Abstract
Cytokine-induced apoptosis inhibitor 1 (CIAPIN1) is a protein that regulates apoptosis and programmed cell death. This study aimed to assess the potential function of this molecule in curbing the proliferation, migration, and glycolysis of endometrial cancer cells and to elucidate its molecular mechanism. We obtained endometrial cancer tissue samples from our hospital to examine the CIAPIN1 expression levels. The human endometrial cancer cell line Ishikawa was transfected with CIAPIN1 siRNA. mRNA and protein expression were then measured using RT-qPCR and Western blotting techniques. CIAPIN1 expression was elevated in both endometrial cancer tissues and serum. CIAPIN1 overexpression enhances cell proliferation and migration, whereas CIAPIN1 silencing (siRNA) increases apoptosis. Mechanistically, CIAPIN1 inhibition elevated reactive oxygen species (ROS) production and oxidative stress, as evidenced by increased malondialdehyde (MDA) and reduced superoxide dismutase (SOD) and catalase (CAT) levels. Furthermore, CIAPIN1 promoted glycolysis via the PI3K/Akt pathway, increasing glucose consumption, lactate secretion, ATP production, and the expression of glycolytic enzymes (PKM2 and LDHA). PI3K inhibition (LY294002) reversed these effects, confirming the pathway dependency. These findings highlight CIAPIN1 as a potential oncogenic driver in EC, enhancing tumor progression through PI3K/Akt-mediated glycolysis and oxidative stress modulation. Targeting CIAPIN1 may represent a novel therapeutic strategy for EC.
Similar content being viewed by others
Introduction
Endometrial cancer (EC) is one of the most common gynecological malignancies, with increasing incidence and mortality rates1. It originates in the uterine lining and is the most prevalent cancer of the female reproductive system2. This type of cancer is often diagnosed in women who have undergone menopause, with the endometrium recognized as a site for cancer development3. In recent years, the incidence of EC has increased in both premenopausal and postmenopausal women. Despite this increase, little progress has been made in improving the 5-year overall survival rate of women with endometrial cancer over the past few decades4. Although there have been advancements in diagnostic and therapeutic strategies, the molecular mechanisms underlying EC progression remain inadequately understood, necessitating further research into the key regulatory molecules and signalling pathways.
CIAPIN1 (Cytokine-Induced Apoptosis Inhibitor 1), initially identified as an anti-apoptotic protein, has emerged as a critical player in various cancers, including gastric, colorectal, and hepatocellular carcinomas5,6. However, its role in EC has not been extensively explored. CIAPIN1 regulates cellular processes such as survival, proliferation, and migration, which are hallmarks of cancer progression7. Recent studies suggest that CIAPIN1 exerts its oncogenic effects through the PI3K/Akt pathway, a well-established signaling cascade implicated in tumorigenesis and metabolic reprogramming8. However, the expression, biological roles, and mechanisms of CIAPIN1 in EC remain unclear. However, its involvement in EC remains unknown.
The PI3K/Akt pathway is a central regulator of cell survival, growth, and metabolism, and its dysregulation is frequently observed in EC9. Activation of this pathway promotes glycolysis, a metabolic shift known as the “Warburg effect” which supports the rapid proliferation and survival of cancer cells, even under hypoxic conditions10. Glycolysis not only provides energy but also generates biosynthetic intermediates that are essential for tumor growth11. Given the critical role of the PI3K/Akt pathway in EC progression, understanding its interaction with CIAPIN1 may provide novel insights into the molecular mechanisms that drive this malignancy.
This study aimed to investigate the role of CIAPIN1 in EC, focusing on its effects on cell survival, proliferation, migration, and glycolysis. We hypothesized that CIAPIN1 promotes these oncogenic processes via the PI3K/Akt pathway, thereby contributing to EC progression. By elucidating the molecular mechanisms underlying CIAPIN1 function in EC, this study may identify potential therapeutic targets for improving patient outcomes. Our findings may pave the way for the development of targeted therapies that disrupt the CIAPIN1/PI3K/Akt axis, thereby offering new hope to patients with advanced or recurrent EC.
Materials and methods
Clinical specimens
We obtained 113 newly diagnosed endometrioid cancer cases, including paraffin-embedded tumor tissues and frozen serum samples, from the pathology archives of our hospital. Adjacent normal tissues (> 3 cm from the tumor lesion margin) were used as controls. Additionally, serum samples were collected from 60 healthy participants undergoing routine physical examinations. To assess CIAPIN1 mRNA and protein expression levels, we analyzed ten frozen paired tumor and adjacent normal tissue samples. All 123 patients with endometrial cancer had not previously received radiotherapy or chemotherapy when obtaining specimens. This study was approved by the Research Ethics Committee of Shanghai Pudong Hospital.
Collection of clinical data
Various clinical data were collected from 113 patients with paraffin-embedded tumor tissue, including patient age, menstrual status, tumor size, histological grade, depth of myometrial invasion, lymph node metastasis, and P53 and Ki-67 levels (Table 1). Tumor stage was determined according to the 2009 International Federation of Gynecology and Obstetrics (FIGO) guidelines12.
Histology and IHC analysis
Endometrial cancer and adjacent tissues were stained with hematoxylin and eosin (HE). Primary antibodies against CIAPIN1 (1:100; ab154904; rabbit polyclonal, reacts with: mouse, human, Abcam, UK) were used. An immunoreactivity score was applied using the following standards: (1) the proportion score was based on the proportion of tumor cells with positive cytoplasmic staining (0, none; 1, ≤ 10%; 2, 10 to ≤ 25%; 3, 26 to ≤ 50%; and 4, > 50%). (2) An intensity score was assigned to the average intensity of positive tumor cells (colourless scored 0, pallid scored 1, yellow scored 2, and brown 3)13. The total CIAPIN1 score is the product of the proportion and intensity scores, ranging from 0 to 12. The total immunoreactivity score was divided into low expression (score 1–6) and high expression (score 7–12).
Enzyme-linked immunosorbent assay (ELISA)
Serum was collected from 113 endometrioid cancer patients and 60 healthy controls. Serum CIAPIN1 levels (P9446; FineTest, Wuhan, China) were measured by ELISA. The concentrations are expressed in µg/mL.
Cell culture
The human endometrial cell lines HEC-1B, RL95-2, and Ishikawa were sourced from the American Type Tissue Culture Collection (ATCC; Rockville, MD, USA). The cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Hyclone, Beijing, China) and 1% streptomycin/penicillin at 37 °C with 5% CO2.
Cell transfection
Ishikawa cells in the logarithmic growth phase were seeded in 6-well plates (1.0 × 105 cells/well) until they reached 70–80% confluence. pcDNA3.1-CIAPIN1 (OE-CIAPIN1) or its negative control (pcDNA3.1 vector) and CIAPIN1 siRNA (si-CIAPIN1) or its negative control (si-NC) were introduced into Ishikawa cells using Lipofectamine 2000 (Invitrogen, Carlsbad, USA). siRNAs were purchased from GenePharma (Shanghai, China). Transfection efficiency was verified by RT-qPCR and Western blotting 48 h after transfection.
Cell grouping
Ishikawa cells were divided into the following groups: (1) control group (without any treatment), (2) si-NC group (transfected with siRNA negative control), (3) si-CIAPIN1 group (transfected with siRNA for CIAPIN1), (4) vector group (transfected with pcDNA3.1 vector), (5) OE-CIAPIN1 group (transfected with pcDNA3.1-CIAPIN1), (6) vector + LY group (simultaneously transfected with pcDNA3.1 and treated with 10 µM LY294002), and (7) OE-CIAPIN1 + LY group (simultaneously transfected with pcDNA3.1-CIAPIN1 and treated with 10 µM LY294002).
MTT assay
Endometrial cancer cells were seeded in 96-well plates at a density of 1 × 104 cells/well. MTT solution (1 mg/mL, M1020, Solarbio, Beijing, China) was added to the cells and incubated for 4 h at 37 °C. The resulting formazan crystals were dissolved in 100 µL of DMSO in each well. Finally, absorbance was measured at 570 nm using a microplate reader.
Cell migration assay
A Transwell assay was used to examine the migration potential of endometrial cancer cells. Cells were placed in Transwell chambers (8 μm, 1 × 105 cells/mL) containing 100 µL of DMEM without FBS. The lower chamber contained 600 µL of DMEM with 10% FBS. After a 72-hour incubation period, the cells in the upper chamber were removed using a cotton swab. The moving cells were stained with 0.1% crystal violet. To determine the average number of migrating cells, stained cells were counted in five different fields using a light microscope at 100x magnification.
Cell apoptosis
An Annexin V-FITC assay kit (CA1020, Solarbio, Beijing, China) was used to assess apoptotic cells. Endometrial cancer cells were cultured in 24-well plates at a density of 5 × 104 cells/well and treated for 48 h. Trypsin was used to digest the cells, which were then incubated with Annexin FITC and propidium iodide (PI) for 15 min at room temperature. Flow cytometry was performed to determine the rate of apoptosis.
Cellular Immunofluorescence
The cellular immunofluorescence assay was conducted as previously described14. Endometrial cancer cells underwent a 20-minute fixation with 4% paraformaldehyde, followed by a 15-minute treatment at room temperature (RT) with 0.5% Triton X-100. After a 2-hour RT blocking period with 5% goat serum, the cells were incubated overnight at 4 °C stained with TUNEL (C1086, Beyotime) and DAPI (S0063, Beyotime). Finally, the samples were examined under an inverted microscope (IX51 Olympus, Tokyo, Japan) at a magnification of ×200.
Intracellular ROS level evaluation
Cellular ROS production was identified using dihydroethidium (DHE; Beyotime; cat. no. S0063) staining. When superoxide anions penetrate living cells, they convert DHE to ethidium, which binds to RNA or DNA, resulting in red fluorescence. The intensity of the red fluorescence correlated with the level of intracellular superoxide production. Endometrial cancer cells were seeded in 24-well plates at a density of 1 × 105 cells/well. DHE was added to the cells at a concentration of 10 M, and the cells were incubated at 37 °C for 30 min. Subsequently, the cells were treated with 100 nM Se and 200 mM HCY for 24 h. After rinsing with PBS, the fluorescence of the cells was assessed using a fluorescence microscope at a magnification of x200.
Measurement of oxidative stress indicators
Endometrial cancer cells were homogenized at a concentration of 10% (w/v) in ice-cold PBS to measure oxidative stress biomarkers. The resulting cell lysate was stored at -80 °C. The activities of MDA (S0131S; Beyotime, Shanghai, China), SOD (S0109; Beyotime), and CAT (S0051; Beyotime) were evaluated using commercial kits, and the level of oxidative stress was determined following a previously established method15. Simultaneously, protein in cell lysate was quantified. The MDA content was expressed as mmol/mg protein. The SOD and CAT activities were expressed as U/mg protein.
Cell metabolism assay
Glucose consumption: Glucose uptake was assessed using a Glucose Uptake Colorimetric Assay Kit (K676; BioVision, Mountain View, CA, USA). Following transfection, the cells were maintained at 37 °C for 48 h, collected, and plated to 96-well plates at a density of 1 × 104 cells per well. The cells were then deprived of glucose by pre-incubating for 40 min with 100 µL of Krebs-Ringer-Phosphate-HEPES buffer, followed by a 20-minute incubation with 10 µL of 2-deoxyglucose (10 mM) and Reaction Mix A (for NADPH production) at 37 °C for one hour. Subsequently, the cells were treated with 90 µL of extraction buffer per well for 40 min at 90 °C and then placed in an ice bath for 5 min. Reaction Mix B (for recycling amplification) was then added to each well, and the mixture was centrifuged at 4°C and 16,000 × g for 2 min. The optical density of the supernatant was measured at 412 nm using a Varioskan LUX microplate reader (Thermo Fisher Scientific).
Lactate production: In 6-well plates, 2 × 105 cells were seeded and incubated at 37 °C for 48 h to assess lactate production using the Lactate Colorimetric Assay Kit (K627, BioVision). Following this, the cells were placed in serum-free medium at 37 °C for an additional hour, after which the supernatant was collected for lactate production analysis. The reaction mixture was incubated at room temperature for 30 min in the dark, and the optical density of the supernatant was measured at 450 nm using a microplate reader (Varioskan LUX, Thermo Fisher Scientific).
ATP level: ATP levels were measured using an ATP Colorimetric Assay Kit (MAK1900; Sigma-Aldrich; Merck KGaA) according to the manufacturer’s instructions. In summary, cells (5 × 105) were mixed with 100 µL of ATP Assay Buffer and centrifuged at room temperature for 5 min at 16,000×g. The optical density of the supernatant was determined at 570 nm using a Varioskan LUX microplate reader (Thermo Fisher Scientific). In the results of glucose consumption, lactate production, and ATP level, the colorimetric values of each group were normalized to those of the control group (set as 1)16.
RNA isolation from endometrial cancer cell and tissue samples
RNA was extracted from 90 to 120 mg of individual endometrial cancer cell samples using the RNeasy Lipid Mini Kit (QIAGEN, Valencia, CA), following the manufacturer’s protocol. For each endometrial cancer and normal tissue sample, RNA was isolated from 5 to 10 sections, each 20 μm thick, with a combined weight of approximately 400 mg. These sections were divided into five microcentrifuge tubes for RNA extraction using the RNeasy Lipid Tissue Mini Kit (QIAGEN, Valencia, CA, USA) according to the manufacturer’s instructions. The concentration (ng/µL) and purity (A260/280) of each RNA sample were assessed using Nanodrop 1000 (ThermoFisher Scientific, Wilmington, DE). After this assessment, all RNA samples were stored at -80 °C until qRT-PCR analysis.
Real-time quantitative PCR (RT-qPCR)
To synthesize cDNA, 1 µg of total RNA was reverse transcribed using the High Capacity cDNA Reverse Transcriptase (RT) Kit, adhering to the manufacturer’s instructions (ThermoFisher Scientific, Foster City, CA), in a final reaction volume of 20µL. The qRT-PCR reaction was carried out in a total volume of 50µL, which included 2µL of the cDNA sample, 1µL of each primer mix, 25µL of iQ SYBR Green Supermix, and 22µL of nuclease-free water. GAPDH was used as an internal reference. The primer sequences were as follows: human CIAPIN1 (forward, 5′-CAC CAA GAA GTC TTC TCC TTC AGT G-3′; reverse, 5′-GCT GAG AGG GTC CAC AGC T-3′). The RT-qPCR protocol involved an initial denaturation at 95 °C for 7 min, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Relative mRNA levels were determined using the 2−ΔΔCt method17.
Western blotting
Complete proteins were extracted using RIPA lysis buffer, while nuclear proteins were isolated using Extraction Reagents from Pierce Biotechnology, Inc. (Rockford, IL, USA). Protein concentrations were measured using BCA protein assay kit (Beyotime Biotechnology, China). Protein samples (50 µg) were separated using 10% SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with a 5% low-fat milk solution and then incubated with primary antibodies against CIAPIN1 (1:500, ab154904, rabbit polyclonal, Abcam), p-PI3K (Tyr458) (1:500, ab278545, rabbit monoclonal, Abcam), PI3K (1:500, ab302958, rabbit monoclonal, Abcam), p-Akt (Ser473) (1:500, ab81283, rabbit monoclonal, Abcam), Akt (1:500, ab8805, rabbit polyclonal, Abcam), PKM2 (1:500, ab85555, rabbit polyclonal, Abcam), and LDHA (1:500, sc-137243, mouse monoclonal, Santa Cruz). The membranes were then treated with horseradish peroxidase-conjugated secondary antibodies. GAPDH (1:2000, ab8245, mouse monoclonal, Abcam) was used as an internal control. Finally, bands were visualized using ECL (Thermo Fisher Scientific, Waltham, MA, USA) and analyzed using ImageJ software.
Statistical analysis
Quantitative variables are expressed as mean ± SD, while categorical variables are presented as numbers (percentages). All statistical analyses were conducted using the SPSS Software 20.0. The Student’s t-test was used to analyze quantitative data, and the χ2 test was employed for categorical data. Pearson’s correlation analysis was used to examine the relationships between CIAPIN1 and clinical indicators. In cellular studies, differences among three or more groups were assessed using one-way analysis of variance (ANOVA) followed by the Bonferroni post-hoc test. A p-value of less than 0.05 indicated a statistically significant difference.
Results
CIAPIN1 expression was upregulated in endometrial cancer tissues and serum
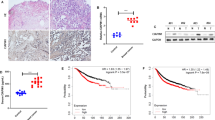
To assess CIAPIN1 levels in endometrial cancer tissues, various tests, such as hematoxylin and eosin (HE) staining and immunohistochemistry, were performed. The findings revealed that CIAPIN1 exhibited strong (#04) and moderate (#01, #02, and #03) expression in four cancerous tissues, whereas all four non-cancerous tissues displayed weak CIAPIN1 expression. This suggests that CIAPIN1 expression is elevated in endometrial tissues compared to that in non-cancerous tissues (Fig. 1A). ELISA was used to evaluate and compare the serum concentrations of CIAPIN1 between patients with endometrial cancer (n = 113) and healthy controls (n = 60), as well as between patients with low CIAPIN1 expression (n = 42) and those with high CIAPIN1 expression (n = 71). The results indicated that CIAPIN1 concentrations were higher in endometrial cancer patients than in controls and were higher in patients with high CIAPIN1 expression than in those with low expression (Fig. 1B and C). The mRNA levels of CIAPIN1 in endometrial cancer tissues (n = 10) and adjacent normal tissues (n = 10) were examined using RT-qPCR, which revealed increased mRNA levels of CIAPIN1 in endometrial cancer tissues (Fig. 1D). Western blot analysis demonstrated elevated protein levels of CIAPIN1 in endometrial cancer tissues (n = 4) compared to those in adjacent normal tissues (n = 4) (Fig. 1E and Fig. S1).
CIAPIN1 expression was upregulated in endometrial cancer tissue and serum samples. (A) Endometrial cancer and adjacent normal tissues were stained with HE and CIAPIN1 expression was evaluated using immunohistochemistry (magnification, 200×). Typical images of CIAPIN1 low and CIAPIN1 high expression are shown. ELISA was performed to measure and compare the serum concentrations of CIAPIN1 (B) between patients with endometrial cancer (n = 113) and healthy controls (n = 60), and (C) between patients with endometrial cancer with CIAPIN1 low expression (n = 42) and CIAPIN1 high expression (n = 71). (D) RT-qPCR was performed to determine the mRNA expression of CIAPIN1 in frozen endometrial cancer tissues (n = 10) and adjacent normal tissues (n = 10). (E) Western blotting was performed to determine the protein expression of CIAPIN1 in endometrial cancer tissues (n = 4) and adjacent normal tissues (n = 4). Data are expressed as mean ± SD and were analyzed using the Student’s t-test. ***P < 0. 001 vs. the control or CIAPIN1 low-expression group.
Overexpression of CIAPIN1 in endometrial cancer cells enhances cell proliferation and migration
The mRNA expression of CIAPIN1 in three endometrial cancer cell lines (HEC-1B, RL95-2, and Ishikawa) was measured using RT‑qPCR. We observed higher levels of CIAPIN1 expression in RL95-2 cells and moderate levels of CIAPIN1 expression in Ishikawa cells than in HEC-1B cells (Fig. 2A). The protein expression of CIAPIN1 in these three endometrial cancer cell lines was measured using western blotting. The results showed higher levels of CIAPIN1 expression in RL95-2 cells, and moderate levels of CIAPIN1 expression in Ishikawa cells than in HEC-1B cells (Fig. 2B and Fig. S2). Ishikawa cells with moderate CIAPIN1 expression were selected for further experiments. Ishikawa cells were transfected with pcDNA3.1-CIAPIN1 or the vector. The levels of CIAPIN1 mRNA and protein were detected by RT-qPCR and Western blotting, respectively, 48 h after transfection. The results indicated that CIAPIN1 mRNA and protein expression levels were elevated in overexpression-CIAPIN1 (OE-CIAPIN1) (Fig. 2C and D, and Fig. S2). Cell viability was determined using the MTT assay in Ishikawa cells transfected with pcDNA3.1-CIAPIN1 or the vector for 12, 24, 48, and 72 h, and an improved cell viability rate was observed with increasing transfection time (Fig. 2E). The migratory ability of Ishikawa cells was evaluated using a transwell assay, and the migrated cells were stained with 0.1% crystal violet. The results demonstrated that CIAPIN1 overexpression enhanced the migratory ability of Ishikawa cells (Fig. 2F). Similar trends were observed after quantifying of the relative number of migrated cells (p < 0.001) (Fig. 2G).
CIAPIN1 overexpression enhances endometrial cancer cell proliferation and migration. (A) The mRNA expression of CIAPIN1 in three endometrial cancer cell lines (HEC-1B, RL95-2, and Ishikawa) was measured using RT‑qPCR. (B) Protein expression of CIAPIN1 in the three endometrial cancer cell lines was measured using western blotting. The Ishikawa cell line with moderate CIAPIN1 expression was chosen for further experiments. Ishikawa cells were transfected with pcDNA3.1-CIAPIN1 or the vector. (C) CIAPIN1 mRNA levels were detected by RT-qPCR 48 h after transfection. (D) CIAPIN1 protein levels were detected by western blotting. (E) Cell viability was determined by MTT assay in Ishikawa cells transfected with pcDNA3.1-CIAPIN1 or vector for 12, 24, 48, and 72 h. (F) The migratory ability of Ishikawa cells was evaluated using the Transwell assay, and the migrated cells were stained with 0.1% crystal violet (magnification 100×). (G) Quantification of the number of migrating cells. Data are expressed as mean ± SD in triplicates (n = 6) and analyzed using the Student’s t-test. ***P < 0.01 vs. Vector group.
Silencing of CIAPIN1 improves endometrial cancer cells apoptosis
Ishikawa cells were transfected with siRNA targeting CIAPIN1 (si-CIAPIN1) or control siRNA (si-NC) and cultured for 48 h. The levels of CIAPIN1 mRNA and protein were detected by RT-qPCR and Western blotting in Ishikawa cells 48 h after transfection. The results showed that siCIAPIN1-3 markedly suppressed CIAPIN1 mRNA and protein expression compared to siCIAPIN1-1 and siCIAPIN1-2 in Ishikawa cells (p < 0.001) (Fig. 3A and B, and Fig. S3). Cell apoptosis was assessed using Annexin V-FITC and PI double staining and analyzed by flow cytometry. The results demonstrated that inhibition of CIAPIN1 expression increased the apoptosis rate in Ishikawa cells (Fig. 3C). In addition, a consistently increased apoptosis rate was observed after calculating the apoptotic rate using the sum of early and late apoptotic cells (p < 0.001) (Fig. 3D). Moreover, Ishikawa cells stained with TUNEL and DAPI showed that inhibition of CIAPIN1 expression increased the TUNEL positive cells (Fig. 3E and F).
CIAPIN1 silencing enhances apoptosis in endometrial cancer cells. Ishikawa cells were transfected with siRNA targeting CIAPIN1 (si-CIAPIN1) or control siRNA (si-NC) and cultured for 48 h. (A) RT‑qPCR was performed to assess the mRNA levels of CIAPIN1 in cells transfected with three siRNAs. (B) Western blotting was performed to assess protein levels of CIAPIN1. (C) Cell apoptosis was assessed by Annexin V-FITC and PI double staining and analyzed using flow cytometry. Representative flow cytometry images are shown. (D) The apoptotic rate was calculated as the sum of the lower right and upper right quadrants. (E) Cells were stained with TUNEL and DAPI and photographed under a fluorescence microscope (magnification 200×). (F) Quantification of the percentage of TUNEL-positive cells relative to DAPI-positive cells. Data are expressed as mean ± SD in triplicates (n = 6), and analyzed using Student t test. ***P < 0.001 vs. control group or si-NC group.
Inhibition of CIAPIN1 in endometrial cancer cells improves cellular ROS production and oxidative stress
To explore how CIAPIN1 inhibition affects the production of cellular ROS and oxidative stress in endometrial cancer cells, we performed dihydroethidium (DHE) staining of Ishikawa cells. These findings indicate that blocking CIAPIN1 led to an increase in cellular ROS production in these cells (Fig. 4A). When we quantified DHE-positive cells in comparison to DAPI-positive cells, similar patterns of cellular ROS production were noted (p < 0.001) (Fig. 4B). We assessed three markers of oxidative stress in Ishikawa cell lysates using a colorimetric approach: malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT). Our results revealed that CIAPIN1 inhibition resulted in elevated MDA levels and reduced SOD and CAT levels in Ishikawa cells (all p < 0.001) (Fig. 4C-E).
CIAPIN1 inhibition in endometrial cancer cells enhances cellular ROS production and oxidative stress. (A) Representative images of dihydroethidium staining of Ishikawa cells. (B) Quantification of dihydroethidium-positive cells relative to DAPI-positive cells. Three oxidative stress indicators were measured in the lysate of Ishikawa cells using the colorimetric method: (C) MDA, (D) SOD, and (E) CAT. Data are expressed as mean ± SD in triplicates (n = 6), and analyzed using Student t test. ***P < 0.001 vs. si-NC group.
CIAPIN1 promotes Glycolysis in endometrial cancer cells via the PI3K/Akt pathway
To investigate the role of CIAPIN1 in regulating glycolysis in endometrial cancer cells, we examined its effect on the PI3K/Akt pathway and key glycolytic indicators in Ishikawa cells. Western blotting analysis revealed that CIAPIN1 overexpression significantly increased PI3K and Akt phosphorylation, whereas CIAPIN1 silencing had the opposite effect (Fig. 5A and Fig. S4). Quantitative analysis confirmed that p-PI3K (normalized to total PI3K) and p-Akt (normalized to total Akt) levels were elevated following CIAPIN1 overexpression (Fig. 5B). Notably, treatment with the PI3K inhibitor LY294002 (10 µM, 24 h) abolished this activation, confirming PI3K/Akt pathway dependence.
CIAPIN1 overexpression in endometrial cancer cells promotes glycolysis by activating the PI3K/Akt pathway. (A) Representative bands of PI3K/Akt pathway proteins by western blot in Ishikawa cells with CIAPIN1 overexpression or silencing. (B) Quantification of p-PI3K (normalized to total PI3K protein) and p-Akt (normalized to total Akt protein). Ishikawa cells were transfected with pcDNA3.1-CIAPIN1 or vector, and or treated with a PI3K inhibitor LY294002 (10 µM) for 24 h. Glycolysis-related indicators were measured in the lysate of Ishikawa cells: (C) glucose consumption, (D) lactate secretion, and (E) ATP levels. (F) Representative bands of glycolysis-related proteins in Ishikawa cells, as determined by western blotting. (G, H) Quantification of PKM2 and LDHA in Ishikawa cells. ***P < 0.001 vs. Vector group; ###P < 0.001 vs. si-NC group. ***P < 0.001 vs. control group (transfected with Vector); ###P < 0.001 vs. OE-CIAPIN1 group.
Ishikawa cells were transfected with pcDNA3.1-CIAPIN1 or vector and treated with the PI3K inhibitor LY294002 (10 µM) for 24 h. Glycolysis-related indicators were measured in the lysates of Ishikawa cells based on glucose consumption, lactate secretion, and adenosine triphosphate (ATP) levels. CIAPIN1-overexpressing cells exhibited increased glucose consumption, lactate secretion, and ATP production, which is indicative of increased glycolysis (Fig. 5C-E). Conversely, PI3K inhibition (LY294002) reversed these effects, suggesting that CIAPIN1-mediated glycolysis depends on PI3K/Akt signaling. Western blotting demonstrated that CIAPIN1 overexpression increased the expression of key glycolytic enzymes including pyruvate kinase M2 (PKM2) and lactate dehydrogenase A (LDHA) (Fig. 5F and Fig. S4). Densitometric analysis confirmed the significant upregulation of PKM2 and LDHA in CIAPIN1-overexpressing cells (Fig. 5G and H). These findings demonstrated that CIAPIN1 promotes glycolysis in endometrial cancer cells by activating the PI3K/Akt pathway, leading to elevated glucose metabolism, lactate production, and ATP generation, along with increased expression of glycolytic enzymes. PI3K inhibition abrogates these effects, supporting the critical role of PI3K/Akt signaling in CIAPIN1-driven glycolysis.
Discussion
We carried out research investigated the role of CIAPIN1 in endometrial cancer. Our results indicated that CIAPIN1 levels were higher in endometrial cancer tissues, cells, and serum. CIAPIN1 overexpression enhances the proliferation and migration of endometrial cancer cells. Conversely, silencing of CIAPIN1 increased apoptosis in these cancer cells. Additionally, CIAPIN1 inhibition elevates ROS production and oxidative stress in endometrial cancer cells. Furthermore, CIAPIN1 overexpression in these cells stimulated glycolysis via the activation of the PI3K/Akt pathway. Consequently, our study suggests that CIAPIN1 may serve as a promising therapeutic target for the identification and improvement of endometrial cancer treatment.
CIAPIN1 is a regulatory molecule located on the long arm of chromosome 16q21. According to Shibayama et al.5, it is involved in the rat sarcoma signal transduction pathway, which is separate from apoptotic B-cell lymphoma 2 or the cysteine-dependent aspartate-directed protease family. CIAPIN1 is present in various normal fetal and adult tissues and is expressed in differentiated and metabolically active tissues18. However, its expression is suppressed in certain malignant tissues, such as gastric cancer and clear cell renal cell carcinoma19,20. Recent research has shown that CIAPIN1 is upregulated in endometrial cancer tissues and the serum of patients. Additionally, elevated CIAPIN1 levels have been detected in endometrial cancer cell lines, including RL95-2 and Ishikawa cells.
Tumor development is affected by several factors, including cell migration, invasion, cell cycle, and proliferation. In clear cell renal cell carcinoma, DLGAP5 is overexpressed, and reducing its expression decreases cell viability, proliferation, migration, and invasion21. In ovarian cancer cells, microRNA-409-5p has been identified to suppress cell proliferation and causes G2/M phase arrest and apoptosis by targeting DLGAP522. Additionally, research has indicated that reducing DLGAP5 expression inhibits the proliferation of breast cancer cells23. In this study, we performed an experiment in which Ishikawa cells were transfected with either pcDNA3.1-CIAPIN1 or a vector. To assess cell proliferation, we conducted a cell proliferation assay 24 h post-transfection. Our findings indicate that CIAPIN1 overexpression leads to increased proliferation of Ishikawa cells. Additionally, we explored the impact of CIAPIN1 overexpression on cell migration using a Transwell assay with Ishikawa cells. These results demonstrate that CIAPIN1 overexpression enhanced the migration capability of these cells (Fig. 2F). This trend was consistent when the relative number of migrated cells was quantified (Fig. 2G). Conversely, reducing CIAPIN1 expression improved cellular apoptosis (Fig. 3), ROS production, and oxidative stress in breast cancer cells (Fig. 4).
The PI3K/Akt pathway is a well-established oncogenic signaling pathway in endometrial cancer. It is frequently dysregulated in EC, often due to mutations in genes such as PIK3CA (encoding the p110α catalytic subunit of PI3K) or loss of function of the tumor suppressor PTEN, which negatively regulates this pathway. Activation of this pathway promotes cell survival, proliferation, metabolism, and metastasis, making it a critical driver of endometrial cancer progression24. This study demonstrated that CIAPIN1 promotes survival, proliferation, migration, and glycolysis in endometrial cancer cells, which are hallmark features of cancer progression. These findings are consistent with the known role of CIAPIN1 in other cancers, where it enhances tumorigenesis and metastasis by modulating key signaling pathways, including the PI3K/Akt pathway7,25.
This study specifically linked CIAPIN1 to the activation of the PI3K/Akt pathway in endometrial cancer. This is significant because the PI3K/Akt pathway is a central regulator of cellular processes, such as glucose metabolism (glycolysis), cell growth, and survival. The finding that CIAPIN1 enhances glycolysis and cell proliferation through PI3K/Akt activation is consistent with the known role of this pathway in promoting the Warburg effect (a metabolic shift to glycolysis even in the presence of oxygen) and supporting tumor growth in EC24. The findings of this study reinforce the importance of targeting the PI3K/Akt pathway for the treatment of endometrial cancer. Given that CIAPIN1 appears to be an upstream activator of this pathway, it may serve as a potential therapeutic target and biomarker for EC. However, this study had certain limitations. First, we assessed the role of CIAPIN1 in endometrial cancer using only three cell lines: HEC-1B, RL95-2, and Ishikawa. Second, additional in vivo validation is required to confirm the function of CIAPIN and its association with the PI3K/Akt pathway.
Conclusion
This study demonstrated that CIAPIN1 is significantly upregulated in endometrial cancer tissues and serum, correlating with disease progression. Functional assays revealed that CIAPIN1 overexpression enhanced cancer cell proliferation, migration, and glycolysis via the PI3K/Akt pathway, whereas its suppression induced apoptosis and oxidative stress (Fig. 6). These findings highlight CIAPIN1 as a potential oncogenic driver in endometrial cancer and suggest that targeting CIAPIN1 or its downstream signaling pathways could be a promising therapeutic strategy. Further research is required to explore its clinical applicability in endometrial cancer treatment.
Data availability
The data from this study is available upon a reasonable request from Dr. Jiashi Gu atgujiashiycl@163.com.
References
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73 (1), 17–48 (2023).
Koh, W. J. et al. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 16 (2), 170–199 (2018).
Aoki, Y. et al. Adjuvant treatment of endometrial cancer today. Jpn J. Clin. Oncol. 50 (7), 753–765 (2020).
Connor, E. V. & Rose, P. G. Management strategies for recurrent endometrial cancer. Expert Rev. Anticancer Ther. 18 (9), 873–885 (2018).
Shibayama, H. et al. Identification of a cytokine-induced antiapoptotic molecule Anamorsin essential for definitive hematopoiesis. J. Exp. Med. 199 (4), 581–592 (2004).
Li, X., Wu, K. & Fan, D. CIAPIN1 as a therapeutic target in cancer. Expert Opin. Ther. Targets. 14 (6), 603–610 (2010).
Wang, X. B. et al. CIAPIN1 is a potential target for apoptosis of multiple myeloma. Mater. Express. 9 (9), 1106–1111 (2019).
Fruman, D. A. et al. The PI3K pathway in human disease. Cell 170 (4), 605–635 (2017).
Mendoza, M. C., Er, E. E. & Blenis, J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem. Sci. 36 (6), 320–328 (2011).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324 (5930), 1029–1033 (2009).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell. Metab. 23 (1), 27–47 (2016).
Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 108 (2), 176 (2010).
Cai, X., Wang, J. & Xin, X. CIAPIN1 nuclear accumulation predicts poor clinical outcome in epithelial ovarian cancer. World J. Surg. Oncol. 10, 112 (2012).
Sun, J. et al. Ginsenoside re inhibits myocardial fibrosis by regulating miR-489/myd88/NF-κB pathway. J. Ginseng Res. 47, 218–227 (2023).
Ma, J. Y. et al. The protective effects of Echinacoside on oxidative stress injury in vascular dementia rats. Chin. Pharmacol. Bull. 30, 638–642 (2014).
Suo, S. et al. Mechanism of PTPN18 for regulating the migration and invasion of endometrial cancer cells via the MYC/PI3K/AKT pathway. Histol. Histopathol. 40 (2), 215–223 (2025).
Zhu, X. et al. ANGPTL4 suppresses the profibrogenic functions of atrial fibroblasts induced by angiotensin II by up-regulating PPARγ. Iran. J. Basic. Med. Sci. 26 (5), 587–593 (2023).
Hao, Z. et al. Subcellular localization of CIAPIN1. J. Histochem. Cytochem. Off J. Histochem. Soc. 54, 1437–1444 (2006).
Li, X., Hao, Z. & Fan, R. CIAPIN1 inhibits gastric cancer cell proliferation and cell cycle progression by downregulating CyclinD1 and upregulating P27. Cancer Biol. Ther. 6, 1539–1545 (2007).
He, L., Wang, H. & Jin, H. CIAPIN1 inhibits the growth and proliferation of clear cell renal cell carcinoma. Cancer Lett. 276, 88–94 (2009).
Feng, Y. et al. Pan-cancer analysis and experiments with cell lines reveal that the slightly elevated expression of DLGAP5 is involved in clear cell renal cell carcinoma progression. Life Sci. 287, 120056 (2021).
Li, W. et al. MicroRNA-409-5p inhibits cell proliferation, and induces G(2)/M phase arrest and apoptosis by targeting DLGAP5 in ovarian cancer cells. Oncol. Lett. 24, 261 (2022).
Zhu, K., Yi, C. & Tong, C. circ_0058063 promotes breast cancer progression by upregulating DLGAP5 via sponging miR-557. Cancer Biomark. 39 (1), 1–13 (2024).
Slomovitz, B. M. & Coleman, R. L. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin. Cancer Res. 18 (21), 5856–5864 (2012).
Shi, H. et al. Expression of CIAPIN1 in human colorectal cancer and its correlation with prognosis. BMC Cancer. 10, 477 (2010).
Acknowledgements
We thank the patients for their specimens and consent.
Funding
Key Specialty Construction Project of Pudong Health and Family Planning Commission of Shanghai (Grant No.PWZzk2022-21) supported this study.
Author information
Authors and Affiliations
Contributions
H.S. conceptualization, methodology, investigation, formal analysis, and data curation. J.S. and Y.D. conceptualization, methodology, investigation, formal analysis. J.G. conceptualization, methodology, supervision. All authors contributed to the writing of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The experimental protocols were approved by the Shanghai Pudong Hospital of Fudan University Review Board (IRB), which granted this study a waiver from a full IRB review. All methods were conducted in accordance with the relevant guidelines and regulations.
Consent to participate
Written informed consent was obtained from all subjects involved in the study.
Consent to publish
Patients was agreed to publish their specimen data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, H., Shao, J., Duan, Y. et al. CIAPIN1 promotes survival, proliferation, migration and glycolysis of endometrial cancer cells through PI3K/Akt pathway. Sci Rep 15, 27739 (2025). https://doi.org/10.1038/s41598-025-13471-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13471-9