Abstract
A simple and highly chemoselective heteroannulation protocol for the synthesis of polysubstituted heterocycles is reported. In this work, various oxa-aza[3.3.3]propellanes were synthesized via a sequential one-pot reaction of ninhydrin, malononitrile and nitroketene acetal or nitroketene aminals in water at ambient temperature. This successive Knoevenagel condensation/Michael addition/cyclization sequence features high atom economy, excellent efficiency, and the use of water as a solvent without the need for metal catalysts or external activators. This method can be presented as a good example of group-assisted purification (GAP), in which traditional purification techniques such as recrystallization and column chromatography are avoided and pure products are obtained simply by washing the crude products.
Similar content being viewed by others
Introduction
Propellanes are defined as tricyclic systems in which three rings are connected through a central carbon-carbon single bond. Since their discovery in 1965, these multi-ring structures have attracted the attention of many organic chemists and continue to be fascinating synthetic targets. The importance of these compounds undoubtedly lies in their challenging and unusual architecture, unique topology, physical properties and wide range of applications1,2,3,4. Nitrogen- and oxygen-containing propellanes are a class of prominent pharmacophores found in both bioactive natural compounds and synthetic pharmaceutical products5,6,7. They have been found in a large group of alkaloids, containing aza[4.4.3]propellane scaffolds 1–8 (known as hasubanans), aza[4.3.3]propellanes 9–11 (known as acutumines), oxa-aza[4.4.3]propellane frameworks (fendleridines 12 and 1-acetylaspidoalbidine 13) as well as oxa-aza[4.3.3]propellanes (aspidohylline 14 and aspidodasycarpine 15) (Fig. 1)8,9.
In modern synthetic chemistry, the design and use of multicomponent reactions (MCRs) have attracted the attention of many researchers in fields such as medicinal, biological and organic chemistry. Since these reactions enable the formation of multiple bonds in a single operation, they eliminate the need for purification processes after each separate step. As a result, MCRs offer significant advantages such as: saving time and cost, improving reaction efficiency and reducing waste generation10,11,12,13,14,15,16,17.
In this work, we succeeded in synthesizing a series of heterocyclic [3.3.3]propellanes through a highly efficient cascade multicomponent reaction. The resulting products feature two heterocyclic moieties (furan and pyrrole rings) integrated within the propellane framework. The following are selected examples of reported MCRs used for the synthesis of oxa-aza[3.3.3]propellanes:
In 2014 a three-component cascade reaction was developed for the diastereoselective synthesis of polycyclic pyrroles containing four quaternary stereocenters. The reactions were carried out with acenaphthylene1,2-dione, ethyl trifluoroacetylacetate and heterocyclic ketene aminals (HKAs) as starting materials (Fig. 2, A)18. In another work, an efficient domino four-component reaction between ninhydrin, malononitrile, malonate compounds and aryl isothiocyanates in the presence of NaH in DMF was reported in 2014. This approach led to the formation of oxa-aza[3.3.3]propellanes (Fig. 2, B)19. In 2013 a one-pot domino reaction of ninhydrin, malononitrile and 3-arylamino-2-cyclohexenones for the synthesis of fused oxa-aza[3.3.3]propellanes was developed (Fig. 2, C)20,21. In 2015 A chemoselective protocol for the synthesis of heterocyclic [3.3.3]propellanes was described via four-component cascade reaction of ninhydrin, malononitrile, dialkyl acetylenedicarboxylates and primary amines (Fig. 2, D)22. In another study reported in 2018 a two-step four-component reaction for the synthesis of oxa-aza[3.3.3]propellanes was presented by the condensation reaction between acenaphthenequinone, malononitrile derivatives, β-ketoester or β-diketone and primary amines (Fig. 2, E)23. In 2012 heterocyclic [3.3.3]propellanes were prepared using a sequential four-component reaction between ninhydrin, malononitrile and various ketene aminals (Fig. 2, F)24,25.
Continuing our research on the synthesis of novel nitrogen-containing heterocycles using green chemistry protocols26,27, here we report an efficient approach for the synthesis of highly functionalized oxa-aza[3.3.3]propellanes via a sequential one-pot reaction between ninhydrin 1, malononitrile 2 and NMSM 3 or nitroketene aminals 6 (Figs. 3 and 4).
The highlight in comparing existing methods with the present method is the use of water as the sole solvent, which makes this synthesis valuable and unique.
The development of multicomponent reactions utilizing water as a green reaction medium has attracted much attention. Water is a safe, non-flammable, abundant, nontoxic, stable, and inexpensive solvent, so its use in organic synthesis is of particular importance22,26,27,28.
Furthermore, the catalyst-free conditions and easy isolation method (without the need for conventional techniques such as crystallization and chromatography or GAP purification) make this synthesis significantly practical. High atom economy, remarkable product purity and yield, and favorable chemoselectivity are other advantages of the designed reactions.
Results and discussion
Reactions design
In line with our ongoing interest and expertise in one-pot multicomponent reactions, we were trying to explore the possibility of trapping a Knoevenagel adduct, generated in situ from ninhydrin and malononitrile, with a ketene N, S-acetal to form a heterocyclic product. So, a three-component reaction involving ninhydrin 1, malononitrile 2 and N-methyl-1-(methylthio)-2-nitroethenamine 3 was designed in ethanol under reflux conditions, during which a mixture of two products 4,5 was obtained (Fig. 3, Reaction 1). At first, it was difficult to identify the structure of the products from the NMR spectrums of the mixture. So, a two-component reaction between ninhydrin 1 and NMSM 3 (Fig. 3, Reaction 2) was carried out under the same conditions and its NMR spectrums was compared with the spectrums of above mixture. In this investigation, it was clarified that one of the products of the first reaction was the same two-component product, dihydroxy-indeno[1,2-b]pyrrole 4, that does not involve malononitrile in its structure. Then, by changing the reaction conditions, an attempt was made to form and identify the unknown product 5. The use of other organic solvents such as methanol, acetonitrile, and chloroform did not improve the reaction in favor of the formation of pure product 5. Even the use of acidic and basic catalysts did not help. The experimental results showed that when water was used as the solvent, the reaction proceeded quickly with excellent yields and oxa-aza[3.3.3]propellane 5 was obtained in pure form in 86% yield (Fig. 3, Reaction 3).
In general, Knoevenagel condensation reactions are accelerated using aqueous media (due to hydrophobic effect of water, product precipitation, strong solvation of intermediates). The indeno[1,2-b]pyrrole 4 is probably more thermodynamically stable than propellane 5, which is favored under thermal conditions in a solvent like ethanol.
Water is a highly polar protic solvent and may favor hydrogen bonding, stabilization of polar intermediates, and kinetic control. Room temperature favors kinetically controlled pathways, and propellane 5 likely follows a faster, lower-energy pathway, while the compound 4 may require thermal input. So, under these mild conditions, only the kinetic product (propellane 5) forms, and the reaction does not proceed to compound 4.
The structure of 5 was later clearly confirmed by single-crystal X-ray analysis. The structure of 4 was predicted based on NMR spectroscopy similarities29,30.
Optimization of the conditions for further syntheses
Based on the success of the above reaction, various reactions were done using cyclic nitroketene aminals 6a-g, and in all of them, the polysubstituted oxa-aza[3.3.3]propellane derivatives 7a-g were synthesized with high efficiency and purity (Fig. 4). These reactions were carried out sequentially in two steps. In the first step, according to the reported methods, active cyclic enamines 6 were synthesized from the reaction between nitroketene dithioacetal (1,1-bis(methylthio)-2-nitroethylene) and diamines, ethanol amine or cysteamine hydrochloride in ethanol at reflux for 5–6 h31,32,33,34,35,36 and then, after removing the solvent, ketene aminal 6 was used as the starting material in a three-component one-pot cascade reaction. As previously mentioned and can be seen in Table 1, in these reactions, the yield was also low using ethanol and the main product was obtained without the participation of malononitrile. Other organic solvents were also not suitable for this synthesis (Entry 1–4, Table 1). The desired propellane product 7a was obtained with significant yield and purity using water as the reaction medium (Entry 7, Table 1). In fact, water increases the selectivity in these reactions. Based on previous observations and experiences, condensation reactions in water are very fast and it seems that water in addition to increasing selectivity, also plays a catalytic role in these reactions.
To the best of our knowledge, the presented method is novel and among the synthesized derivatives, three products 5, 7e and 7f are completely new.
The reactions were completed in total after 10 h to afford the corresponding heterocyclic products 7a–g in high yields (76–88%). The results are given in Table 2.
Scope and limitations
To further expand the reaction scope, it was also performed with other linear ketene acetals (synthesized from the reaction of 1,1-bis(methylthio)-2-nitroethene with various amines), in which cases the desired product was not synthesized and a mixture of products was observed in TLC. Additionally, an attempt was made to use acenaphthylene1,2-dione instead of ninhydrin, but in this case, the propellane product was not obtained. Malononitrile derivatives (ethyl/methyl cyanoacetate) were also tested in these reactions, but did not lead to the desired product.
Structure determination
The structures of products 5, 7a-g were confirmed based on their IR, 1H NMR, 13C NMR and Mass spectroscopic data (see the supporting information, Fig. S2-S28). Definitive evidence for the structure of 5 was obtained through single-crystal X-ray analysis (CCDC 234884). The ORTEP diagram of 5 is shown in supporting information, Fig S1. The physical properties of the crystal are also presented in Tables S1-S9.
Here we take a closer look at the spectra of one of the products. As an example the 1H NMR spectrum of 5 showed two sharp signals at δ 2.49 and δ 3.36 ppm were related to thiomethyl and aminomethyl protons respectively. The NH2 group appeared at δ 7.73 ppm. The protons of aromatic ring were seen at δ 7.78–8.16 ppm. In the 1H-decoupled 13C NMR spectrum, characteristic signals corresponding to the SMe, NMe, CN, and CO groups of 5 were observed at δ 17.8, 32.0, 117.6 and 192.9 ppm respectively (Fig. 5). The mass spectrum of 5 showed a molecular ion peak at m/z = 356. The IR spectrum of 5 exhibited stretching absorption bands corresponding to the NH2 group at 3399 and 3315 cm− 1, the nitrile group at 2187 cm− 1 and the carbonyl group at 1724 cm− 1. Two absorption bands related to nitro group appeared at 1477 and 1331 cm− 1.
Mechanism
A plausible mechanism for the formation of propellane 5 is illustrated in Fig. 6. The reaction begins with the formation of a Knoevenagel adduct A between ninhydrin 1 and malononitrile 24, which then produces intermediate B by Michael addition of N-methyl-1-(thiomethyl)-2-nitroethanamine 3. This intermediate undergoes imine-enamine tautomerism, resulting in intermediate C. Subsequently, intramolecular nucleophilic addition of the NH to the carbonyl group leads to the formation of intermediate D. This mechanism proceeds with intramolecular cyclization via oxygen attack on the carbon of nitrile group, leading to the formation of the propellane system E. Finally, product 5 is obtained through a proton shift and imine-enamine tautomerization (Fig. 6). As an example, the steps for the formation of 7b is shown in Fig. 7. As can be seen the mechanism for the synthesis of products 7 are also similar, only initially the reaction between nitroketene dithioacetal and aliphatic diamine forms a nitroketene aminal product 6b (with the elimination of two moles of methane-thiol)31,36, which reacts with the condensation intermediate A as an active enamine (Fig. 7).
Experimental
General remarks
The chemicals utilized in the experiments, including malononitrile, ninhydrin, 1,1-bis(methylthio)-2-nitroethane, ethylenediamine, diaminopropane, 1,4-butanediamine and N-methyl-1-(thiomethyl)-2-nitroethanamine, as well as the solvents, were sourced from Aldrich and were used as received. The nuclear magnetic resonance (NMR) spectra for hydrogen (1H) and carbon (13C) nuclei were obtained using Bruker DRX-300 Avance spectrometers, operating at 300 MHz and 75.4 MHz, respectively, in deuterated DMSO-d6 solvent. Chemical shifts are reported in ppm (δ) and coupling constant (J) are given in hertz (Hz). Melting points were determined with an electrotherma1 9100 apparatus. Mass spectra were recorded with an Agilent 5975 C VL MSD with Triple-Axis detector operating using an ionization potential of 70 eV. IR spectra were measured using Bruker Tensor 27 spectrometer (ῡ in cm− 1) in the form of KBr tablets. The progress of the reactions was monitored by thin-layer chromatography (TLC) using Merck silica gel-coated plates, with detection achieved using a 254 nm ultraviolet lamp.
Synthesis of 2-(methylthio)dihydro(epoxyethano)indeno[1,2-b]pyrrole (5)
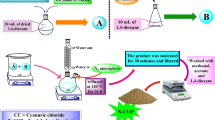
First Step: In a reaction vessel (2.5 cm diameter test tube, 20 cm length), a one-to-one mixture of ninhydrin (1 mmol, 0.189 g) and malononitrile (1 mmol, 0.066 g) was stirred in 8 mL of water at room temperature. After 3 min, a yellow precipitate formed, indicating the consumption of the starting materials, as confirmed by thin-layer chromatography (TLC). Second Step: After 30 min, without isolating the ninhydrin-malononitrile condensation precipitate (A), N-methyl-1-(thiomethyl)-2-nitroethanamine (1 mmol, 0.148 g) was added to the mixture. The reaction was stirred for 3 h at room temperature. The completion of the reaction was monitored by TLC, using an ethyl acetate/n-hexane (1:1) solvent system. Once the reaction was complete, the solid precipitate was filtered and washed with water, followed by hot ethanol, to yield the pure product.
Synthesis of fused proppellane derivatives (7a-g)
First Step: In a reaction container, a mixture of one mmol of the desired diamine (or ethanolamine/cysteamine) and 1,1-bis(methylthio)-2-nitroethene (1 mmol, 0.165 g) was stirred in 6 mL of ethanol under reflux conditions for 5 h. After completion, the solution was cooled in an ice bath, leading to the crystallization of the product. The crystals were then filtered and washed with cold ethanol. Second Step: In a separate reaction vessel, a one-to-one mixture of ninhydrin (1 mmol, 0.189 g) and malononitrile (1 mmol, 0.066 g) was stirred in 8 mL of water at room temperature. After 3 min, a yellow precipitate formed, indicating the consumption of the starting materials as confirmed by thin-layer chromatography (TLC). Third Step: The ketene aminal compound synthesized in the first step was added in the form of dry crystals to the mixture of the second step (ninhydrin-malononitrile condensation precipitation in water). The reaction was stirred for 5 h at room temperature. For TLC analysis, more polar solvent systems, such as ethyl acetate/n-hexane (2:1) or pure ethyl acetate, were used to observe the product spots. The final product was isolated by filtration and washed with hot water and ethanol to yield the pure compound.
10-Amino-1-methyl-2-(methylthio)-3-nitro-4-oxo-1,4-dihydro-8b,3a-(epoxyetheno)indeno[1,2-b]pyrrole-11-carbonitrile (5)
Yellow solid; yield: 0.306 g (86%); mp: 255–257 °C; IR (KBr) (ῡmax /cm− 1): 3399, 3315, 3251, 3198, 2187, 1724, 1648, 1580, 1331, 1018; 1H NMR (300 MHz, DMSO): δ 2.49 (3 H, s, SCH3), 3.36 (3 H, s, NCH3), 7.73 (2 H, s, NH2), 7.78–8.16 (4 H, m, ArH); 13C{1H} NMR (75.4 MHz, DMSO): δ 17.8 (SCH3), 32.0 (NCH3), 53.1 (C-CN), 65.7 (Csp3-C = O), 107.5 (Csp3-O), 117.6 (CN), 124.4 (C-C = NO2), 125.8, 126.1, 132.6, 136.6, 136.9, 142.5 (Ar), 162.0 (C = C-SMe), 167.6 (C-NH2), 192.9 (C = O); MS (EI, 70 eV): m/z (%) = 356 (1) [M]+, 339 (3), 313 (64), 268 (23), 222 (36), 179 (19), 154 (25), 126 (38), 94 (100), 79 (70), 61 (30).
12-Amino-10-nitro-9-oxo-1,2,3,9-tetrahydro-4a,9a-(epoxyetheno)indeno[2ʹ,1ʹ:4,5]pyrrolo[1,2-a]imidazole-11-carbonitrile (7a)24
Green solid; yield: 0.287 g (85%); mp: 229–231 °C; 1H NMR (300 MHz, DMSO): δ 3.44–3.54 (2 H, m, CH2NH), 3.90–3.94 (2 H, m, CH2N), 7.60 (2 H, s, NH2), 7.77–7.95 (4 H, m, ArH), 9.16 (1H, s, NH); 13C{1H} NMR (75.4 MHz, DMSO): δ 41.8 (CH2NH), 49.0 (CH2N), 52.2 (C-CN), 69.4 (Csp3-C = O), 101.4 (Csp3-O), 101.9 (C-NO2), 117.5 (CN), 125.1, 125.5, 132.1, 135.7, 136.5, 143.1 (Ar), 159.6 (C = C-NO2), 167.4 (C-NH2), 193.4 (C = O).
13-Amino-11-nitro-10-oxo-2,3,4,10-tetrahydro-1H-5a,10a-(epoxyetheno)indeno[2ʹ,1ʹ:4,5]pyrrolo[1,2-a]pyrimidine-12-carbonitrile (7b)24
Dark yellow solid; yield: 0.263 g (76%); mp: 255–257 °C; IR (KBr) (ῡmax /cm− 1): 3309, 3144, 2879, 2204, 1723, 1645, 1521, 1395, 1189, 1060, 828; 1H NMR (300 MHz, DMSO): δ 1.97–2.08 (2 H, m, CH2), 3.19–3.23 (1H, m, CH2NH), 3.35–3.36 (1H, m, CH2NH), 3.50–3.54 (1H, m, CH2N), 3.78–3.81 (1H, m, CH2N), 7.58 (2 H, s, NH2), 7.75–8.04 (4 H, m, ArH), 9.13 (1H, s, NH); 13C{1H} NMR (75.4 MHz, DMSO): δ 19.2 (CH2), 37.6 (CH2NH), 38.5 (CH2N), 52.4 (C-CN), 63.3 (Csp3-C = O), 104.3 (Csp3-O), 104.7 (C-NO2), 117.6 (CN), 125.0, 125.2, 132.1, 135.5, 136.3, 142.1 (Ar), 152.2 (C = C-NO2), 167.3 (C-NH2), 193.1 (C = O).
13-Amino-3,3-dimethyl-11-nitro-10-oxo-2,3,4,10-tetrahydro-1 H-5a,10a-(epoxyetheno)indeno[2ʹ,1ʹ:4,5]pyrrolo[1,2-a]pyrimidine-12-carbonitrile (7c)24
Dark yellow solid; yield: 0.345 g (85%); mp: 269–272 °C; IR (KBr) (ῡmax /cm− 1): 3434, 3254, 2966, 2193, 1724, 1659, 1528, 1444, 1318, 1281, 1041; 1H NMR (300 MHz, DMSO): δ 0.91 (3 H, s, CH3), 1.03 (3 H, s, CH3), 3.03 (2 H, AB quartet, δA = 2.98, δB = 3.09, CH2N), 3.42–3.50 (2 H, ABX system, CH2NH), 7.59 (2 H, s, NH2), 7.78–8.08 (4 H, m, ArH), 9.14 (1H, s, NH); 13C{1H} NMR (75.4 MHz, DMSO): δ 23.3 (CH3), 23.7 (CH3), 27.3 (CMe2), 48.9 (CH2NH), 49.4 (CH2N), 52.4 (C-CN), 63.5 (Csp3-C = O), 104.1 (Csp3-O), 104.5 (C-NO2), 117.6 (CN), 125.0, 125.3, 132.1, 135.4, 136.4, 142.2 (Ar), 151.4 (C = C-NO2), 167.4 (C-NH2), 193.1 (C = O); MS (EI, 70 eV): m/z (%) = 379 (0.1) [M]+, 367 (5), 332 (6), 303 (13), 248 (12), 187 (6), 165 (17), 127 (20), 104 (26), 76 (72), 56 (100).
14-Amino-12-nitro-11-oxo-1,2,3,4,5,11-hexahydro-6a,11a-(epoxyetheno)indeno[2ʹ,1ʹ:4,5]pyrrolo[1,2-a]1,3diazepine-13-carbonitrile (7d)24
Pink solid; yield: 0.340 g (88%); mp: 225–227 °C; IR (KBr) (ῡmax /cm− 1): 3304, 2195, 1725, 1625, 1533, 1432, 1333, 1135, 1083; 1H NMR (300 MHz, DMSO): δ 1.45–1.89 (4 H, m, 2CH2), 3.45–3.55 (2 H, m, CH2NH), 3.92–3.96 (2 H, m, CH2N), 7.60 (2 H, s, NH2), 7.75–7.96 (3 H, m, ArH), 8.14 (1H, d, J = 7.8 Hz, ArH), 9.50 (1H, s, NH); 13C{1H} NMR (75.4 MHz, DMSO): δ 25.7 (CH2), 26.1 (CH2), 42.5 (CH2NH), 44.2 (CH2N), 52.6 (C-CN), 63.4 (Csp3-C = O), 105.0 (Csp3-O), 105.7 (C-NO2), 117.5 (CN), 125.0, 125.1, 132.1, 135.5, 136.5, 143.1 (Ar), 158.2 (C = C-NO2), 167.1 (C-NH2), 192.9 (C = O).
12-Amino-10-nitro-9-oxo-3,9-dihydro-2 H-4a,9a-(epoxyetheno)indeno[2ʹ,1ʹ:4,5]pyrrolo[2,1-b]oxazole-11-carbonitrile (7e)
Yellow solid; yield: 0.263 g (78%); mp: 255–257 °C; 1H NMR (300 MHz, DMSO): δ 4.13 (2 H, t, J = 8.4 Hz, CH2N), 4.97 (2 H, t, J = 8.4 Hz, CH2O), 7.27 (2 H, s, NH2), 8.16 (4 H, m, ArH); 13C{1H} NMR (75.4 MHz, DMSO): δ 44.5 (CH2N), 52.6 (C-CN), 55.7 (CH2O), 72.3 (Csp3-C = O), 105.2 (Csp3-O), 105.8 (C-NO2), 117.6 (CN), 123.3, 125.1, 136.2, 136.9, 139.7, 142.4 (Ar), 158.6 (C = C-NO2), 167.2 (C-NH2), 199.1 (C = O).
12-Amino-10-nitro-9-oxo-3,9-dihydro-2H-4a,9a-(epoxyetheno)indeno[2ʹ,1ʹ:4,5]pyrrolo[2,1-b]thiazole-11-carbonitrile (7f)
Brown solid; yield: 0.285 g (80%); mp: 209–211 °C; IR (KBr) (ῡmax /cm− 1): 3453, 3349, 2187, 1715, 1639, 1441, 1276, 1188, 1018; 1H NMR (300 MHz, DMSO): δ 3.48 (2 H, t, J = 7.8 Hz, CH2S), 4.34 (2 H, t, J = 7.8 Hz, CH2N), 7.19 (2 H, s, NH2), 8.02–8.09 (4 H, m, ArH); 13C{1H} NMR (75.4 MHz, DMSO): δ 28.0 (CH2S), 51.5 (CH2N), 56.7 (C-CN), 57.2 (Csp3-C = O), 117.7 (CN), 118.5 (Csp3-O), 123.4, 137.0, 139.6 (Ar), 151.0 (C = C-S), 161.8 (C-NH2), 198.7 (C = O).
12-Amino-2-methyl-10-nitro-9-oxo-1,2,3,9-tetrahydro-4a,9a-(epoxyetheno)indeno[2ʹ,1ʹ:4,5]pyrrolo[1,2-a]imidazole-11-carbonitrile (7 g)24
Yellow solid; yield: 0.275 g (77%); mp: 215–217 °C; IR (KBr) (ῡmax /cm− 1): 3306, 3176, 2202, 1718, 1653, 1443, 1315, 1252, 1079; 1H NMR (300 MHz, DMSO): δ 1.33 (3 H, d, J = 6.3 Hz, CH3), 3.48–3.51 (1H, m, CH), 4.06–4.09 (2 H, m, CH2N), 7.58 (2 H, s, NH2), 7.88–7.96 (4 H, m, ArH), 9.36 (1H, s, NH); 13C{1H} NMR (75.4 MHz, DMSO): δ 19.3 (CH3), 48.1 (CH2N), 52.2 (C-CN), 57.8 (CHNH), 69.2 (Csp3-C = O), 101.9 (Csp3-O), 102.3 (C-NO2), 117.5 (CN), 124.9, 125.0, 132.0, 135.5, 136.5, 140.9 (Ar), 158.6 (C = C-NO2), 167.4 (C-NH2), 193.3 (C = O).
Conclusion
In this research, we developed an efficient and green method for the synthesis of functionalized heterocyclic [3.3.3]propellanes containing two moieties: 2-aminofuran and indenopyrrole or indopyrroloimidazole/pyrimidines/diazepine using ninhydrin, malononitrile and various nitroketene aminals. These reactions were carried out in water in two steps without the need for a catalyst and led to the formation of new oxa-aza[3.3.3]propellane products in high yields. Notably, this synthetic approach offers significant advantages, including a straightforward procedure, quick product separation, high chemoselectivity, absence of toxic solvents, sequentional one-pot method and high atom economy.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information file.
References
Dilmaç, A. M., Wezeman, T., Bär, R. M. & Bräse, S. Occurrence, synthesis and applications of natural and designed [3.3.3]propellanes. Nat. Prod. Rep. 37, 224–245 (2020).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Wiberg, K. B. Inverted geometries at carbon. Acc. Chem. Res. 17, 379–386 (1984).
Yavari, I., Malekafzali, A. & Skoulika, S. Tandem synthesis of trichloromethylated [3.3.3]propellanes from trichloroacetamidines and a ninhydrin-malononitrile adduct. Tetrahedron Lett. 55, 3154–3156 (2014).
Yavari, I., Khajeh-Khezri, A. & Halvagar, M. R. A synthesis of thioxo[3.3.3]propellanes from acenaphthoquinone-malononitrile adduct, primary amines and CS2 in water. Arab. J. Chem. 11, 188–195 (2017).
Kheilkordi, Z., Ziarani, G. M. & Mohajer, F. Application of multi-component reaction in the synthesis of heterocyclic [3.3.3]propellane derivatives. Curr. Org. Chem. 26, 287–298 (2022).
Dilmaç, A. M., Spuling, E., Meijere, A. & Bräse, S. Propellanes-from a chemical curiosity to explosive materials and natural products. Angew Chem. 56, 5684–5718 (2017).
Sokolenko, Y. M. et al. Far away from flatland. Synthesis and molecular structure of dihetera[3.3.n]propellanes and trihetera[3.3.n]propellanes: advanced analogues of morpholine/piperazine. J. Org. Chem. 84, 13908–13921 (2019).
Zu, L., Boal, B. W. & Garg, N. K. Total synthesis of (±)-aspidophylline A. J. Am. Chem. Soc. 133, 8877–8879 (2011).
Abdel-Lateef, H. M. et al. Green synthesis of novel pyridines via one-pot multicomponent reaction and their anti-inflammatory evaluation. ACS Omega. 8, 11326–11334 (2023).
De Lima, H. B., Das Neves, G. M., Gonçalves, I. L., Merlo, A. A. & Eifler-Lima, V. L. Multicomponent reactions in the last 30 years: how are we today? J. Braz Chem. Soc. 35, 1–33 (2024).
Shen, X., Hong, G. & Wang, L. Recent advances in green multi-component reactions for heterocyclic compound construction. Org. Biomol. Chem. 23, 2059–2078 (2025).
Mohlala, R. L., Rashamuse, T. J. & Coyanis, E. M. Highlighting multicomponent reactions as an efficient and facile alternative route in the chemical synthesis of organic-based molecules: a tremendous growth in the past 5 years. Front. Chem. 12, 1469677 (2024).
Nandi, S. et al. One-pot multicomponent reaction: A highly versatile strategy for the construction of valuable nitrogen‐containing heterocycles. Chemistryselect 7, e202201901 (2022).
Yi, R. N. & He, W. M. Photocatalytic inisci-type multicomponent reaction for the synthesis of 1-(halo)alkyl-3-heteroaryl bicyclo[1.1.1]pentanes. Chin. Chem. Lett. 35, 110115 (2024).
Zhang, M., Liu, Y. H., Shang, Z. R., Hu, H. C. & Zhang, Z. H. Supported molybdenum on graphene oxide/Fe3O4: an efficient, magnetically separable catalyst for one-pot construction of spiro-oxindole dihydropyridines in deep eutectic solvent under microwave irradiation. Catal. Commun. 88, 39–44 (2017).
Zhang, M. et al. Catalyst-free, visible-light promoted one-pot synthesis of spirooxindole-pyran derivatives in aqueous ethyl lactate. ACS Sustain. Chem. Eng. 5, 6175–6182 (2017).
Chen, X. et al. Highly diastereoselective convergent synthesis of polycyclic pyrroles with consecutive quaternary stereocenters: cascade construction of multiple C–C and C–hetero bonds. ACS Sustain. Chem. Eng. 2, 2391–2398 (2014).
Alizadeh, A., Bayat, F. & Zhu, L. G. Regioselective multicomponent sequential synthesis of oxa-aza[3.3.3]propellanes. Aust J. Chem. 67, 949–952 (2014).
Zhang, L. J. & Yan, C. G. One-pot domino reactions for synthesis of heterocyclic [3.3.3]propellanes and spiro[cyclopenta[b]pyridine-4,20-indenes]. Tetrahedron 69, 4915–4921 (2013).
Kheilkordi, Z., Ziarani, G. M., Badiei, A., Mohajer, F. & Luque, R. Fe3O4@SiO2@Pr–Oxime-(BuSO3H)3 synthesis and its application as magnetic nanocatalyst in the synthesis of heterocyclic [3.3.3]propellanes. J. Iran. Chem. Soc. 20, 591–597 (2023).
Alizadeh, A., Rezvanian, A. & Zhu, L. G. Synthesis of heterocyclic [3.3.3]propellanes via a sequential four-component reaction. J. Org. Chem. 77, 4385–4390 (2012).
Beyrati, M. & Hasaninejad, A. One-pot, sequential four-component synthesis of novel heterocyclic [3.3.3] propellane derivatives at room temperature. RSC Adv. 8, 14171–14176 (2018).
Alizadeh, A. & Rezvanian, A. Powerful approach to synthesis of fused oxa-aza[3.3.3]propellanes via chemoselective sequential MCR in a single pot. Tetrahedron 68, 10164–10168 (2012).
Chen, N. et al. Bridged heterocyclic neonicotinoid analogues: design, synthesis, and insecticidal activity. Res. Chem. Intermed. 41, 5293–5300 (2015).
Rahimi, Z., Bayat, M. & Hosseini, H. New multicomponent reactions in water: A facile synthesis of 1,3-dioxo-2-indanilidene-heterocyclic scaffolds and indenoquinoxalines through reaction of ninhydrin-malononitrile adduct with diverse N–binucleophiles. RSC Adv. 12, 33772–33779 (2022).
Hosseini, H. & Bayat, M. An efficient and ecofriendly synthesis of highly functionalized pyridones via a one-pot three-component reaction. RSC Adv. 8, 27131–27143 (2018).
Nasiriani, T. et al. Isocyanide-based multicomponent reactions in water: advanced green tools for the synthesis of heterocyclic compounds. Top. Curr. Chem. 380, 1–69 (2022).
Alizadeh, A., Zarei, A. & Rezvanian, A. A. Novel and one-pot multicomponent approach to the synthesis of dihyroindeno[1,2-b]pyrroles and indeno[2′,1′:4,5]pyrrolo[1,2-a]-fused 1,3-diazaheterocycles. Synthesis 42, 497–501 (2011).
Jeyachandran, V., Muthu, M. & Kumar, R. R. Efficient green protocol for the synthesis of novel dihydroindeno[1,2-b]pyrroles. Synth. Commun. 45, 1137–1144 (2015).
Yang, P. H. Recent developments in the heterocyclic ketene aminal-based synthesis of heterocycles. Res. Chem. Intermed. 42, 5617–5637 (2016).
Bayat, M. & Hosseini, F. S. Tetrahedron Lett. 58, 1616–1621 (2017).
Rouzban, H., Bayat, M. & Hosseini, H. Efficient regioselective five-component synthesis of novel thiazolo[3,2-a]pyridine carbohydrazides and oxazolo[3,2-a]pyridine carbohydrazides. Mol. Divers. 27, 667–678 (2023).
Chen, D. et al. Iridium/acid dual-catalyzed enantioselective aza-ene-type allylic alkylation of nitro ketene aminals with racemic allylic alcohols. Org. Lett. 26, 508–513 (2024).
Alizadeh, A., Mokhtari, J. & Ahmadi, M. Synthesis of the novel pyrimido[1,6-a] pyrimidine and imidazo[1,2-c]pyrimidine derivatives based on heterocyclic ketene aminals. Tetrahedron 68, 319–322 (2012).
Kazemi Movahed, S., Dabiri, M. & Bazgir, A. An efficient one-pot four‐component synthesis of functionalized imidazo[1,2‐a]pyridines. Helv. Chim. Acta. 96, 525–532 (2013).
Acknowledgements
Financial support of this research from Imam Khomeini International University, Iran is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
H. H. and M. B. designed the reactions and Z. R. carried them out. The entire process was done under the supervision of M. B. and the writing of the paper was done by all members. The analysis of the spectra and the identification of the structures were done by H. H. and M. B.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rahimi, Z., Bayat, M. & Hosseini, H. A highly chemo- and regioselective synthesis of heterocyclic [3.3.3]propellanes via sequential multicomponent reactions in water. Sci Rep 15, 27969 (2025). https://doi.org/10.1038/s41598-025-13577-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13577-0
Keywords
This article is cited by
-
DFT studies on the mechanism of one-pot α,γ-difunctionalization of β-ketoesters: regio-, chemo-, and stereoselectivity promoted by DBU/MeOH
Journal of Molecular Modeling (2025)