Abstract
The association between chronic kidney disease (CKD) and the risk of age-related macular degeneration (AMD) is unclear. Our study aimed to evaluate this relationship considering the potential impact of proteinuria. This retrospective cohort study used a large representative population sample from the Korean National Health Insurance Service database (2009–2019) of individuals who participated in a national health screening program in 2009. CKD was determined by estimated glomerular filtration rate (eGFR). Proteinuria was assessed using dipstick urinalysis. AMD was identified according to International Classification Disease, Tenth Revision, codes in claims data. The Cox regression hazards model was used to estimate the association between CKD and risk of AMD. Among 4,005,946 participants, 400,189 (10.0%) had CKD. There was no significant association between CKD and AMD, but a positive relationship was identified between proteinuria and AMD. In stratification analysis with age and sex, the risk of AMD was more evident in younger (< 65 years) than older individuals (P-interaction < 0.001) and in men than women (P-interaction < 0.001). A positive association between proteinuria and AMD risk was observed and was prominent in younger males.
Similar content being viewed by others
Introduction
The association between renal disease and blindness was first reported by Richard Bright in 1836,1 marking a significant milestone in our understanding of the intricate relationship between the kidney and the eye. Over the years, extensive research has revealed that these two organs share not only anatomical similarities, but also developmental, physiological, and pathogenic pathways2. Notably, the glomerulus, a vital component of the kidney, and the choroid, a crucial part of the eye, possess vascular networks that exhibit striking structural resemblance. Moreover, a range of oculo-renal syndromes, including von Hippel–Lindau syndrome and Alport syndrome, with genetic links has been identified, further highlighting the connection between these two organs3.
Chronic kidney disease (CKD) is a major public health problem increasing rapidly around the world, and much of this increasing burden is expected to occur in Asia4. CKD has been consistently linked to various eye disorders, including retinopathy, glaucoma, and cataract5. Age-related macular degeneration (AMD), an important cause of blindness6,7,8, has also been investigated in relation to CKD9,10. However, a meta-analysis of 12 observational studies investigating CKD and AMD yielded inconclusive results due to statistical and methodological heterogeneity11. In addition, results have been inconsistent among individual studies5,9,10,11,12.
Although current CKD diagnosis in clinical practice largely relies on reduced estimated glomerular filtration rate (eGFR), proteinuria per se is a marker of structural kidney damage even in patients with normal eGFRs. Patients with proteinuria possess a greater risk of progressive kidney failure compared to those without proteinuria13. Thus, the current CKD guideline includes not only eGFR-based criteria, but also albuminuria criteria14. Interestingly, Nitsch suggested proteinuria rather than eGFR as a main factor independently related to AMD15. Given that overt proteinuria indicates the presence of glomerular disease, which may share a pathology with AMD, an association between proteinuric kidney disease and AMD can be hypothesized. However, there is little information about the relationship between proteinuria and AMD except for a single case–control study.
Our study aimed to provide a comprehensive analysis of the relationship between CKD and AMD, considering the potential impact of proteinuria, ultimately seeking to improve the understanding of the interplay between these two conditions.
Methods
Study setting and data source
In Korea, the National Health Insurance Service (NHIS) provides mandatory universal medical insurance to around 97% of the population, while the remaining 3% are Medicaid beneficiaries. The NHIS maintains a comprehensive database that contains demographic information (like age, sex, disability, and income) and health claims data, such as clinical visit date, prescription, and diagnosis according to International Classification of Disease, Tenth Revision (ICD-10) codes based on medical bills for reimbursement16.
The biennial NHIS health screening program includes anthropometric measurements (such as blood pressure and body mass index), laboratory tests (such as fasting glucose and serum lipid levels), and a self-administered questionnaire on medical history and lifestyle behaviors (such as smoking, drinking, and physical activity)17. Fasting serum glucose, lipid levels, and hemoglobin were measured after an overnight fast. Urine samples were collected randomly during the early morning following an overnight fast. Detailed information on lifestyle was obtained by self-questionnaire.
Given its comprehensive linkage of demographic, mortality, health claims, and health screening data, the mentioned population-based nationwide NHIS database is widely used in epidemiological studies18.
Study population
This retrospective cohort study included 4,471,011 individuals ≥ 50 years of age who participated in a national health screening program in 2009. Subjects who had any missing data (n = 253,148), end-stage renal disease diagnosis at baseline (n = 10,673), or AMD diagnosis at baseline (n = 154,131) were excluded. We applied a one-year lag time to reduce the effect of reverse causality (n = 47,113). A total of 4,005,946 participants was included for the final analysis (Fig. 1).
The Institutional Review Board of Soongsil University approved this study (SSU-202007-HR-236-01), this study was conducted in accordance with the Declaration of Helsinki. Ethical approval was waived in this study by the Institutional Review Board of Soongsil University, Seoul, South Korea. Informed consent was waived by the Institutional Review Board of Soongsil University because the data in the NHIS were anonymized and de-identified.
Definitions of CKD and proteinuria
The NHIS had been using the Modification of Diet in Renal Disease (MDRD) equation for CKD screening19,20.
CKD was defined as eGFR < 60 mL/min/1.73 m2which measures the filtering efficiency of the kidneys.
Separately, proteinuria was assessed using dipstick urinalysis. The levels of proteinuria were categorized as negative (−), trace (±), 1+, 2+, 3+, and 4+, corresponding to urinary protein levels of undetectable, 10 mg/dL, 30 mg/dL, 100 mg/dL, 300 mg/dL, and 1000 mg/dL or greater, respectively.
Study outcomes and follow-up
The endpoints of the study were incident AMD. Newly diagnosed AMD was operationally defined based on claim records collected within 1 year before the health screening examination. AMD was identified based on ICD-10 code H35.3, in consideration of previous studies21,22,23. All participants were followed from the index date of the health screening examination in 2009 and were censored on the date of AMD occurrence, death, or at the end of the study period (December 31, 2019).
Covariates
In Korea, insurance premium levels are determined based on income. Household income was categorized based on the lowest 25 percentile of health insurance premium level. Comorbidities were defined based on the medical claims data before screening according to ICD-10 codes and relevant medication use (hypertension, I10–I13 and I15; diabetes mellitus, E11–E14; dyslipidemia, E78). The Charlson comorbidity index (CCI) was calculated based on the diagnosis code24. Body mass index was calculated as weight in kilograms divided by the square of height in meters. Smoking status was classified as non-smoker, ex-smoker, or current smoker. Alcohol consumption was categorized as none, mild (< 30 g/day), or heavy (≥ 30 g/day). Regular physical activity was defined as strenuous exercise performed at least three times a week for ≥ 20 min per session or moderate physical activity performed at least five times per week for > 30 min.
Statistical analysis
The comparison of baseline characteristics based on the presence of CKD and proteinuria was conducted using the chi-square test for categorical variables or the t test for continuous variables. The hazard ratio (HR) for AMD accompanying 95% CI was calculated using Cox regression analysis. We used Schoenfeld’s global test to test the proportional hazards assumption in the Cox proportional hazard models. We adjusted for multiple confounding factors, mainly the shared common risk factors for CKD and AMD9,11,25. Model 1 was unadjusted; model 2 was adjusted for age and sex; model 3 was further adjusted for income, smoking, alcohol consumption, regular physical activity, and BMI; and model 4 was further adjusted for hypertension, diabetes mellitus, dyslipidemia, and CCI. Kaplan–Meier curves were used to illustrate the cumulative incidence probabilities of AMD. Furthermore, to assess the potential effect modification by age, we examined sex, comorbidities, and BMI through stratified analysis. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). P < 0.05 was considered statistically significant.
Results
Baseline characteristics
Among the participants, 400,189 (10.0%) had CKD and were more likely to be older, female, non-smokers, and non-drinkers. They also had a greater prevalence of comorbid conditions such as hypertension, diabetes, and dyslipidemia, as well as higher CCI scores, compared to the non-CKD group (Table 1).
When comparing the groups with proteinuria to the group without proteinuria, the former groups tended to be older, with the exception of the 4 + proteinuria group, which comprised the youngest patients (mean ± standard deviation, 52.0 ± 8.6 years). The total proteinuria group also demonstrated a higher likelihood of unfavorable traits, such as current smoking and/or heavy drinking, and a higher prevalence of comorbidities. These findings were accompanied by elevated measurements of BMI, waist circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting glucose, lipid levels, and CCI scores, all of which increased as the severity of proteinuria increased (Table 2).
Risk of AMD according to CKD and proteinuria
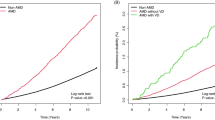
During the mean follow-up period of 8.6 years, 468,838 AMD events were reported. The CKD group was associated with a higher risk of AMD (unadjusted HR 1.26, 95% confidence interval [CI] 1.25–1.27) than the non-CKD group based on eGFR. However, the increased risk was attenuated after adjustment for confounding factors (Model 2, adjusted HR [aHR] 1.02, 95% CI 1.01–1.02; Model 3, aHR 1.01, 95% CI 1.00–1.02; Model 4, aHR 0.99, 95% CI 0.98–1.00). Notably, a positive relationship was identified between proteinuria and AMD (trace, aHR 1.01, 95% CI 1.00–1.03; 1+, aHR 1.04, 95% CI 1.02–1.06; 2+, aHR 1.13, 95% CI 1.10–1.16; 3+, aHR 1.17, 95% CI 1.10–1.23; and 4+, aHR 1.32, 95% CI 1.17–1.48) (Table 3; Fig. 2). Kaplan–Meier analysis also demonstrated a significant trend in the risk of AMD according to severity of proteinuria (Fig. 3).
Stratified analysis
When stratified by age, the risk of AMD in individuals with higher proteinuria was more prominent in younger individuals (< 65 years) than older individuals (P-interaction < 0.001). Also, the risk of AMD was more evident in men than women (P-interaction < 0.001). However, there was no significant difference in the relationship between proteinuria and the risk of AMD according to the presence of comorbidities (P-interaction = 0.1403) or obesity (P-interaction = 0.4475) (Table 4).
Discussion
The present study demonstrated a risk of AMD according to the CKD and proteinuria using a large population-based nationwide sample. There was an association between CKD and the risk of AMD with a fixed, narrow confidence interval, indicating highly accurate estimates. However, it became no longer significant after adjusting for multiple confounding factors, such as hypertension, diabetes, and dyslipidemia. Remarkably, a clear positive relationship according to the degree of proteinuria was noted even after adjusting for the shared common risk factors of CKD and AMD. When performing a stratified analysis, the association between proteinuria and AMD was more prominent among younger and male individuals.
There are several possible mechanisms that could explain our findings. First, epidemiological studies have demonstrated that the kidneys and eyes are genetically and epigenetically connected2,3. Since they share common developmental processes, the two exhibit an anatomical resemblance to glomerular-like vascular structures2 and likely will be similarly affected. For example, in dense deposit disease, also known as membranoproliferative glomerulonephritis (MPGN), which affects both the kidneys and eyes, immune system proteins attack and adhere to the glomerular basement membrane in thick patches called dense deposits. Over time, these deposits interfere with the kidney’s ability to filter fluids, and the individual experiences kidney failure. Also, deposits in the eye appear as drusen, which are a hallmark of AMD and result in breakdown of the blood–retinal barrier by interfering with the retinal pigment epithelium (RPE). Macular changes from drusen-like deposits with RPE alteration could facilitate the onset of choroidal neovascularization, an advanced form of AMD26,27. In addition, potentially underlying this renal–ocular association, mutations have been found in the complement factor H (CFH) gene. CFH is expressed in the RPE and is critical for normal retinal development28. Previous studies have revealed that its absence is linked to AMD29,30, and urinary CFH is an indicator of renal damage31,32. When renal function declines, mutated CFH is excreted into the urine and can cause AMD.
Second, there is a plausible hypothesis of common pathogenic pathways between CKD and AMD. For example, the renin–angiotensin–aldosterone system (RAAS), which regulates blood volume and systemic vascular resistance, is found in both the kidneys and various ocular tissues. Proteinuria is associated with activation of the RAAS33, which contracts glomerular afferent arterioles to aggravate renal ischemia and reduce glomerular filtration function34. Interestingly, a local RAAS and its component have been detected in many structures of the human eye, with possible roles in aqueous humor dynamics and intraocular pressure, as well as retinal vascular implications in concert with AMD in hypertension and diabetes35. RAAS hyperactivity promotes an inflammatory condition that causes macrophage infiltration resulting in ocular choroidal neovascularization, which is one of the most important characteristics of wet AMD36. In stratified analysis, the association between proteinuria and AMD risk was remarkable in younger individuals and men. AMD is an age-related disease with higher probability of occurrence in older than younger people. Thus, younger subjects with proteinuria might have a strong genetic predisposition or an association with systemic diseases of the kidney. The high AMD risk in young people is consistent with findings from a Taiwanese nationwide population-based study37. Interestingly, the onset age of cuticular drusen, which is a subtype marked by extracellular deposits in AMD and a well-known association with MPGN type 2, is generally younger than that for other age-related drusen38,39.
The effect of proteinuria on AMD risk was prominent in males. A previous study demonstrated that the relationship between proteinuria and AMD was prominent only in men but was limited by measurement errors in women15. Furthermore, several studies have suggested that AMD progression might follow different processes in women than men due to the protective effect of estrogen, which may lead to favorable alterations in serum lipid levels and may exert antioxidant properties40. Thus, further evaluation is required to enhance our understanding of the complex relationship between proteinuria and AMD development among the sexes.
While our study showed a clear positive correlation between proteinuria with AMD, we found no significant association between eGFR determined by creatinine measurement and AMD (Supplementary Table 1). This is consistent with previous studies that serum creatinine was not associated with AMD in the Korean population41 and another recent study of the ‘Asian Eye Epidemiology Consortium’ reporting that CKD defined by eGFR was associated with only late AMD, but not with early AMD42. This might be because eGFR is temporary and highly time-sensitive43. On the other hand, proteinuria reflects progressive renal injury and is a better and earlier marker of local kidney inflammation and increased complement production44.
Clinical implications
The finding that CKD could elevate the risk of AMD is important for the clinical management of patients with CKD. Strict management of common shared risk factors of CKD and AMD, such as hypertension, diabetes, and dyslipidemia, should be recommended. In addition, subjects who reported proteinuria in their health examination should be considered as having a risk of development of AMD, as well as the risk of renal disease. Since proteinuria is a modifiable risk factor of renal disease, the use of a renin–angiotensin system (RAS) inhibitor, such as an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, could help to protect patients from AMD. In addition, SGLT2 inhibitors45 and non-steroidal Mineralocorticoid Receptor antagonists46 can decrease proteinuria. Previous studies suggested47,48 that the utilization of RAS inhibitors could protect against AMD in hypertensive patients and retinal vascular diseases such as retinopathy in at-risk patients with diabetes. Further basic or translational research may reveal the pathophysiology and underlying mechanisms between proteinuria and AMD.
Study limitations
There are several potential limitations in this study. First, selection bias may be present because individuals who undergo health screening examinations tend to have healthier lifestyles than the general population. Second, AMD was defined based on the H35.3 ICD-10 diagnostic code using medical claims data, and there is a greater chance for a false-positive diagnosis compared to that of an ophthalmologic examination. H35.3 includes not only AMD, but also non-AMD such as macular cyst, hole and toxic maculopathy. The association between CKD and non-AMD included in H35.3 was not well established. Thus, the results might be underestimated because non-AMD unrelated to CKD were also included as study outcomes. Besides, AMD can be underdiagnosed due to patients not seeking healthcare services, which may lead to underestimation of the results. Furthermore, Dry and wet types of AMD could not be separated because the medical claim code did not include that distinction. Further analysis with distinction of dry and wet AMD is needed to demonstrate the clear impact of CKD on the development of AMD. Third, considering AMD is a degenerative disease, the duration of our study may not be sufficient to fully elucidate its association with CKD. Fourth, proteinuria was categorized based on the dipstick method, which can be influenced by various factors. For example, dehydration, exercise, and infection could cause false positives, while dilute urine and non-albumin proteins could cause false negatives49. Thus, careful consideration is required to interpret the results, and future studies using albumin creatinine ratios or 24-h urine albumin levels are warranted to validate the dose-dependent relationship. Fifth, the impact of other eye diseases was not considered. For example, cataract surgery might accelerate the progression of AMD, particularly for the Asians50. To date, cataract and glaucoma are not direct risk factors for the development of AMD51,52but there might be interfering effects since they share common risk factors, such as age, smoking and family history. In addition, AMD can be overdiagnosed due to patients seeking healthcare services for the other eye diseases. Also, there might be residual confounding variables, such as environmental and nutritional factors which was unavailable. Sixth, we could not consider the effect of RAS-related medication use, which might affect the relationship between proteinuria and AMD risk. Finally, this study could not determine causal inference due to its observational nature. Further mechanistic studies are needed to confirm our findings.
Conclusion
The present study findings enhance our understanding of the relationship between the eyes and kidneys. In young, male patients with persistent high-grade proteinuria, an ophthalmic examination could be helpful. Future translational and prospective research are needed to validate our results and determine the underlying mechanisms.
Data availability
The data that support the findings of this study are available from the Korean National Health Insurance Service (KNHIS) and were used under license for the current study (http://nhiss.nhis.or.kr). Restrictions apply to their availability (data are not publicly available). Data are available from the authors with permission from the KNHIS upon reasonable request to corresponding authors (Shin and Han).
References
Bright, R. Tubular view of the morbit appearance in 100 cases connected with albuminous urine with observations. Guy’s Hos Rep. 1, 380–400 (1836).
Farrah, T. E., Dhillon, B., Keane, P. A., Webb, D. J. & Dhaun, N. The eye, the kidney, and cardiovascular disease: old concepts, better tools, and new horizons. Kidney Int. 98, 323–342. https://doi.org/10.1016/j.kint.2020.01.039 (2020).
Izzedine, H., Bodaghi, B., Launay-Vacher, V. & Deray, G. Eye and kidney: from clinical findings to genetic explanations. J. Am. Soc. Nephrol. 14, 516–529. https://doi.org/10.1097/01.asn.0000051705.97966.ad (2003).
Liyanage, T. et al. Prevalence of chronic kidney disease in asia: a systematic review and analysis. BMJ Glob Health. 7 https://doi.org/10.1136/bmjgh-2021-007525 (2022).
Wong, C. W. et al. Increased Burden of Vision Impairment and Eye Diseases in Persons with Chronic Kidney Disease - A Population-Based Study. EBioMedicine 5, 193–197 (2016). https://doi.org/10.1016/j.ebiom.2016.01.023
Causes of blindness and vision impairment. In 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the global burden of disease study. Lancet Glob Health. 9, e144–e160. https://doi.org/10.1016/s2214-109x(20)30489-7 (2021).
Burton, M. J. et al. The lancet global health commission on global eye health: vision beyond 2020. Lancet Glob Health. 9, e489–e551. https://doi.org/10.1016/s2214-109x(20)30488-5 (2021).
Roth, F., Bindewald, A. & Holz, F. G. Keypathophysiologic pathways in age-related macular disease. Graefes Arch. Clin. Exp. Ophthalmol. 242, 710–716. https://doi.org/10.1007/s00417-004-0976-x (2004).
Liew, G., Mitchell, P., Wong, T. Y., Iyengar, S. K. & Wang, J. J. CKD increases the risk of age-related macular degeneration. J. Am. Soc. Nephrol. 19, 806–811. https://doi.org/10.1681/asn.2007080844 (2008).
Choi, J., Moon, J. W. & Shin, H. J. Chronic kidney disease, early age-related macular degeneration, and peripheral retinal Drusen. Ophthalmic Epidemiol. 18, 259–263. https://doi.org/10.3109/09286586.2011.602509 (2011).
Chen, Y. J. et al. Age-Related macular degeneration in chronic kidney disease: A Meta-Analysis of observational studies. Am. J. Nephrol. 48, 278–291. https://doi.org/10.1159/000493924 (2018).
Chong, E. W. et al. Is renal function associated with early age-related macular degeneration? Optom. Vis. Sci. 91, 860–864. https://doi.org/10.1097/opx.0000000000000288 (2014).
Hemmelgarn, B. R. et al. Relation between kidney function, proteinuria, and adverse outcomes. Jama 303, 423–429. https://doi.org/10.1001/jama.2010.39 (2010).
Andrassy, K. M. Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int. 84, 622–623. https://doi.org/10.1038/ki.2013.243 (2013).
Nitsch, D., Evans, J., Roderick, P. J., Smeeth, L. & Fletcher, A. E. Associations between chronic kidney disease and age-related macular degeneration. Ophthalmic Epidemiol. 16, 181–186. https://doi.org/10.1080/09286580902863064 (2009).
Cheol Seong, S. et al. Data resource profile: the National health information database of the National health insurance service in South Korea. Int. J. Epidemiol. 46, 799–800. https://doi.org/10.1093/ije/dyw253 (2017).
Shin, D. W., Cho, J., Park, J. H. & Cho, B. National general health screening program in korea: history, current status, and future direction. Precis Future Med. 6, 9–31. https://doi.org/10.23838/pfm.2021.00135 (2022).
Lee, Y. H., Han, K., Ko, S. H., Ko, K. S. & Lee, K. U. Data Analytic Process of a Nationwide Population-Based Study Using National Health Information Database Established by National Health Insurance Service. Diabetes Metab. J. 40, 79–82 (2016). https://doi.org/10.4093/dmj.2016.40.1.79.
Lamb, E. J., Tomson, C. R. & Roderick, P. J. Estimating kidney function in adults using formulae. Ann. Clin. Biochem. 42, 321–345. https://doi.org/10.1258/0004563054889936 (2005).
Choi, R., Lee, S. G. & Lee, E. H. Comparative analysis of seven equations for estimated glomerular filtration rate and their impact on chronic kidney disease categorization in Korean patients at local clinics and hospitals. J. Clin. Med. 13 https://doi.org/10.3390/jcm13071945 (2024).
Schuster, A. K. et al. Epidemiology of diagnosed Age-related macular degeneration in germany: an evaluation of the prevalence using AOK PLUS claims data. Ophthalmol. Ther. 13, 1025–1039. https://doi.org/10.1007/s40123-024-00901-6 (2024).
Lehrer, S. & Rheinstein, P. H. Cannabis smoking and age-related macular degeneration in the UK biobank cohort. J. Fr. Ophtalmol. 45, 756–761. https://doi.org/10.1016/j.jfo.2022.01.004 (2022).
Yoon, B., Sa, H. S. & Kim, H. J. Incidence and risk factors of age-related macular degeneration in patients with parkinson’s disease: a population-based study. Front. Aging Neurosci. 16, 1331786. https://doi.org/10.3389/fnagi.2024.1331786 (2024).
Kim, K. H. [Comparative study on three algorithms of the ICD-10 Charlson comorbidity index with myocardial infarction patients]. J. Prev. Med. Public. Health. 43, 42–49. https://doi.org/10.3961/jpmph.2010.43.1.42 (2010).
Jung, W. et al. Increased end-stage renal disease risk in age-related macular degeneration: a nationwide cohort study with 10-year follow-up. Sci. Rep. 13, 183. https://doi.org/10.1038/s41598-022-26964-8 (2023).
McAvoy, C. E. & Silvestri, G. Retinal changes associated with type 2 glomerulonephritis. Eye (Lond). 19, 985–989. https://doi.org/10.1038/sj.eye.6701697 (2005).
Mansour, A. M. et al. Retinal findings in membranoproliferative glomerulonephritis. Am. J. Ophthalmol. Case Rep. 7, 83–90. https://doi.org/10.1016/j.ajoc.2017.06.011 (2017).
Sivapathasuntharam, C. et al. Complement factor H regulates retinal development and its absence May Establish a footprint for age related macular degeneration. Sci. Rep. 9, 1082. https://doi.org/10.1038/s41598-018-37673-6 (2019).
Armento, A., Ueffing, M. & Clark, S. J. The complement system in age-related macular degeneration. Cell. Mol. Life Sci. 78, 4487–4505. https://doi.org/10.1007/s00018-021-03796-9 (2021).
Nozaki, M. et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. U S A. 103, 2328–2333. https://doi.org/10.1073/pnas.0408835103 (2006).
Liu, M. et al. Implication of urinary complement factor H in the progression of Immunoglobulin A nephropathy. PLoS One. 10, e0126812. https://doi.org/10.1371/journal.pone.0126812 (2015).
Tamano, M. et al. Urinary complement factor H in renal disease. Nephron 92, 705–707. https://doi.org/10.1159/000064090 (2002).
Park, J. et al. Associations between kidney function, proteinuria, and the risk of kidney cancer: A nationwide cohort study involving 10 million participants. Am. J. Epidemiol. 190, 2042–2052. https://doi.org/10.1093/aje/kwab140 (2021).
Ma, K., Gao, W., Xu, H., Liang, W. & Ma, G. Role and mechanism of the Renin-Angiotensin-Aldosterone system in the onset and development of cardiorenal syndrome. J. Renin Angiotensin Aldosterone Syst. 2022 (3239057). https://doi.org/10.1155/2022/3239057 (2022).
Holappa, M., Vapaatalo, H. & Vaajanen, A. Local ocular renin-angiotensin-aldosterone system: any connection with intraocular pressure? A comprehensive review. Ann. Med. 52, 191–206. https://doi.org/10.1080/07853890.2020.1758341 (2020).
Wang, T. et al. Hypertension affects the treatment of wet age-related macular degeneration. Acta Ophthalmol. 99, 871–876. https://doi.org/10.1111/aos.14791 (2021).
Chen, C. Y. et al. Association between macular degeneration and mild to moderate chronic kidney disease: A nationwide population-based study. Med. (Baltim). 96, e6405. https://doi.org/10.1097/md.0000000000006405 (2017).
Boon, C. J. et al. Basal laminar Drusen caused by compound heterozygous variants in the CFH gene. Am. J. Hum. Genet. 82, 516–523. https://doi.org/10.1016/j.ajhg.2007.11.007 (2008).
van de Ven, J. P. et al. Clinical evaluation of 3 families with basal laminar Drusen caused by novel mutations in the complement factor H gene. Arch. Ophthalmol. 130, 1038–1047. https://doi.org/10.1001/archophthalmol.2012.265 (2012).
Heesterbeek, T. J., Lorés-Motta, L., Hoyng, C. B. & Lechanteur, Y. T. E. Den hollander, A. I. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 40, 140–170. https://doi.org/10.1111/opo.12675 (2020).
Park, S. J. et al. Age-related macular degeneration: prevalence and risk factors from Korean National health and nutrition examination survey, 2008 through 2011. Ophthalmology 121, 1756–1765. https://doi.org/10.1016/j.ophtha.2014.03.022 (2014).
Xue, C. C. et al. Is kidney function associated with Age-Related macular degeneration?? Findings from the Asian eye epidemiology consortium. Ophthalmology 131, 692–699. https://doi.org/10.1016/j.ophtha.2023.12.030 (2024).
Hirst, J. A. et al. Change in glomerular filtration rate over time in the Oxford renal cohort study: observational study. Br. J. Gen. Pract. 72, e261–e268. https://doi.org/10.3399/bjgp.2021.0477 (2022).
Zandi-Nejad, K., Eddy, A. A., Glassock, R. J. & Brenner, B. M. Why is proteinuria an ominous biomarker of progressive kidney disease? Kidney Int Suppl, S76-89 (2004). https://doi.org/10.1111/j.1523-1755.2004.09220.x
Kalay, Z. et al. SGLT-2 inhibitors in nephrotic-range proteinuria: emerging clinical evidence. Clin. Kidney J. 16, 52–60. https://doi.org/10.1093/ckj/sfac189 (2023).
Currie, G. et al. Effect of mineralocorticoid receptor antagonists on proteinuria and progression of chronic kidney disease: a systematic review and meta-analysis. BMC Nephrol. 17, 127. https://doi.org/10.1186/s12882-016-0337-0 (2016).
Fletcher, E. L., Phipps, J. A., Ward, M. M., Vessey, K. A. & Wilkinson-Berka, J. L. The renin-angiotensin system in retinal health and disease: its influence on neurons, glia and the vasculature. Prog Retin Eye Res. 29, 284–311. https://doi.org/10.1016/j.preteyeres.2010.03.003 (2010).
Ren, C., Liu, W., Yin, X., Zhang, B. & Lu, P. Renin-Angiotensin System Inhibitor Usage and Age-Related Macular Degeneration among Hypertensive Patients: Results from the National Health and Nutrition Examination Survey, 2005–2008. J Ophthalmol 4252031 (2020). (2020). https://doi.org/10.1155/2020/4252031
Kojima, C., Umemura, H., Shimosawa, T. & Nakayama, T. Sex differences in the evaluation of proteinuria using the urine dipstick test. Front. Med. (Lausanne). 10, 1148698. https://doi.org/10.3389/fmed.2023.1148698 (2023).
Liu, Y. & Cai, Q. Does Cataract Surgery Improve the Progression of Age-Related Macular Degeneration? A Meta-Analysis. J Ophthalmol 7863987 (2020). (2020). https://doi.org/10.1155/2020/7863987
Seo, J. H. & Lee, Y. Causal associations of Glaucoma and Age-Related macular degeneration with cataract: A bidirectional Two-Sample Mendelian randomisation study. Genes (Basel). 15. https://doi.org/10.3390/genes15040413 (2024).
Martínez-Velasco, A., Miralles-Pechuán, M. V. L., Perez-Ortiz, L., Zenteno, A. C. & Estrada-Mena, J. C. The relevance of cataract as a risk factor for Age-Related macular degeneration: A machine learning approach. Appl. Sci. 9, 5550. https://doi.org/10.3390/app9245550 (2019).
Funding
This research was partially supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant no. HI20C1073).
Author information
Authors and Affiliations
Contributions
Conception and design: Park, Han, Kim, Lee, Jang, Yoon, Lim, Shin; Analysis and interpretation: Park, Han, Kim, Lee, Jang, Yoon, Lim, Shin; Data collection: Park, Han, Kim, Shin; Overall responsibility: Han, Shin.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The Institutional Review Board of Soongsil University approved this study (SSU-202007-HR-236-01), this study was conducted in accordance with the Declaration of Helsinki. Ethical approval was waived in this study by the Institutional Review Board of Soongsil University, Seoul, South Korea. Informed consent was waived by the Institutional Review Board of Soongsil University because the data in the NHIS-NSC were anonymized and de-identified.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, J., Han, K., Kim, B. et al. Risk of age-related macular degeneration according to the chronic kidney disease and proteinuria in Korea: a 10-year nationwide cohort study. Sci Rep 15, 26595 (2025). https://doi.org/10.1038/s41598-025-12297-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12297-9