Abstract
Utilize Quantitative Computed Tomography (QCT) to acquire quantitative parameters of bone, muscle, and adipose tissue in subjects, and subsequently investigate the disparities in these parameters based on classifications of Bone Mineral Density (BMD) and gender. Employ the average values of various parameters at the age of 30 as the baseline to compute the growth rate every five years and analyze the variations in these parameters of different tissues with respect to age and gender. Collect subjects who underwent combined chest CT and QCT scans at our hospital. Following parameters were obtained: volumetric bone mineral density (vBMD) of T12-L2 vertebrae, fat fractions (FF) of liver, pancreas, and paraspinal muscles, the content (including mass and area) of cortical mineral, cancellous mineral, abdominal total adipose tissue, visceral adipose tissue, subcutaneous adipose tissue, splanchnic soft tissue (excluding visceral adipose tissue), paraspinal intramuscular adipose tissue, paraspinal intramuscular muscle tissue at the central level of L2 vertebrae. Based on vBMD, the subjects were categorized into three groups: normal, osteopenia, and osteoporosis group. They were further subgrouped by BMD and gender to compare differences in various parameters. Taking the average values of these parameters at age 30 as the baseline, we calculated the growth rate every five years, observing the age-related changes in these parameters of different tissues in both males and females. Among males, no notable disparities were observed in the splanchnic soft tissue and paraspinal intramuscular muscle content across various vBMD categories, whereas other parameters exhibited significant variations in accordance with vBMD. In females, all parameters, with the exception of liver fat fraction, demonstrated significant changes across different vBMD categories. Males exhibit a higher liver FF, as well as greater content of total adipose tissue, visceral adipose tissue, splanchnic soft tissue, cortical mineral, cancellous mineral, and paraspinal intramuscular muscle compared to females. Conversely, females demonstrate higher muscle FF, paraspinal intramuscular adipose tissue, and subcutaneous adipose tissue content than males. In age-related changes, there is an increase in FF of the liver, pancreas, and paraspinal muscles, accompanied by an augmentation in the content of total adipose tissue, visceral adipose tissue, and paraspinal intramuscular adipose tissue. Conversely, there is a decline in BMD, cortical and trabecular content, as well as in the content of paraspinal intramuscular muscle tissue. The difference lies in the reduction of subcutaneous fat content in males, as opposed to an increase in females. The trend of variations in mass and area within the same tissue does not always align consistently. Furthermore, the content of both cortical and cancellous mineral decreased with age, but cortical mineral loss occurs earlier and faster than cancellous mineral. As vBMD diminishes, muscle content decreases concurrently with an augmentation in fat infiltration. In males, visceral fat infiltration prevails, whereas in females, both muscle loss and fat accumulation are pronounced. The depletion of cortical minerals may transpire earlier and at a quicker rate compared to the loss of cancellous minerals, indicating that clinical attention to osteoporosis cannot ignore changes in the cortical bone.
Similar content being viewed by others
Introduction
On a global scale, the issue of population aging is becoming increasingly prominent. Projections indicate that by 2030, the number of people aged 65 and above worldwide will surpass 1 billion1. China is among the countries experiencing rapid population aging. The health issues of its elderly population are placing a significant strain on society, the economy, and healthcare resources. The skeletal muscle system occupies a pivotal role in the human body2,3,4,5, with osteoporosis, sarcopenia, and an array of associated complications being particularly prevalent among the elderly population6,7. Osteoporosis is a condition marked by reduced bone density and quality, deterioration of bone microstructure, and heightened bone fragility. With the deepening of global population aging, there has been a notable rise in the prevalence of osteoporosis, with osteoporotic compression fractures, a significant complication, posing a grave threat to the health and wellbeing of older adults8. Sarcopenia refers to the loss of skeletal muscle mass and strength due to aging. The process of muscle loss often involves infiltration by adipose tissue, including both intracellular and intramuscular fats9. Osteosarcopenia refers to the concomitant occurrence of osteoporosis and sarcopenia, a geriatric syndrome characterized by the co-existence of these two chronic musculoskeletal conditions6. This concurrent degeneration of bone and muscle, characterized by fatty infiltration, significantly increases fragility fracture risk and adversely impacts functional mobility10,11. Therefore, it is both imperative and time-sensitive to employ straightforward approaches for rapid analysis of interconnections among bone, muscle, and adipose tissue, which is importance for early prevention and treatment of osteoporosis and its related complications in clinical practice.
Bone mineral density (BMD) stands as the most prevalently employed approach for evaluating vertebral osteoporosis at present. Quantitative Computed Tomography (QCT), on the other hand, is an authentic three - dimensional measurement technique. It has the remarkable advantage of measuring vertebral volumetric bone mineral density (vBMD). Furthermore, the post - processing software of QCT possesses a multi - dimensional measurement capacity. It can concurrently quantify the content of cortical mineral, trabecular mineral, abdominal adipose tissue, and muscle in any cross - sectional plane within the scanning scope7,12. Owing to these capabilities, QCT is recognized as a trustworthy method for appraising abdominal fat distribution and paraspinal muscle content13,14. Meanwhile, QCT post-processing software enables the measurement of fat fractions in the liver, pancreas, and paraspinal muscles through region-of-interest (ROI) delineation. This innovative feature synergizes with QCT’s core functionality to enable simultaneous acquisition of multiple quantitative tissue parameters—BMD, muscle attenuation, and adipose distribution—without necessitating additional radiation exposure. Such integrated assessment represents a paradigm shift in musculoskeletal research, offering a unified platform for concurrent evaluation of osteoporosis and sarcopenia pathophysiology.
This study aims to derive quantitative parameters of bone, muscle, and adipose tissues via QCT, and to evaluate variations in these parameters across different groups stratified by BMD, gender, and age—thereby providing comprehensive references for the early prevention and management of clinical osteoporosis. We hypothesize that with the decline in vBMD, bone and muscle content will decrease, whereas adipose tissue content will increase.
Methods
Participants
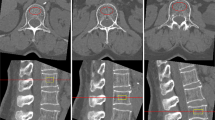
The data from participants who underwent a combined chest CT and QCT one-stop examination at our hospital between January 2020 and July 2024 were collected. Inclusion criteria were as follows: (1) Aged 30–80 years; (2) Absence of compression fractures at T12-L2 vertebral bodies, metal implants, or bone cement fillings; (3) No history of medications affecting bone metabolism; (4) No history of liver disease, kidney disease or type 2 diabetes. Exclusion criteria included: (1) Incomplete scanning of the T12-L2 vertebral bodies; (2) Severe image artifacts impairing quantitative measurements; (3) Deformities such as scoliosis; (4) Vertebral space-occupying lesions such as metastases and myeloma. Based on the inclusion and exclusion criteria, 966 participants were finally included in this study (shown in Fig. 1). This study was reviewed and approved by the Ethics Committee of Hebei Medical University Third Hospital (2023-058-1).
CT scans
All CT images were acquired using dual-energy CT (IQon, Philips Healthcare). The scanning parameters included a tube voltage of 120 kV, tube current of 66 mAs, slice thickness of 1.0 mm, matrix size of 512 × 512, and a field of view (FOV) 215 × 215 mm². The scan extended from the thoracic inlet to the lung base.
QCT post-processing analysis
vBMD measurements
Following image acquisition, chest CT datasets were transferred to a dedicated QCT post-processing workstation (QCTPro V6.1, Mindways Technologies) for vBMD analysis. The vBMD values of the T12 - L2 vertebrae were meticulously measured. Subsequently, the average of these values was computed to serve as the outcome. In accordance with the international diagnostic criteria for osteoporosis established by lumbar spine QCT, the subjects were classified into three distinct groups according to their vBMD values: normal bone mass (vBMD > 120 mg/cm³), osteopenia (vBMD ranging from 80 to 120 mg/cm³), and osteoporosis (vBMD < 80 mg/cm³). During the vBMD measurement, the ROI was positioned as centrally as feasible within the vertebral body. Care was taken to avoid the cortical bone and intervertebral venous areas, and the ROI area was kept between 3 and 5 cm². Throughout the entire study, a calibration phantom was employed for quality control purposes. The results demonstrated that the calibration was successfully accomplished. The raw vBMD data were then used for additional in - depth analysis.
Measurement of liver, pancreas, and paraspinal muscle fat fraction (FF)
ROI measurements were systematically performed in multiple anatomical locations. In the liver, ROIs were placed in the left lobe, right anterior lobe, and right posterior lobe while carefully avoiding major blood vessels and bile ducts. For pancreatic evaluation, ROIs were positioned at the head, body, and tail regions, steering clear of visible vascular structures and pancreatic ducts. Additionally, bilateral paraspinal muscle ROIs were obtained, with careful attention to avoid muscle edges. FF measurements were obtained from all ROIs.
Measurements of bone, muscle, and adipose tissue content
Tissue composition analysis was performed on the central cross-section of the L2 vertebral body using dedicated software. The analysis quantified the mass and area of the following components: bone tissue (including both cortical and cancellous mineral), total abdominal adipose tissue (TAT), visceral adipose tissue (VAT), paraspinal intramuscular adipose tissue (IMAT), paraspinal intramuscular muscle tissue (IMMT), and splanchnic soft tissue (excluding VAT). Additionally, subcutaneous adipose tissue (SAT) content was derived by subtracting VAT from TAT (SAT = TAT - VAT).
Statistical analysis
Statistical analyses were performed using SPSS 27.0 (IBM Corp., Armonk, NY, USA). Outliers were identified and excluded based on Z-score criteria (threshold: |Z| > 3). Due to unequal group sizes and non-normal distributions of variables, continuous data are presented as medians with interquartile ranges (IQR). For reliability assessment, imaging measurements of 100 randomly selected participants were independently evaluated by two radiologists with 3 years of clinical experience. One radiologist repeated all measurements twice within a two-week interval to assess intra-observer reliability. Intraclass correlation coefficients (ICC) were calculated to evaluate both inter- and intra-observer agreement. Participants were stratified by volumetric bone mineral density (vBMD) categories. Between-group comparisons of parameters were conducted using Kruskal-Wallis tests with post hoc Dunn’s tests, adjusted for multiple comparisons via Bonferroni correction. Sex-based differences were analyzed using Mann-Whitney U tests. Five-year growth rates of parameters were calculated using sex-specific values at age 30 as baseline references. Correlation analyses were performed using Spearman’s rank correlation coefficient to examine: (1) associations between BMD, muscle, and adipose tissue measures, and (2) relationships between mass and area measurements of the same tissue type. A two-tailed p-value < 0.05 was considered statistically significant for all analyses.
Results
Demographic characteristics of the participants
The study included 966 participants, comprising 478 males (median age: 57 years; IQR: 47–66) and 488 females (median age: 57 years; IQR: 48–65). Based on vBMD classification, 458 participants had normal bone density, 311 had osteopenia, and 197 were diagnosed with osteoporosis. Reproducibility assessments for vBMD, FF, and tissue composition measurements demonstrated good to excellent reliability, with ICC ranging from 0.698 to 0.983 (shown in Table 1).
Statistical comparisons across vBMD categories
All measured parameters in males demonstrated significant associations with vBMD categories, except for the mass and area of splanchnic soft tissue and paraspinal IMMT (shown in Table 2). Post hoc Dunn’s test with Bonferroni correction revealed significant differences in pancreatic FF, paraspinal muscle FF, cortical mineral mass, cortical mineral area, cancellous mineral mass, cancellous mineral area, and paraspinal IMAT mass across all pairwise group comparisons. The osteopenia group exhibited significantly greater values compared to the normal group for liver FF, VAT mass and VAT area. Both the osteopenia and osteoporosis groups demonstrated significantly increased paraspinal IMAT area compared to the normal group.
For females, all parameters showed significant differences across vBMD categories, with the exception of liver FF (shown in Table 3). Post hoc Dunn’s test with Bonferroni correction revealed significant differences in pancreatic FF, paraspinal muscle FF, VAT mass, VAT area, cortical mineral mass, cortical mineral area, cancellous mineral mass, cancellous mineral area, paraspinal IMAT mass, and paraspinal IMAT area across all pairwise group comparisons. In addition, both the osteopenia and osteoporosis groups exhibited significantly higher TAT mass, TAT area, SAT mass, and SAT area than the normal group. Conversely, the osteoporosis group had a significantly lower splanchnic soft tissue area compared to the normal group.
Gender-specific differences in tissue composition parameters
Statistical analysis revealed significant sex-based differences in nearly all measured parameters (shown in Table 4) with pancreatic FF being the sole exception (p = 0.226). Key gender disparities included male-dominant parameters (all p < 0.01): liver FF, mass/area of TAT, VAT, splanchnic soft tissue, cortical mineral, cancellous mineral and paraspinal IMMT and female-dominant parameters: mass/area of SAT and paraspinal IMAT.
Growth rate of the parameters at every 5 years
Based on the age-30 baseline averages, the 5-year growth rates of each parameter for males and females are shown in Tables 5 and 6. A consistent age-related pattern was observed, characterized by progressive increases in adipose tissue deposition accompanied by declines in the content of muscle and bone, with the notable exception of subcutaneous adipose tissue (SAT) which showed a distinct decreasing trajectory specifically in males.
Discussion
This study leveraged concurrent chest CT and QCT scans to opportunistically assess multiple body composition parameters, enabling a comprehensive analysis of the interrelationships between bone, muscle, and adipose tissue distribution. Specifically, we analyzed variations in fat fraction (liver, pancreas, paraspinal muscle) and tissue content (including cortical mineral, cancellous mineral, TAT, VAT, SAT, paraspinal IMAT, visceral soft tissue, and paraspinal IMMT. Our findings demonstrate that declining vBMD is associated with reduced bone mineral content, decreased visceral soft tissue and paraspinal intramuscular muscle mass, and a general increase in adipose tissue deposition across multiple anatomical regions. These observations align with and corroborate existing literature on metabolic and musculoskeletal changes related to bone mineral density loss2,10,12,15,16,17,18.
The influence of gender on tissue composition varies significantly across different anatomical regions. When categorized by vertebral trabecular bone mineral density (vBMD), distinct gender-specific patterns emerged. For males, no significant differences were observed in visceral soft tissue or paraspinal IMMT content. Instead, changes were primarily limited to increased VAT and paraspinal IMAT. For females, declining vBMD was associated not only with TAT, VAT, SAT, and paraspinal IMAT but also with a pronounced reduction in visceral soft tissue and paraspinal IMMT. These findings indicate that females undergo concurrent muscle degeneration and fat infiltration with decreasing vBMD, whereas males predominantly exhibit fat infiltration without significant muscle loss. When stratified by gender, nearly all measured parameters—except pancreatic fat fraction —exhibited significant differences, underscoring the critical role of gender in studying the interplay between bone, muscle, and adipose tissue. Our analysis revealed that males generally exhibit higher levels of bone mineral content, muscle content, and adipose tissue deposition compared to females. Notably, males demonstrated greater paraspinal IMMT but lower IMAT content than females. This suggests that females experience substantially higher degrees of fat infiltration in the paraspinal muscles, which may contribute to their increased susceptibility to fractures.
Our analysis reveals significant temporal variations in tissue composition changes across different age groups. To precisely characterize these changes, we established age 30 as the baseline reference point and systematically evaluated five-year growth rate in tissue parameters19. This approach allowed us to identify critical transition periods in body composition alterations. As demonstrated in Tables 5 and 6, bone and muscle tissue exhibit a progressive decline with advancing age, whereas adipose tissue shows a marked increase. Notably, the rate of positive change in adipose tissue appears more pronounced in females, suggesting that aging females may be particularly susceptible to accelerated fat infiltration. Further analysis reveals that TAT, VAT, and SAT content increase significantly with age in females. In contrast, among males, only SAT displays a significant age-dependent variation, with a paradoxical decrease observed over time. This pattern implies that visceral adipose accumulation predominates in aging males, while females experience concurrent visceral and subcutaneous adipose deposition. Interestingly, hepatic fat fraction remains relatively stable across age groups, implying that liver abnormalities may be driven primarily by non-age-related factors such as pharmacological agents, viral infections, or genetic predisposition.
Osteoporosis is globally defined and diagnosed using dual-energy X-ray absorptiometry (DXA), which remains the gold standard for assessing BMD. However, DXA has several well-documented limitations, including challenges in accounting for abdominal aortic calcifications overlapping the lumbar spine, the inherent two-dimensional (2D) projection of three-dimensional (3D) structures, interference from surrounding soft tissues, and limited predictive accuracy for individual fracture risk. Furthermore, studies have questioned the precision of DXA in evaluating localized vertebral BMD20,21. For instance, Ling et al. demonstrated that while DXA-derived areal BMD (aBMD) is influenced by confounding factors such as body weight, body mass index (BMI), and spinal thickness—potentially misleadingly suggesting that individuals with losing weight face an elevated risk of BMD loss—QCT-measured vBMD did not exhibit such biases22. Given these discrepancies, QCT has gained increasing recognition as a more reliable alternative for assessing vertebral vBMD23,24,25. Our study comprehensively highlights the clinical advantages of QCT in body composition assessment. Unlike conventional methods, QCT enables simultaneous evaluation of multiple critical indicators including bone density, muscle mass, adipose tissue distribution, and solid organ fat fraction (e.g., liver and pancreas) without exposing patients to additional radiation exposure. This comprehensive, multi-parametric approach provides clinicians with valuable imaging data to support personalized treatment strategies. Moreover, this technique offers significant benefits for patients. By comparing their body composition metrics with age-matched reference values and tracking longitudinal changes, individuals can gain actionable insights to guide targeted lifestyle modifications and preventive health measures.
While BMI is widely utilized as a population-level indicator of obesity, its accuracy in individual assessments remains limited. For instance, individuals with normal-range BMI values may still exhibit elevated abdominal adiposity, which would clinically classify them as obese despite their ostensibly normal BMI. Current research demonstrates only weak correlations between BMI and key body composition parameters, including skeletal muscle mass index and vBMD2,26. Furthermore, multiple studies have established that BMI serves as an unreliable predictor of both BMD and fracture risk17,22. In light of these well-documented limitations, our study protocol minimized consideration of BMI ranges during subject enrollment.
Current osteoporosis research has predominantly focused on trabecular bone, while cortical bone remodeling remains relatively understudied. Cortical bone serves as a critical structural component in maintaining vertebral morphology and mechanical strength. While QCT-derived vBMD primarily assesses trabecular bone, the current focus of osteoporosis evaluation, cortical bone analysis has been largely overlooked in clinical practice. Our study addresses this gap by examining both bone compartments. Through longitudinal assessment over five years, we observed that although both cortical and trabecular bone demonstrate age-related declines in both genders, cortical bone exhibits more pronounced deterioration. Specifically, cortical bone loss initiates at age 36 in males and females, whereas trabecular bone reduction begins later at age 51. These findings suggest that cortical bone deterioration precedes and progresses more rapidly than trabecular bone loss, corroborating our previous research on age-related bone changes27. Notably, to the best of our knowledge, this represents the first comprehensive investigation highlighting the significant contribution of vertebral cortical bone to osteoporosis pathogenesis. This novel perspective underscores the need for incorporating cortical bone assessment in clinical osteoporosis evaluation protocols.
The detection for mass of bone, muscle, and adipose tissue is another novelty. While previous investigations have relied on cross-sectional area (CSA) as a surrogate for tissue mass3,23,28, the advanced QCT post-processing software employed in our research enables direct measurement of both mass and area for each tissue type at any anatomical level. Through simultaneous assessment of mass and area within identical tissue regions, we observed that mass differences consistently paralleled area variations when stratified by vBMD. This consistent relationship persisted across gender-based classifications. Subsequent correlation analysis demonstrated strong linear associations between tissue mass and cross-sectional area, with correlation coefficients ranging from 0.711 to 0.994 in males and 0.775 to 0.991 in females. It substantiates the conventional approach of estimating tissue mass from cross-sectional area measurements. However, longitudinal analysis of 5-year growth rates revealed important discrepancies. In males, the mass and area of SAT and cancellous mineral showed divergent trends, as did cortical mineral mass and area in females. These findings indicate that tissue area and mass changes are not always synchronous29, suggesting that CSA-based mass estimation may occasionally yield inaccurate results, particularly in longitudinal assessments.
The musculoskeletal system exhibits complex interrelationships among bone, muscle, and adipose tissue. Emerging evidence demonstrates that bone loss is linked to alterations in muscle composition, abdominal adiposity, and bone marrow fat content10. Muscle activity stimulates osteogenic activity and promotes bone formation2,4. This relationship is particularly evident in the paraspinal musculature, which plays a vital role in maintaining spinal stability and function3. At the cellular level, Jin et al.5 elucidated the critical role of skeletal muscle satellite cells in osteoporotic fracture repair, mediated through the β-catenin signaling pathway5. Conversely, pathological fat infiltration represents a hallmark feature of muscle degeneration and functional impairment17. Clinical studies have consistently demonstrated a significant inverse correlation between the degree of paraspinal muscle fat infiltration and vertebral vBMD15,16, highlighting the intricate interplay between muscular degeneration and skeletal integrity. Our Spearman correlation analysis revealed significant negative correlations between vertebral vBMD and several fat-related parameters, including liver FF, pancreas FF, paraspinal muscle FF, as well as TAT, VAT, and SAT. Notably, bone marrow fat, as a relatively independent fat region, is equally important for the diagnosis and treatment of osteoporosis18,30. This finding aligns with the work of Ling et al.10, who employed MR chemical shift-encoded imaging to quantify vertebral bone marrow fat content and established its inverse relationship with vBMD10.
Recent systematic reviews indicate that the quadriceps, lumbar, and paraspinal muscles demonstrate the most pronounced age-related atrophy in individuals aged 25–75 years31. Lei et al. reported no significant association between psoas muscle mass and vertebral vBMD in postmenopausal women with type 2 diabetes mellitus29. In contrast, Zhou et al. conducted detailed analyses of fat infiltration in three paraspinal muscle groups (erector spinae, multifidus, and psoas), revealing that vBMD showed stronger correlations with the erector spinae and multifidus muscles than with the psoas muscle15, with the multifidus muscle demonstrating particularly pronounced fat infiltration and the strongest association with vBMD. These findings have been corroborated by studies16,24, suggesting that the anatomical position and unique fiber orientation of the multifidus muscle may contribute to its stronger relationship with vertebral BMD. Based on this evidence, we selected both the erector spinae and multifidus muscles for paraspinal muscle ROI analysis in our study. This approach is supported by several investigations: Li et al.17 similarly focused on these muscle groups when examining paraspinal muscle fat infiltration and vBMD correlations, while Zhao et al.9 explicitly recommended combining analysis of these muscles due to their similar relationship with vBMD and close anatomical proximity.
This investigation boasts several notable strengths, including a large sample size and comprehensive assessment of multiple bone, muscle, and adipose tissue parameters. Our analytical approach employed vBMD, gender and age as primary stratification criteria to systematically examine variations across these parameters. Nevertheless, several limitations should be acknowledged when interpreting our findings. Firstly, due to the limitations of retrospective studies, the factors of daily lifestyle of the participants, such as comorbidities, pharmacotherapies, drinking and smoking were not taken into account. A small number of patients with myosteatosis may also not be excluded. Secondly, our participant population was geographically restricted, limiting the generalizability of our findings across diverse ethnic groups and geographic regions.
Conclusion
The changes in body composition associated with decreased vBMD exhibit different gender specific patterns. In males, adipose tissue infiltration, particularly visceral adipose deposition, emerges as the predominant alteration. In contrast, females demonstrate a more complex phenotype characterized by widespread adipose infiltration across multiple depots, concurrent with progressive loss of both muscle and splanchnic soft tissue. Notably, these metabolic changes occur in parallel with cortical bone deterioration, highlighting the necessity of evaluating cortical bone in comprehensive osteoporosis management.
Data availability
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants but are available from the corresponding author JZ (E-mail: 37400408@hebmu.edu.cn) upon reasonable request.
References
Chen, L-K. Urbanization and population aging: converging trends of demographic transitions in modern world. Archives Gerontol. Geriatrics 101. (2022).
Li, Z., Chen, J., Yang, J., Wang, R. & Wang, W. Relationship between paraspinal muscle properties and bone mineral density based on QCT in patients with lumbar disc herniation. BMC Musculoskelet. Disorders 25(1). (2024).
Hida, T. et al. Effect of race, age, and gender on lumbar muscle volume and fat infiltration in the degenerative spine. J. Orthop. Sci. 26 (1), 69–74 (2021).
Li, Y., Liu, C., Lu, J., Sun, H. & Li, Y. Relationship between muscle and subcutaneous adipose tissue size and density and proximal femur bone in elderly women with hip fracture. Aging Clin. Experimental Research 36(1). (2024).
Jin, Z. et al. Role of skeletal muscle satellite cells in the repair of osteoporotic fractures mediated by β-catenin. J. Cachexia Sarcopenia Muscle. 13 (2), 1403–1417 (2022).
Clynes, M. A., Gregson, C. L., Bruyère, O., Cooper, C. & Dennison, E. M. Osteosarcopenia: where osteoporosis and sarcopenia collide. Rheumatology 60 (2), 529–537 (2021).
Tu, Y. et al. A preliminary study on degenerate characteristics of lumbar and abdominal muscles in middle-aged and elderly people with varying bone mass. BMC Musculoskelet. Disorders 24(1). (2023).
Iwata, S. et al. Osteoporosis, spinal degenerative disorders, and their association with low back pain, activities of daily living, and physical performance in a general population. Scientific Reports 14(1). (2024).
Zhao, Y. et al. Fatty infiltration of paraspinal muscles is associated with bone mineral density of the lumbar spine. Archives Osteoporosis 14(1). (2019).
Wang, L. et al. Greater bone marrow fat and myosteatosis are associated with lower vBMD but not asymptomatic vertebral fracture. Eur. Radiol. 33 (1), 578–586 (2022).
Gandham, A. et al. Falls, fractures, and areal bone mineral density in older adults with sarcopenic obesity: A systematic review and meta-analysis. Obesity Reviews 22(5). (2021).
Ji, R. et al. Correlation between musculoskeletal mass and perfusion in patients with Gastrointestinal malignancy: a preliminary study based on quantitative CT and CT perfusion. BMC Musculoskelet. Disorders 23(1). (2022).
Khil, E. K., Choi, J-A., Hwang, E., Sidek, S. & Choi, I. Paraspinal back muscles in asymptomatic volunteers: quantitative and qualitative analysis using computed tomography (CT) and magnetic resonance imaging (MRI). BMC Musculoskelet. Disorders 21(1). (2020).
Zhang, X. et al. Body compositions differently contribute to BMD in different age and gender: a pilot study by QCT. Archives Osteoporosis 14(1). (2019).
Zhou, S. et al. Associations between paraspinal muscles fatty infiltration and lumbar vertebral bone mineral density – An investigation by fast kVp switching dual-energy CT and QCT. European J. Radiol. Open 9. (2022).
Li, X. et al. Relationship between Oseteoporosis with fatty infiltration of paraspinal muscles based on QCT examination. J. Bone Miner. Metab. 40 (3), 518–527 (2022).
Li, X. et al. Correlation between bone mineral density (BMD) and paraspinal muscle fat infiltration based on QCT: A Cross-Sectional study. Calcif. Tissue Int. 110 (6), 666–673 (2022).
Xu, L. et al. Risk factors associated with bone marrow adiposity deposition in postmenopausal women in the CASH China study. Diabetes Metabolic Syndrome Obes. 16, 1167–1176 (2023).
Wu, W. et al. Reference values for paravertebral muscle size and myosteatosis in Chinese adults, a nationwide multicenter study. Acad. Radiol. 31 (7), 2887–2896 (2024).
Di, M. et al. Cortical endplate bone density measured by novel Phantomless quantitative computed tomography May predict cage subsidence more conveniently and accurately. Orthop. Surg. 15 (12), 3126–3135 (2023).
Maldonado, G. et al. Common errors in dual-energy X-ray absorptiometry scans in imaging centers in Ecuador. Archives Osteoporosis 15(1). (2020).
Wang, L. et al. Adjustment of DXA BMD measurements for anthropometric factors and its impact on the diagnosis of osteoporosis. Archives Osteoporosis 15(1). (2020).
Chiapparelli, E. et al. The association between lumbar paraspinal muscle functional cross-sectional area on MRI and regional volumetric bone mineral density measured by quantitative computed tomography. Osteoporos. Int. 33 (12), 2537–2545 (2022).
Peng, X. et al. Age-related fatty infiltration of lumbar paraspinal muscles: a normative reference database study in 516 Chinese females. Quant. Imaging Med. Surg. 10 (8), 1590–1601 (2020).
Li, C., Xu, B., Chen, M. & Zhang, Y. Evaluation for performance of body composition index based on quantitative computed tomography in the prediction of metabolic syndrome. Metab. Syndr. Relat. Disord. 22 (4), 287–294 (2024).
Kong, M. et al. Age-specific reference values for low Psoas muscle index at the L3 vertebra level in healthy populations: A multicenter study. Frontiers Nutrition 9. (2022).
Bai, L. et al. Cortex or cancellous—which is early for the decrease of bone content for vertebral body in health? Endocrine 78 (3), 597–604 (2022).
Liu, Z. et al. Quantitative study of vertebral body and paravertebral muscle degeneration based on Dual-Energy computed tomography: correlation with bone mineral density. J. Comput. Assist. Tomogr. 47 (1), 86–92 (2023).
Gao, L. et al. Relationship between body composition and bone mineral density in postmenopausal women with type 2 diabetes mellitus. BMC Musculoskelet. Disorders 23(1). (2022).
Imani, M. et al. Validation of a semiautomatic image analysis software for the quantification of musculoskeletal tissues. Calcif. Tissue Int. 110 (3), 294–302 (2021).
Naruse, M., Trappe, S. & Trappe, T. A. Human skeletal muscle-specific atrophy with aging: a comprehensive review. J. Appl. Physiol. 134 (4), 900–914 (2023).
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Jian Z provided the overall design ideas and specific implementation steps for the completion of the manuscript. All authors contributed to the study conception and design. Material preparation were performed by Wei Z and Xuesong Z, data collection was performed by Fan Y. Data analysis was performed by Junfei L and Ying L. The first draft of the manuscript was written by Jujia L. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Hebei Medical University Third Hospital (2023-058-1).
Informed consent
Due to the retrospective nature of the study, the Ethics Committee of Hebei Medical University Third Hospital waived the need of obtaining informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Zhang, W., Zhang, X. et al. Opportunistic assessment of variations in tissue composition content using chest QCT. Sci Rep 15, 27428 (2025). https://doi.org/10.1038/s41598-025-13001-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13001-7