Abstract
Certolizumab pegol (CZP), a PEGylated anti-tumor necrosis factor alpha (TNF-α) biologic, is approved for treating autoimmune disorders, including psoriatic arthritis (PsA). Despite its good therapeutic effects, its real-world safety remains limited. This study aims to evaluate the real-world safety of CZP using the FDA Adverse Event Reporting System (FAERS). We collected FAERS data from Q2 2008 to Q4 2024 related to adverse event reports associated with CZP. Four disproportionality analysis methods were used to explore the relationship between CZP and adverse events (AEs), including: reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN), and multi-item gamma Poisson shrinker (MGPS). Additionally, the study analyzed the time to onset of AEs. A total of 78,143 reports listed CZP as the primary suspect (PS), with 216,522 associated AEs. CZP-induced AEs spanned 24 system organ classes (SOCs). The median time to onset for AEs was 92 days (interquartile range: 3–409 days). At the PT level, a total of 322 AE signals were identified as positive, with known positive events including infections and infestations, general disorders, administration site conditions, and musculoskeletal and connective tissue disorders. Additionally, unexpected positive AEs, such as spontaneous abortion, premature birth, pemphigus, and basal cell carcinoma, were identified. This study systematically evaluated the safety of CZP using the FAERS database, confirming some known AEs and revealing unexpected AEs. This post-marketing safety evaluation has deepened our understanding of the safety profile of CZP, and prospective studies are needed to validate the findings of this study.
Similar content being viewed by others
Introduction
Psoriasis is a chronic skin disease affecting approximately 1–3% of the global population, imposing significant personal and societal burdens. Psoriatic arthritis (PsA) develops in up to 30% of individuals with psoriasis1. PsA is a systemic inflammatory disorder that affects joints and skin throughout the body. Additionally, it causes inflammation at entheseal sites, digits (dactylitis), and the axial skeleton1,2. Beyond musculoskeletal and skin manifestations, PsA patients frequently experience fatigue, physical function limitations, sleep disturbances, and reduced work capacity3.
The onset of PsA results from the interaction of genetic predisposition, immune dysregulation, and environmental factors. Traditional treatments include nonsteroidal anti-inflammatory drugs (NSAIDs) and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) such as methotrexate, sulfasalazine, cyclosporine, and leflunomide4. NSAIDs may be used as monotherapy for mild PsA but only for short-term symptom relief. For peripheral arthritis, early initiation of csDMARDs, particularly methotrexate, is recommended5,6. If these strategies fail, biologic therapies—such as tumor necrosis factor inhibitors (TNFi), interleukin-12/23 or interleukin-17 inhibitors, and Janus kinase inhibitors (JAKi)—should be introduced7. However, the efficacy of biologics in psoriasis management may diminish over time8,9. Given that fewer than one-third of patients achieve minimal disease activity (MDA) in most clinical trials, additional treatment options are needed. Early and aggressive intervention is crucial to prevent irreversible joint damage10.
Certolizumab pegol (CZP), commercially known as Cimzia®, is a subcutaneously administered biologic11. It consists of a polyethylene glycolated (PEGylated) antigen-binding fragment of a recombinant human monoclonal antibody that selectively neutralizes TNFα12,13. CZP exhibits dose-proportional pharmacokinetics following subcutaneous administration. Absorption is slow, with maximum plasma concentrations reached in approximately 2–7 days14. The drug has a half-life of approximately 14 days after a single dose, consistent across tested doses in healthy volunteers15. This prolonged half-life is attributed to PEGylation, which extends tissue distribution time and promotes sustained therapeutic effects16. After cleavage from the Fab’ fragment, the PEG moiety is not further metabolized and is eliminated primarily via urinary excretion14. The absence of the Fc region prevents CZP from crossing the placenta, making it a safe option for pregnant women17. Additionally, CZP has demonstrated the ability to inhibit structural damage progression and improve patient-reported outcomes (PROs)18,19. Pivotal phase III trials, including CIMPASI-1 and CIMPASI-2, have shown that CZP significantly enhances both clinical outcomes and quality of life in PsA patients20. While generally well-tolerated, CZP has a safety profile comparable to other TNF inhibitors, with reported adverse events (AEs) including injection site reactions and increased susceptibility to infections21,22,23. Thus, while CZP is a valuable therapeutic option for PsA, comprehensive risk assessment and vigilant monitoring are essential to optimize its clinical use and mitigate potential adverse outcomes.
Most safety data on CZP come from clinical trials and systematic reviews, leaving gaps in real-world safety evidence. The FDA Adverse Event Reporting System (FAERS) collects spontaneous AE reports from healthcare professionals, patients, and legal representatives, providing valuable insights into drug safety in real-world settings24,25. Due to its accessibility and openness, FAERS is increasingly being utilized by researchers for pharmacovigilance and drug safety analyses26,27. This study aims to analyze the safety of CZP using disproportionality analysis through the FAERS database, providing initial safety insights for clinicians and regulatory agencies regarding the drug’s real-world use.
Methods
Data sources and process
FAERS is a cornerstone of post-marketing safety surveillance, serving as a comprehensive database for AE reports. It collects data from a wide range of stakeholders, including healthcare professionals, pharmaceutical companies, and patients. Updated quarterly, FAERS is globally recognized for its robust data collection and strict standardization requirements, making it a widely accepted pharmacovigilance tool.
FAERS data files consist of seven key components: patient demographic and administrative information (DEMO), drug information (DRUG), AE coding (REAC), patient outcomes (OUTC), report sources (RPSR), therapy start and end dates (THER), and indications for drug administration (INDI). The relationship between a drug and an AE is categorized as primary suspect (PS), secondary suspect (SS), concomitant (C), or interacting (I).
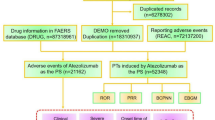
Given the FDA approval timeline for CZP28, we conducted a retrospective pharmacovigilance study using FAERS data from Q2 2008 to Q4 2024. To enhance the study’s reliability, only AE reports where CZP was listed as the primary suspect (PS) were included. The reports were then deduplicated and standardized.
For deduplication, we selected PRIMARYID, CASEID, and FDA_DT fields from the DEMO table and sorted them by CASEID, FDA_DT, and PRIMARYID. Following FDA recommendations, duplicate cases were removed as follows: (1) If CASEID was identical, the most recent FDA_DT was retained; (2) If both CASEID and FDA_DT were identical, the record with the highest PRIMARYID was selected.
To standardize AE coding, we utilized the Medical Dictionary for Regulatory Activities (MedDRA, version 27.0) to map AEs to system organ class (SOC) and preferred term (PT) levels.
A detailed flowchart (Fig. 1) illustrates the step-by-step process of data extraction, processing, and analysis, ensuring transparency and methodological rigor.
Data analysis
This study employs multiple disproportionality analysis methods to identify potential risk signals between CZP and AEs. These methods include the reporting odds ratio (ROR)29, proportional reporting ratio (PRR)30, Bayesian confidence propagation neural network (BCPNN)31, and multi-item gamma Poisson shrinker (MGPS), which assess associations between CZP and reported AEs32.
It is a commonly accepted principle that an elevated ROR value is indicative of a stronger association between AEs and the target drug. The PRR is a statistical instrument utilized in the domain of pharmacovigilance for the purpose of evaluating the relative frequency of AEs for a particular pharmaceutical agent33. The BCPNN algorithm uses Bayesian principles to calculate the information component (IC) based on the four-grid table, which reports the drug’s adverse event ratio34. MGPS is an approach that focuses on signal detection in large datasets, using shrinkage techniques to adjust effect sizes and minimize false positives35.
Research shows ROR and PRR are suitable for rapid screening of high-frequency events and have high sensitivity in signal detection. BCPNN is better for rare events and has better specificity. MGPS has been demonstrated to be a suitable method for signal detection in large datasets. When employed in conjunction with BCPNN, the synergistic benefits of these two methodologies can further mitigate random fluctuations in the data, thereby enhancing the accuracy of the signal36. Combining these methods reduces random data fluctuations and improves signal detection.
As shown in Supplementary Table S1, these methods utilize a 2 × 2 contingency table to compute ROR, PRR, BCPNN, and EBGM values. Supplementary Table S2 provides the corresponding equations and threshold criteria for each algorithm. A SOC is considered a positive signal if it meets the threshold for at least one disproportionality analysis method, while a PT must meet the threshold for all four disproportionality analysis methods to be classified as a positive signal. All analyses were conducted using R software (version 4.4.2).
Results
Descriptive analysis
A comprehensive extraction of all reports from the FAERS database between Q2 2008 and Q4 2024 identified a total of 20,211,660 reports. After deduplication and screening, a total of 78,143 CZP-related AE reports were identified and analyzed. The general characteristics of these AE reports are summarized in Table 1.
Among the reported AE reports, women accounted for 74.44%, significantly higher than men (21.86%). Regarding age distribution, the 45–65 age group accounted for the highest proportion (21.64%), followed by the 18–45 age group (19.11%). Geographically, most reports originated from the United States (67.63%), followed by Canada (9.14%), the United Kingdom (6.85%), Brazil (2.50%), and Japan (2.49%). The majority of reports were submitted by healthcare professionals (57.84%). From 2008 to 2024 (Fig. 2), the highest number of reports was recorded in 2023 (19.47%).
Signal detection related to SOC levels
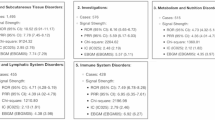
The signal intensity analysis of CZP at the SOC level revealed that AE were primarily concentrated in 24 SOCs, as shown in Table 2 (Fig. 3). The heatmap of SOC categorized by ROR is shown in Fig. 4.
The SOC with the highest number of reports was general disorders and administration site conditions (n = 47,258), followed by infections and infestations (n = 30,100). The strongest signal intensity was observed in pregnancy, puerperium, and perinatal conditions (n = 3,228, ROR = 3.54). Several other SOCs also exhibited positive signal, including: ① Infections and infestations (n = 30,100, ROR = 2.75); ② Musculoskeletal and connective tissue disorders (n = 24,449, ROR = 2.17); ③ Skin and subcutaneous tissue disorders (n = 15,076, ROR = 1.24); ④ General disorders and administration site conditions (n = 47,258, ROR = 1.24); ⑤ Injury, poisoning, and procedural complications (n = 25,841, ROR = 1.19).
Signal detection at the PT levels
A total of 322 CZP-induced AE positive signals were identified after meeting the criteria across all four algorithms. All AEs that met the positive signal criteria are detailed in Supplementary Table S3.
Table 3 presents the top 50 CZP-related AEs with positive signals, ranked by ROR values at the PT level. Common AEs at the PT level included infection, sinusitis, lower respiratory tract infection, bronchitis, herpes zoster, injection site discoloration, and spontaneous abortion.
Time to onset (TTO) analysis of AEs
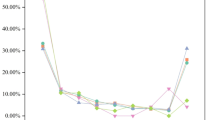
A total of 15,466 AE reports were included in the TTO analysis after excluding cases with missing or incorrect onset data. The median TTO of AEs was 92 days (interquartile range IQR: 3–409 days). As shown in Fig. 5, most cases (37.94%, n = 7,944) occurred within the first month following CZP injection.
Subgroup analysis
Subgroup analyses were conducted to explore AEs with positive signals across different populations, including by age and gender. In both genders, common AEs included infections, sinusitis, lower respiratory tract infections, and herpes zoster. However, certain conditions were reported more frequently in specific genders. Male patients showed higher incidences of product dose omission issues, abdominal pain, nephrolithiasis, and basal cell carcinoma (Supplementary Table S4), while female patients required more attention for maternal exposure during pregnancy, lupus-like syndrome, and kidney infections (Supplementary Table S5).
Among patients under 18 years, common AEs included fetal exposure during pregnancy, premature birth, exposure via breast milk, and small for gestational age (Supplementary Table S6). For patients aged 18–65 years, the most reported AEs were maternal exposure during pregnancy, urinary tract infection, sinusitis, bronchitis, and injection site discoloration (Supplementary Table S7). In patients aged 65 and older, urinary tract infections, herpes zoster, sinusitis, influenza, and cellulitis were most frequently reported (Supplementary Table S8). Additionally, significant reports of malignant tumors, including basal cell carcinoma, lymphoma, malignant melanoma, and squamous cell carcinoma, were noted in older patients.
Discussion
CZP is an important biologic treatment for various autoimmune diseases. As its use and approval have expanded, reports of AEs have also increased, reaching 15,212 cases in 2023. This highlights the need for continued monitoring. This study analyzes CZP-related AEs using data from the FAERS database, covering the period from its approval in Q2 2008 to Q4 2024. To our knowledge, this is the first comprehensive post-marketing study on CZP’s AEs based on the FAERS database.
Our findings confirm the AEs listed in the drug’s prescribing information, such as infections, musculoskeletal disorders, and injection site reactions. Additionally, we identified new AEs, including spontaneous abortion, frequent bowel movements, SARS-CoV-2 test positivity, low birth weight infants, basal cell carcinoma, and pemphigus. Women and those aged 45–65 had the highest incidence of AEs following CZP treatment. Real-world data shows that more than half of anti-TNF therapies are used to treat rheumatoid arthritis (RA)37, which is more common in women (3:1 ratio) and usually develops between the ages of 35–5538. This likely explains our findings.
Infections were a significant concern in our study. Both clinical and real-world evidence have raised infections as a major safety issue for CZP users39. An analysis of 163 randomized controlled trials (RCTs) showed CZP significantly increases the likelihood of serious infections40. Compared to other biologics like etanercept and adalimumab, CZP was linked to a higher risk of infections. However, it showed a slightly lower risk of tuberculosis reactivation compared to infliximab and adalimumab41. Patients on CZP should be closely monitored for infections, with screenings for tuberculosis before and during treatment.
Injection site reactions, such as rash, discoloration, urticaria, and pallor, were also common but typically not severe42. These side effects may affect patient adherence to treatment, especially over long periods. Addressing these symptoms quickly and improving communication between patients and healthcare providers may help enhance treatment outcomes. Musculoskeletal and connective tissue disorders were notable AEs, with conditions like lupus-like syndrome and juvenile idiopathic arthritis linked to CZP. This may be due to CZP’s mechanism as a TNF-α inhibitor, which could trigger new autoimmune responses or worsen existing conditions43,44.
We also identified previously unreported pregnancy-related AEs, including spontaneous abortion, preterm birth, and low birth weight. Although existing evidence on the safety of CZP during pregnancy remains limited, a review analysis indicated that compared to the general population, CZP does not exhibit teratogenic effects or increased risk of fetal mortality45. Multiple prospective studies have reported no consistent association between CZP exposure and major congenital malformations46,47. Conversely, a large cohort study documented the occurrence of spontaneous abortions and congenital malformations in pregnancies exposed to CZP48. Notably, the incidence of low birth weight among singleton infants treated with CZP was higher than the U.S. population baseline49. Our findings are consistent with a recent pharmacovigilance study based on EudraVigilance data, which also reported spontaneous abortion as a common AE among pregnant patients treated with CZP50. Additionally, this study demonstrated that the overall maternal and neonatal safety profile of CZP is comparable to other TNF-α inhibitors. Overall, CZP exhibits a favorable safety profile during pregnancy, but these findings underscore the necessity of vigilant perinatal monitoring. The observed associations with spontaneous abortion, preterm birth, and low birth weight highlight the critical balance required between maternal disease management and fetal risk mitigation during pregnancy. Further large-scale longitudinal studies are warranted to refine risk stratification protocols for CZP use in pregnancy.
Cancer risks, particularly skin cancers, were also raised in our study. Basal cell carcinoma, lung adenocarcinoma, and other malignancies were more common in CZP-treated patients. A systematic review of five TNF-α inhibitors identified skin cancer, breast cancer, and basal cell carcinoma as the most prevalent malignancy-associated AEs21. Previous studies on the safety profile of CZP in clinical trials have illustrated that in RA, the incidence of lymphatic and hematopoietic cancers (mainly lymphomas) has increased compared to the general population but remains low in other indications51. Analysis of 48 FDA-reported pediatric cases linked to TNF-α inhibitors showed lymphomas accounted for 50% of malignancy events52. The tumorigenic potential of CZP, a TNF-α inhibitor, operates through dual mechanisms. Primarily, by blocking TNF-α binding to TNFR1/2 receptors, it disrupts normal signaling pathways, impairing NF-κB-mediated proliferation control and causing cellular dysregulation53,54,55. Secondarily, TNF-α's dose-dependent duality—anti-angiogenic at high concentrations but pro-angiogenic at low levels—creates a disrupted equilibrium under CZP therapy56. These intersecting pathways elucidate why TNF-α inhibition may suppress inflammation while paradoxically elevating oncogenic risk. Clinicians should closely monitor for signs of tumor progression, especially in patients with existing cancer or those at high risk.
When analyzing AEs, “TTO” refers to the time that elapses between starting treatment and the occurrence of an AE. Most cases occurred within the first month, highlighting the need for vigilant monitoring of AEs during the first month of treatment. However, the median duration of AEs associated with CZP was 92 days, and there were still a number of AEs that occurred over a year, suggesting that longer follow-up is needed for future clinical trials.
The observed gender differences in AEs among patients treated with CZP may reflect underlying biological and hormonal influences. For instance, males are more prone to developing renal calculi, a phenomenon attributable not only to lifestyle risk factors but also to higher calcium oxalate and uric acid supersaturation in their blood57. Similarly, the association of basal cell carcinoma with male patients might be related to higher sun exposure and a potentially higher baseline risk of skin malignancies due to genetic predispositions58. Conversely, the risk of lupus-like syndrome can be associated with estrogen’s immunomodulatory effects, which have been shown to exacerbate autoimmune responses59. Among patients aged < 18 years, the prevalence of AEs such as fetal exposure during pregnancy, small-for-dates babies, and prematurity underscores the critical consideration of maternal health and exposure risks during pregnancy and lactation. In patients over 65 years of age, careful attention and vigilance should be given to the development of serious infections as well as malignancies (including skin cancers and lymphomas) during the course of the medication. These findings highlight the importance of implementing gender- and age-specific monitoring and management strategies to effectively address these AEs.
Despite providing valuable insights, this study has limitations. The FAERS database relies on self-reporting, which may introduce bias and affect the reliability of the findings60. Data is also predominantly from the U.S., limiting its global applicability. Furthermore, the lack of detailed patient information makes it difficult to establish causal relationships. Future multicenter, longitudinal studies are necessary to provide a more comprehensive safety evaluation of CZP.
Conclusion
This study utilized the FAERS database to evaluate the safety of CZP from multiple perspectives, providing robust evidence for its real-world safety and revealing unexpected AEs. This long-term post-marketing safety evaluation deepened our understanding of CZP’s safety profile, and prospective studies are warranted to validate the findings of this study.
Data availability
Data are publicly available and can be retrieved from the FAERS Publish Dashboard: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDEFAERS.html.
References
Stober, C. Pathogenesis of psoriatic arthritis. Best Pract. Res. Clin. Rheumatol. 35, 101694. https://doi.org/10.1016/j.berh.2021.101694 (2021).
Veale, D. J. & Fearon, U. The pathogenesis of psoriatic arthritis. Lancet 391, 2273–2284. https://doi.org/10.1016/S0140-6736(18)30830-4 (2018).
Orbai, A.-M. et al. International patient and physician consensus on a psoriatic arthritis core outcome set for clinical trials. Ann. Rheum. Dis. 76, 673–680. https://doi.org/10.1136/annrheumdis-2016-210242 (2017).
Michelena, X. et al. How are we addressing axial psoriatic arthritis in clinical practice?. Rheumatol. Ther. 11, 1441–1456. https://doi.org/10.1007/s40744-024-00722-w (2024).
Gossec, L. et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update. Ann. Rheum. Dis. 83, 706–719. https://doi.org/10.1136/ard-2024-225531 (2024).
Mulder, M. L. M. et al. Comparing methotrexate monotherapy with methotrexate plus leflunomide combination therapy in psoriatic arthritis (COMPLETE-PsA): A double-blind, placebo-controlled, randomised, trial. Lancet Rheumatol. 4, e252–e261. https://doi.org/10.1016/S2665-9913(22)00028-5 (2022).
Bonelli, M. et al. Selectivity, efficacy and safety of JAKinibs: New evidence for a still evolving story. Ann. Rheum. Dis. 83, 139–160. https://doi.org/10.1136/ard-2023-223850 (2024).
Gniadecki, R. et al. Comparison of long-term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br. J. Dermatol. 172, 244–252. https://doi.org/10.1111/bjd.13343 (2015).
Menter, A. et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: Results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J. Eur. Acad. Dermatol. Venereol. 30, 1148–1158. https://doi.org/10.1111/jdv.13611 (2016).
Mease, P. J. et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: Results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann. Rheum. Dis. 76, 79–87. https://doi.org/10.1136/annrheumdis-2016-209709 (2017).
Deeks, E. D. Certolizumab pegol: A review in inflammatory autoimmune diseases. BioDrugs 30, 607–617 (2016).
Esposito, M. et al. Certolizumab pegol for the treatment of psoriatic arthritis and plaque psoriasis. Expert Rev. Clin. Immunol. 16, 119–128. https://doi.org/10.1080/1744666X.2020.1713754 (2020).
Campanati, A. et al. Certolizumab pegol for the treatment of psoriasis. Expert Opin. Biol. Ther. 17, 387–394. https://doi.org/10.1080/14712598.2017.1283401 (2017).
Pasut, G. Pegylation of biological molecules and potential benefits: Pharmacological properties of certolizumab pegol. BioDrugs 28, 15–23. https://doi.org/10.1007/s40259-013-0064-z (2014).
Choy, E. H. S. et al. Efficacy of a novel PEGylated humanized anti-TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double-blinded, randomized, dose-escalating trial. Rheumatology 41, 1133–1137. https://doi.org/10.1093/rheumatology/41.10.1133 (2002).
Tokuyama, M. & Mabuchi, T. Summary of certolizumab pegol in psoriasis including structural features, pharmacokinetics and treatment. Immunotherapy 16, 273–285. https://doi.org/10.2217/imt-2023-0058 (2024).
van Gendt, J. et al. Pharmacokinetics of monoclonal antibodies throughout pregnancy: A systematic literature review. Clin. Pharmacokinet. 63, 589–622. https://doi.org/10.1007/s40262-024-01370-7 (2024).
Correction: 4-year results from the RAPID-PsA phase 3 randomised placebo-controlled trial of certolizumab pegol in psoriatic arthritis. RMD Open 4. https://doi.org/10.1136/rmdopen-2017-000582corr1 (2018).
Coates, L. C. et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 68, 1060–1071. https://doi.org/10.1002/art.39573 (2016).
Kerschbaumer, A. et al. Efficacy and safety of pharmacological treatment of psoriatic arthritis: A systematic literature research informing the 2023 update of the EULAR recommendations for the management of psoriatic arthritis. Ann. Rheum. Dis. 83, 760–774. https://doi.org/10.1136/ard-2024-225534 (2024).
Li, M., You, R., Su, Y., Zhou, H. & Gong, S. Characteristic analysis of adverse reactions of five anti-TNFɑ agents: A descriptive analysis from WHO-VigiAccess. Front. Pharmacol. 14, 1169327. https://doi.org/10.3389/fphar.2023.1169327 (2023).
Chen, C., Borrego, M. E., Roberts, M. H. & Raisch, D. W. Comparison of post-marketing surveillance approaches regarding infections related to tumor necrosis factor inhibitors (TNFi’s) used in treatment of autoimmune diseases. Expert Opin. Drug. Saf. 18, 733–744. https://doi.org/10.1080/14740338.2019.1630063 (2019).
Chiu, Y.-M. & Chen, D.-Y. Infection risk in patients undergoing treatment for inflammatory arthritis: Non-biologics versus biologics. Expert Rev. Clin. Immunol. 16, 207–228. https://doi.org/10.1080/1744666X.2019.1705785 (2020).
Liu, H., Yang, Q., Li, Z., Yan, S. & Ming, S. Systematic analysis of sugammadex-related adverse drug reaction signals using FAERS database. Int. J. Surg. 111, 1988–1994. https://doi.org/10.1097/JS9.0000000000002194 (2025).
Chen, H., Yang, G. & Ma, J. Ocular toxicity associated with anti-HER2 agents in breast cancer: A pharmacovigilance analysis using the FAERS database. Int. J. Cancer 154, 1616–1625. https://doi.org/10.1002/ijc.34848 (2024).
Zhao, K., Zhao, Y., Xiao, S. & Tu, C. Assessing the real-world safety of tralokinumab for atopic dermatitis: Insights from a comprehensive analysis of FAERS data. Front. Pharmacol. 15, 1458438. https://doi.org/10.3389/fphar.2024.1458438 (2024).
Su, H., Jia, J., Mao, Y., Zhu, R. & Li, Z. A real-world analysis of FDA Adverse Event Reporting System (FAERS) events for liposomal and conventional doxorubicins. Sci. Rep. 14, 5095. https://doi.org/10.1038/s41598-024-55185-4 (2024).
Bate, A. et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54, 315–321 (1998).
Rothman, K. J., Lanes, S. & Sacks, S. T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 13, 519–523 (2004).
Noguchi, Y., Aoyama, K., Kubo, S., Tachi, T. & Teramachi, H. Improved detection criteria for detecting drug-drug interaction signals using the proportional reporting ratio. Pharmaceuticals 14, 4. https://doi.org/10.3390/ph14010004 (2020).
Tada, K., Maruo, K., Isogawa, N., Yamaguchi, Y. & Gosho, M. Borrowing external information to improve Bayesian confidence propagation neural network. Eur. J. Clin. Pharmacol. 76, 1311–1319. https://doi.org/10.1007/s00228-020-02909-w (2020).
Sakaeda, T., Kadoyama, K., Minami, K. & Okuno, Y. Commonality of drug-associated adverse events detected by 4 commonly used data mining algorithms. Int. J. Med. Sci. 11, 461–465. https://doi.org/10.7150/ijms.7967 (2014).
Evans, S. J., Waller, P. C. & Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 10, 483–486. https://doi.org/10.1002/pds.677 (2001).
Bate, A. Bayesian confidence propagation neural network. Drug Saf. 30, 623–625. https://doi.org/10.2165/00002018-200730070-00011 (2007).
Teng, H., Zhang, S., Yu, J. & Li, F. Adverse event profile differences between maribavir and valganciclovir: Findings from the FDA adverse event reporting system. Front. Pharmacol. 16, 1518258. https://doi.org/10.3389/fphar.2025.1518258 (2025).
Zhang, C., Wen, J. & Li, Y. A real-world disproportionality analysis of Tenofovir Alafenamide (TAF): Data mining of the FDA adverse event reporting system (FAERS). PLoS ONE 20, e0324675. https://doi.org/10.1371/journal.pone.0324675 (2025).
Deepak, P., Stobaugh, D. J., Sherid, M., Sifuentes, H. & Ehrenpreis, E. D. Neurological events with tumour necrosis factor alpha inhibitors reported to the Food and Drug Administration Adverse Event Reporting System. Aliment Pharmacol. Ther. 38, 388–396. https://doi.org/10.1111/apt.12385 (2013).
Smolen, J. S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 82, 3–18. https://doi.org/10.1136/ard-2022-223356 (2023).
Glatt, S. et al. Efficacy and safety of bimekizumab as add-on therapy for rheumatoid arthritis in patients with inadequate response to certolizumab pegol: A proof-of-concept study. Ann. Rheum. Dis. 78, 1033–1040. https://doi.org/10.1136/annrheumdis-2018-214943 (2019).
Singh, J. A. et al. Adverse effects of biologics: A network meta-analysis and Cochrane overview. Cochrane Database Syst. Rev. 2011, CD008794. https://doi.org/10.1002/14651858.CD008794.pub2 (2011).
Harrold, L. R. et al. One-year risk of serious infection in patients treated with certolizumab pegol as compared with other TNF inhibitors in a real-world setting: Data from a national U.S. rheumatoid arthritis registry. Arthritis Res. Ther. 20, 2. https://doi.org/10.1186/s13075-017-1496-5 (2018).
Bykerk, V. P. et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: An integrated analysis from clinical trials. Ann. Rheum. Dis. 74, 96–103. https://doi.org/10.1136/annrheumdis-2013-203660 (2015).
De Stefano, L. et al. Tumor necrosis factor-α inhibitor-related autoimmune disorders. Autoimmun. Rev. 22, 103332. https://doi.org/10.1016/j.autrev.2023.103332 (2023).
Kremenevski, I., Sander, O., Sticherling, M., Raithel, M. & LastName, F. M. Paradoxical reactions to biologicals in chronic inflammatory systemic diseases. Dtsch. Arztebl. Int. 119, 88–95. https://doi.org/10.3238/arztebl.m2022.0067 (2022).
Strain, J., Leis, M., Lee, K. O. & Fleming, P. Certolizumab pegol in plaque psoriasis: Considerations for pregnancy. Skin Therapy Lett. 26, 1–5 (2021).
Hoxha, A. et al. Pregnancy and foetal outcomes following anti-tumor necrosis factor alpha therapy: A prospective multicentre study. Jt. Bone Spine 84, 169–173. https://doi.org/10.1016/j.jbspin.2016.03.014 (2017).
Weber-Schoendorfer, C. et al. Pregnancy outcome after TNF-α inhibitor therapy during the first trimester: A prospective multicentre cohort study. Br. J. Clin. Pharmacol. 80, 727–739. https://doi.org/10.1111/bcp.12642 (2015).
Clowse, M. E. B. et al. Pregnancy outcomes after exposure to certolizumab pegol: Updated results from a pharmacovigilance safety database. Arthritis Rheumatol. 70, 1399–1407. https://doi.org/10.1002/art.40508 (2018).
Clowse, M. E. B. et al. Pregnancy outcomes in subjects exposed to certolizumab pegol. J Rheumatol 42, 2270–2278. https://doi.org/10.3899/jrheum.140189 (2015).
Gaio, M. et al. Pregnancy recommendations solely based on preclinical evidence should be integrated with real-world evidence: A disproportionality analysis of certolizumab and other TNF-alpha inhibitors used in pregnant patients with psoriasis. Pharmaceuticals 17, 904 (2024).
Curtis, J. R. et al. Long-term safety of certolizumab pegol in rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis and Crohn’s disease: A pooled analysis of 11 317 patients across clinical trials. RMD Open 5, e000942. https://doi.org/10.1136/rmdopen-2019-000942 (2019).
Diak, P. et al. Tumor necrosis factor alpha blockers and malignancy in children: Forty-eight cases reported to the Food and Drug Administration. Arthritis Rheum. 62, 2517–2524. https://doi.org/10.1002/art.27511 (2010).
Aggarwal, B. B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 3, 745–756 (2003).
Chen, G. & Goeddel, D. V. TNF-R1 signaling: A beautiful pathway. Science 296, 1634–1635 (2002).
Muppidi, J. R., Tschopp, J. & Siegel, R. M. Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity 21, 461–465 (2004).
Fajardo, L. F., Kwan, H. H., Kowalski, J., Prionas, S. D. & Allison, A. C. Dual role of tumor necrosis factor-alpha in angiogenesis. Am. J. Pathol. 140, 539–544 (1992).
Borghi, L. et al. Essential arterial hypertension and stone disease. Kidney Int. 55, 2397–2406 (1999).
Karagas, M. R. et al. Use of tanning devices and risk of basal cell and squamous cell skin cancers. J. Natl. Cancer Inst. 94, 224–226 (2002).
Hughes, G. C. & Choubey, D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nat. Rev. Rheumatol. 10, 740–751. https://doi.org/10.1038/nrrheum.2014.144 (2014).
Xu, Q. et al. Data mining and analysis of adverse events of Vedolizumab based on the FAERS database. Sci. Rep. 15, 278. https://doi.org/10.1038/s41598-024-75421-1 (2025).
Acknowledgements
This study was performed using the FDA Adverse Event Reporting System (FAERS) database that was provided by the FDA. The information, results, or interpretation of the current study do not represent any opinion of the FDA.
Funding
This work was supported by the National Natural Science Foundation of China (82404140).
Author information
Authors and Affiliations
Contributions
Conceptualization, M.L. and K.Z.; Methodology and formal analysis, P.Y. and R.F.; Writing-review and editing, M.L., P.Y. and K.Z.; Project administration, M.L.; Supervision, K.Z. All authors have read and agreed to the published version of the manuscript. The authors report no conflict of interest.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, M., Yin, P., Fan, R. et al. A real-world pharmacovigilance study of Certolizumab pegol based on FAERS database. Sci Rep 15, 28529 (2025). https://doi.org/10.1038/s41598-025-13502-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13502-5
Keywords
This article is cited by
-
Drug-associated postpartum hemorrhage: a comprehensive disproportionality analysis based on the FAERS database
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)