mar. Environ. Res. 169, 105387 (2021).
Cossarini, G. et al. High-Resolution reanalysis of the mediterranean sea biogeochemistry (1999–2019). Front. Mar. Sci. 8, 741486 (2021).
Zuur, A. F., Ieno, E. N. & Smith, G. M. Analysing Ecological Data (Springer New York, 2007). https://doi.org/10.1007/978-0-387-45972-1
Johnson, R. A., Verrill, S. & Moore, D. H. Two-Sample rank tests for detecting changes that occur in a small proportion of the treated population. Biometrics 43, 641 (1987).
Dufreˆne, M., Dufreˆne, D., Legendre, P. & SPECIES ASSEMBLAGES AND INDICATOR SPECIES: THE NEED FOR A FLEXIBLE ASYMMETRICAL APPROACH. Ecol. Monogr. 67, 345–366 (1997).
R Core Team. R Core Team. version 4.3.1. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (2023). https://www.r-project.org/ (2023).
Posit Team. Posit Team (2023). Rstudio 2023.06.1 Build 524: Integrated Development Environment for R. Posit Software (PBC, 2023). https://posit.co/
Pérès, J. M. & Picard, J. Nouveau Manuel De Bionomie Benthique De La Mer Mediterranéevol. 31 (Recueil des Travaux de la Station Marine d’Endoume,, 1964). 47Marsille (France).
Katsanevakis, S. et al. Spatiotemporal distribution and habitat use of commercial demersal species in the Eastern mediterranean sea. Fish. Oceanogr. 18, 439–457 (2009).
Danovaro, R. et al. Deep-Sea biodiversity in the mediterranean sea: the known, the unknown, and the unknowable. PLoS One. 5, e11832 (2010).
Costello, M. J. & Chaudhary, C. Marine biodiversity, biogeography, Deep-Sea gradients, and conservation. Curr. Biol. 27, R511–R527 (2017).
Borgström, S., Bodin, Ö., Sandström, A. & Crona, B. Developing an analytical framework for assessing progress toward ecosystem-based management. Ambio 44, 357–369 (2015).
Lidström, S. & Johnson, A. F. Ecosystem-based fisheries management: A perspective on the critique and development of the concept. Fish Fish. 21, 216–222 (2020).
Baum, J. K. & Worm, B. Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol. 78, 699–714 (2009).
Blowes, S. A. et al. Mediterranean marine protected areas have higher biodiversity via increased evenness, not abundance. J. Appl. Ecol. 57, 578–589 (2020).
Hiddink, J. G. et al. Cumulative impacts of seabed trawl disturbance on benthic biomass, production, and species richness in different habitats. Can. J. Fish. Aquat. Sci. 63, 721–736 (2006).
Kaiser, M. J. Recent advances in understanding the environmental footprint of trawling on the seabed. Review 97, 755–762. https://doi.org/10.1139/cjz-2018-0248 (2019).
Rogers, S. I., Maxwell, D., Rijnsdorp, A. D., Damm, U. & Vanhee, W. Fishing effects in Northeast Atlantic shelf seas: patterns in fishing effort, diversity and community structure. IV. Can comparisons of species diversity be used to assess human impacts on demersal fish faunas? Fish. Res. 40, 135–152 (1999).
Greenstreet, S. P. R. & Hall, S. J. Fishing and the Ground-Fish assemblage structure in the North-Western North sea: an analysis of Long-Term and Spatial trends. J. Anim. Ecol. 65, 577 (1996).
Prugh, L. R. et al. The rise of the mesopredator. Bioscience 59, 779–791 (2009).
Jennings, S., Greenstreet, S. P. R. & Reynolds, J. D. Structural change in an exploited fish community: a consequence of differential fishing effects on species with contrasting life histories. J. Anim. Ecol. 68, 617–627 (1999).
Lasram, B. R., Hattab, F., Halouani, T. & Romdhane, G. Le loc’h, F. modeling of Beta diversity in Tunisian waters: predictions using generalized dissimilarity modeling and bioregionalisation using fuzzy clustering. PLoS One. 10, e0131728 (2015).
Hatton, I. A., Mazzarisi, O., Altieri, A. & Smerlak, M. Diversity begets stability: sublinear growth and competitive coexistence across ecosystems. Sci. (1979). 383, eadg8488 (2024).
Johnston, E. L. & Roberts, D. A. Contaminants reduce the richness and evenness of marine communities: A review and meta-analysis. Environ. Pollut. 157, 1745–1752 (2009).
Zhang, X. et al. Nutrient enrichment weakens aquatic ecosystem stability via reduced species asynchrony and species richness. Ecol. Indic. 175, 113588 (2025).
Katsanevakis, S., Tempera, F. & Teixeira, H. Mapping the impact of alien species on marine ecosystems: the mediterranean sea case study. Divers. Distrib. 22, 694–707 (2016).
Acknowledgements
TF and SR would like to thank the GES4SEAS project for supporting their research activities.
Author information
Authors and Affiliations
Contributions
DA, SR and SL conceptualization, DA wrote the original draft, DA and IV fromal analysis, all authors revised and edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics statement
The authors were responsible for correct and appropriate use of the data with regard to scientific ethics.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Agnetta, D., Vascotto, I., Panzeri, D. et al. Bottom trawling and environmental variables drive the biodiversity of mediterranean demersal assemblages. Sci Rep 15, 27188 (2025). https://doi.org/10.1038/s41598-025-12258-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12258-2