Abstract
Thyroid disorders are common in patients with autoimmune diseases such as rheumatoid arthritis (RA). Both conditions present significant public health concerns due to their impact on quality of life and increased mortality. The aim of this study was to assess the prevalence of thyroid abnormalities in and investigate their influence on rheumatoid arthritis characteristics. This was a case–control study involving adult female patients with RA and age-matched healthy controls. RA disease activity was evaluated using the Disease Activity Score (DAS28), and functional impact was assessed using the Health Assessment Questionnaire (HAQ). Serum thyroid function tests were performed, including thyroid-stimulating hormone (TSH), free thyroxine (FT4), and antithyroid antibodies (TAAs), including anti-thyroglobulin antibodies (TgAb), anti-thyroperoxidase antibodies (TPOAb), and TSH receptor antibodies (TRAb). The study included 58 female RA patients, with a mean age of 52 ± 14.2 years. The median disease duration was 10.41 years. The median DAS28-CRP was 4.03, and the median HAQ was 1.34. The median FT4 and TSH levels were 15.01 [13.48;16.71] and 1.42 [0.91;2.26], respectively. Thyroid dysfunction was identified in 19% of the participants, with hypothyroidism being the most common disorder (17%). Hyperthyroidism was observed in 2% of patients. Antithyroid antibodies were positive in 15.5% (n = 9) of participants, with TPOAb present in 6 patients (10.3%), TgAb in 3 patients (5.2%), and TRAb in 2 patients (3.4%). No statistically significant association was found between thyroid status and RA disease activity, functional impact, or serological status. Despite the lack of correlation, the high prevalence of thyroid dysfunction underscores the importance of regular thyroid screening to optimize management and prognosis in RA patients.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease of the connective tissue that predominantly affects the synovial membrane, leading to primarily articular manifestations1. Although the exact pathophysiological mechanisms remain unclear, RA is considered a multifactorial condition resulting from the interaction between genetic predisposition and environmental triggers, which activate the immune system and promote systemic inflammation2. This multifactorial nature of RA may also explain its association with other autoimmune diseases, such as autoimmune thyroiditis3,4.
Many studies have investigated the interplay between thyroid function and RA3,5,6. A potential association has been suggested between thyroid disorders, particularly hypothyroidism and autoimmune thyroiditis, and the clinical presentation and progression of RA3. For instance, hypothyroidism may exacerbate fatigue and musculoskeletal symptoms, mimicking or amplifying the manifestations of RA7. Additionally, some studies have reported that the presence of thyroid autoantibodies may correlate with higher disease activity scores6, more severe joint damage8, or greater functional impairment. However, these findings remain inconsistent, with other studies showing no significant relationship between thyroid status and RA disease outcomes9,10.
Although thyroid dysfunction has been widely studied in various populations, data from North African countries, including Tunisia, remain limited. Tunisia is generally considered to have adequate iodine intake due to national salt iodization programs; however, regional variations may still exist11. This study aims to address the data gap by providing region-specific insights that could inform local public health approaches. Despite the potential clinical significance of thyroid dysfunction in rheumatoid arthritis (RA), this association has been insufficiently explored in the Tunisian context. We aimed to determine the prevalence of thyroid abnormalities among RA patients and to evaluate their impact on clinical presentation, disease activity, and functional status.
Methods
Study design
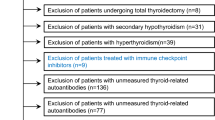
This case–control study was conducted between December 2021 and November 2022 at the Rheumatology Department of La Rabta hospital, a tertiary care center in Tunis, Tunisia. The study included adult female patients diagnosed with rheumatoid arthritis (RA) according to the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria12, and a group of healthy controls. Patients were consecutively recruited during routine outpatient visits or hospitalizations. The source population consisted of RA patients regularly followed in our department, representing a typical North African cohort with established RA. The control group consisted of adult female volunteers recruited from hospital staff, non-blood-related companions of patients, and individuals attending routine health screenings in the community. Controls were matched to RA patients by age (± 2 years) to minimize age-related bias. The exclusive inclusion of females aimed to reduce sex-related confounding. Matching was limited to age and did not extend to other variables such as BMI or comorbidities.
Participants with potential confounding factors that could influence thyroid function were excluded. These included individuals with a history of thyroidectomy or cervical irradiation, those receiving medications affecting thyroid function (such as amiodarone, lithium, or corticosteroids above physiological doses), as well as patients with active infiltrative diseases (e.g., tuberculosis, sarcoidosis), pituitary adenomas, pregnancy or breastfeeding, acute or chronic renal failure, chronic liver disease, known malignancies, or ongoing severe infections. All included participants were screened for thyroid function for the first time during this study.
Data collection
We systematically collected a comprehensive set of data, including demographic information (age and comorbidities), as well as detailed RA characteristics such as the age of onset, disease duration, and the number of swollen and tender joints. We also assessed morning stiffness and nocturnal awakenings to capture the functional burden of the disease. Radiographs of the hands and forefeet were obtained to evaluate the extent of structural damage caused by RA. Disease activity was measured using the Disease Activity Score (DAS28), incorporating C-reactive protein (CRP) as a biomarker for inflammation.
The activity score of patients is graded as follows: Remission (DAS 28 -CRP score of ≤ 2.6), low activity (DAS 28 -CRP > 2.6 and ≤ 3.2), moderate activity (DAS 28 -CRP > 3.2 and ≤ 5.1), and high activity (DAS 28 -CRP > 5.1)13.
To assess the functional impact of the disease, we used the Health Assessment Questionnaire (HAQ).
In addition, we documented functional signs related to thyroid dysfunction, which included symptoms indicative of hypothyroidism such as physical fatigue, hair loss, mental fatigue, dry skin, constipation, and weight gain, along with signs of hyperthyroidism (such as weight loss, nervousness, and anorexia).
Immunological assessments were carried out to measure the presence of rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA). Furthermore, thyroid function was carefully evaluated through the measurement of thyroid-stimulating hormone (TSH), free thyroxine (FT4), and thyroid autoantibodies (TAAs), including anti-thyroglobulin antibodies (TgAb), anti-thyroid peroxidase antibodies (TPOAb), and TSH receptor antibodies (TRAb).
Thyroid dysfunction was defined as any abnormality in serum TSH levels, with or without variations in FT4 levels. Thyroid autoimmunity was considered present if detectable levels of TPOAb, TgAb, and/or TRAb were identified, with or without accompanying thyroid dysfunction.
Statistical study
Descriptive statistics were computed for demographic, clinical, biological, radiographic, and therapeutic characteristics. Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range, IQR) based on data distribution. Categorical variables were expressed as frequencies and percentages.
The Kolmogorov–Smirnov test was used to assess the normality of the data distribution. For normally distributed data, parametric tests were used, including the Student’s t-test for comparing means between two groups. For non-normally distributed data, non-parametric tests, such as the Mann–Whitney U test, were applied.
Associations between categorical variables were evaluated using the Chi-square test or Fisher’s exact test when the expected cell counts were less than 5. Correlation analyses were performed using Pearson’s or Spearman’s correlation coefficients depending on the normality of the data.
The significance level was set at p < 0.05 for all statistical tests.
Results
We collected data from 58 female patients with RA. As shown in Table 1. The mean age was 52.38 ± 14.21 years. The median disease duration was 10.41 years, with more than half (52%) having a disease course of over 10 years. Immunologically, RF was positive in 87% of patients, and ACPA were positive in 73%. Erosive disease was observed in 66% of the patients. The median DAS28-CRP was 4.03, with the following distribution: Remission (8%), low activity (20%), moderate activity (47%), and high activity (25%).
The control group included 58 participants, with a mean age of 52.13 ± 14.77 years. There was no statistically significant difference in age between the two groups (p = 0.929).
Assessment of thyroid function tests
Regarding thyroid biological and immunological status, it showed that thyroid dysfunction was observed in 19% of RA patients (Table 2). The majority had hypothyroidism (17%), with 9% having subclinical hypothyroidism and 2% experiencing hyperthyroidism. Autoimmune thyroid disease was present in 15.5% of patients. Regarding thyroid autoantibodies, 10.3% of patients tested positive for TPOAb, 5.2% for TgAb, and 3.4% for TRAb. Compared to the control group, thyroid dysfunction, particularly hypothyroidism and higher levels of thyroid autoantibodies (TPOAb and TgAb), were significantly more frequent in the RA group.
Functional symptoms related to thyroid dysfunction in RA patients are demonstrated in Table 3.
Comparison of clinical and demographic characteristics of rheumatoid arthritis patients according to thyroid status
As exhibited in Table 4, the comparison of disease characteristics between RA patients with hypothyroidism and euthyroidism showed no significant differences in various clinical and demographic parameters. The mean age was similar between the two groups (54.3 ± 13.7 years versus 51.6 ± 14.4 years, p = 0.585), and there were no significant differences in the prevalence of hypertension, diabetes, dyslipidemia, or overweight/obesity. Disease duration was comparable between hypothyroid and euthyroid patients (13.13 versus 9.19 years, p = 0.428). Similarly, disease activity (DAS28-CRP: 4.49 [3.25–5.28] vesus 3.92 [2.95–5.26], p = 0.629), swollen joint count (SJC: 5.6 ± 5.6 versus 5.2 ± 5.2, p = 0.814), tender joint count (TJC: 7 ± 5.63 versus 6.9 ± 6.6, p = 0.956), C-reactive protein levels (CRP: 11.8 ± 7.8 versus 20.8 ± 34.2, p = 0.414), and pain intensity (VAS: 4.6 ± 2.2 versus 5.3 ± 2.4, p = 0.428) were also similar between groups. Functional impact, assessed by HAQ, was identical in both groups (1.5, p = 0.690). The prevalence of positive ACPA, ANA, and RF was not significantly different between the groups.
Correlation between thyroid hormones, thyroid autoantibodies, and clinical-biological parameters of rheumatoid arthritis
As shown in Table 5, the analysis of correlations between thyroid hormones (FT4, TSH), thyroid autoantibodies (TPOAb, TgAb, TRAb), and clinical-biological parameters of rheumatoid arthritis (RA) revealed no statistically significant associations. Disease duration, disease activity (DAS28), tender joint count, swollen joint count, patient global assessment (VAS), inflammation markers (CRP), as well as structural (NAD, NAT) and global (EGP) assessment parameters, all showed weak and non-significant correlations with thyroid markers. Notably, TRAb levels had a marginally stronger, though still non-significant, correlation with HAQ (p = 0.072).
Similarly in Table 6, across all groups (positive vs. negative for each type of thyroid autoantibody), there were no statistically significant differences observed in disease duration, DAS28, HAQ, and CRP.
Discussion
In our study, thyroid dysfunction was found in 19% of patients with rheumatoid arthritis, with hypothyroidism (clinical and subclinical) being the most common disorder, affecting 17% of patients. Hyperthyroidism was present in 2% of patients. Positive thyroid autoantibodies were detected in 16% of patients, with positivity rates of 10.3% for TPOAb, 5.2% for TgAb, and 3.4% for TRAb. Despite the presence of these autoantibodies, no significant differences were observed between patients with euthyroid and hypothyroid states in terms of disease duration, disease activity (DAS28), functional impact (HAQ), tender joint count, swollen joint count, patient global assessment (VAS) or inflammation markers (CRP). Additionally, no significant correlations were found between thyroid hormone levels, thyroid autoantibodies, and the clinical or biological parameters of RA. Notably, TRAb levels had a marginally stronger, though still non-significant, correlation with HAQ (p = 0.072).
The relation between RA and thyroid function has long been studied14,15. In fact, both disorders share common genetic and environmental factors involved in their pathogenesis, leading to a breakdown in self-tolerance and the development of autoimmune diseases. Several studies have suggested the involvement of shared genes in the susceptibility to developing both RA and autoimmune thyroid disorders. For instance, HLA-DRB1 (Human Leukocyte Antigen DR) is considered a major susceptibility locus for RA14,16, while arginine at position 74 of the HLA-DRB1 chain (DRb-Arg74) has been implicated in the development of Graves’ disease17. The role of thyroid hormones in RA was further supported by an immunohistochemical and genetic study conducted by Pörings et al.18, which demonstrated that the inflammatory synovial environment in RA could reduce TH bioavailability by degrading them in situ via deiodinases.
Li et al.2, in a case–control study and a meta-analysis, reported a significantly higher frequency of both hypothyroidism and hyperthyroidism in patients with RA compared to healthy controls. These findings are further supported by Liu et al.15 in another recent meta-analysis.
A meta-analysis conducted by Pan et al.14, which included 13 studies, suggested that thyroid autoimmunity is significantly more common in patients with RA than in healthy controls. In the literature, the frequency of TAAs positivity in RA ranges from 4 to 32%19, which is consistent with our study, where 9 patients (representing 15.5%) were positive for TAAs. The prevalence of TPOAb ranges between 5 and 37%20,21; in our study, 6 patients (10.3%) tested positive for TPOAb, aligning with the lower range of reported values. Similarly, the prevalence of TgAb between 5 and 31%21,22,23, with our study finding 3 patients (5.2%) positive, which is also at the lower end of this range. Few studies have measured TRAb, with positivity rates ranging from 0 to 7% in RA patients6,22; in our study, 2 patients (3.4%) were positive, consistent with the lower spectrum of reported frequencies. Numerous studies have shown that the prevalence of hypothyroidism (primarily subclinical hypothyroidism) and thyroid autoimmunity increases with age in RA patients24,25,26. In our study, we didn’t find a significant difference in age between patients with thyroid dysfunction and those with normal thyroid function. This finding is consistent with that of Tekaya et al.27 and Atzeni et al.9.
Other than the age of the patients, a link was reported between the occurrence of an autoimmune thyroid disease and the evolution of the rheumatic disease. In fact, Waldenlind et al.28 observed that the risk of autoimmune thyroid disease increased during the five years preceding RA diagnosis, peaked at the time of diagnosis, and then declined two to five years after the RA diagnosis. This suggests a potential impact of antirheumatic therapies on reducing the risk of thyroid dysfunction. In our study, neither the age of onset nor the duration of RA was associated with thyroid dysfunction or the presence of TAAs. Similar findings were reported by Li et al.2, Emamifar et al.29, and Atzeni et al.9, who found no correlation between disease duration and thyroid status in RA.
Studies on the relationship between RA disease activity and thyroid status have reported conflicting results. In our study, no statistically significant association was found between thyroid status and the DAS28 score, nor with the Visual Analog Scale (VAS), swollen and tender joint counts, radiographic scores, or rheumatoid nodules. Similarly, no significant association was found between thyroid status and the functional impact of the disease. There was no correlation between thyroid autoimmunity and disease activity or functional capacity. Like our results, other studies have not demonstrated an association between thyroid status and RA disease activity, including those by Tekaya et al.27, Atzeni et al.9, and Hussein et al.30. In contrast, Waseem et al.31 reported a significant association between thyroid dysfunction and RA disease activity. Disease activity parameters such as VAS for pain, the Global Assessment Scale (GAS), swollen joint counts, DAS28, and erythrocyte sedimentation rate (ESR) were significantly higher in patients with thyroid dysfunction compared to those without. In the study by Waldenlind et al.5, HAQ and pain levels were significantly higher in patients with clinical autoimmune thyroiditis compared to those without at the time of RA diagnosis. The authors suggested that in RA, patient-reported outcomes like VAS and HAQ might be overestimated due to symptoms like fatigue, arthralgia, and myalgia caused by concurrent thyroid disorders. Posselt et al.32 found a significant association between TPOAb, hypothyroidism, and RA disease activity.
In our study, 87% of patients were RF positive, and 73% were positive for ACPA. However, no significant association was found between thyroid status and the serological status of patients. Comparable results have been reported in several studies, such as those by Joshi et al.33, Cardenas Roldan et al.20, and Koszarny et al.6. Nadeem et al.34 also conducted a cross-sectional study on 385 RA patients, reporting a higher frequency of RF positivity in subjects with subclinical hypothyroidism compared to those without. While no significant association was found between ACPA and the thyroid status34. Different results were found in the study by Ghitany et al.35, who compared the thyroid function in RA patients based on seropositivity (RF and/or ACPA) versus Sero negativity. Although no difference was found between the two groups in terms of thyroid dysfunction frequency, a higher prevalence of thyroid autoimmunity was observed in seropositive RA patients compared to seronegative ones. Indeed, TPOAb and TRAb levels were positively correlated with RF and ACPA levels. Similarly, Karakiliç et al.36 found a positive correlation between TPOAb and ACPA levels. These findings support the hypothesis that RA and thyroid dysfunction share a common autoimmune process.
In our study, no association was found between thyroid status and CRP levels in RA patients. These findings are consistent with those of El Attar et al.37 and Atzeni et al.9. However, Koszarny et al.6, in their study on the relationship between RA activity and thyroid autoimmunity, found a positive correlation between plasma TgAb levels, CRP, and ESR. A positive and significant correlation between serum TSH levels and ESR was reported by Azeem et al.38 in a study exploring the impact of hypothyroidism on 1000 RA patients. However, no correlation was found between thyroid hormones and CRP levels. These findings align with those reported by Saqre et al.39, where RA patients with comorbid autoimmune thyroid disease had significantly higher ESR levels than those without thyroid dysfunction, but no difference in CRP levels was found between the two groups.
The strengths of our study include being the first in Tunisia to measure anti-TSH receptor antibodies in RA patients, as well as performing tests for thyroid autoantibodies that are not routinely measured in clinical biochemistry laboratories. However, the small sample size may limit the statistical power of our findings. Genetic predisposition to thyroid disorders was not assessed in this study, which represents another limitation.
In conclusion, thyroid disorders are common in the general population, but they are even more prevalent in patients with autoimmune diseases like RA. The elevated risk of thyroid dysfunction in RA highlights the importance and relevance of conducting thyroid evaluations. However, the thyroid dysfunction and the presence of antithyroid antibodies did not influence the course of the RA. Larger studies should be done before concluding.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to restrictions on ethical and data management approvals. Still, they are available from the corresponding author upon reasonable request.
References
Scott, D. L., Wolfe, F. & Huizinga, T. W. Rheumatoid arthritis. Lancet 376(9746), 1094–1108. https://doi.org/10.1016/S0140-6736(10)60826-4 (2010).
Li, Q. et al. Increased risk of thyroid dysfunction among patients with rheumatoid arthritis. Front. Endocrinol. 9, 799. https://doi.org/10.3389/fendo.2018.00799 (2019).
Lichtiger, A., Fadaei, G. & Tagoe, C. E. Autoimmune thyroid disease and rheumatoid arthritis: Where the twain meet. Clin. Rheumatol. 43(3), 895–905. https://doi.org/10.1007/s10067-024-06888-6 (2024).
Nazary, K. et al. Prevalence of thyroid dysfunction in newly diagnosed rheumatoid arthritis patients. Cureus 13(9), 18204. https://doi.org/10.7759/cureus.18204 (2021).
Waldenlind, K., Delcoigne, B., Saevarsdottir, S. & Askling, J. Does autoimmune thyroid disease affect rheumatoid arthritis disease activity or response to methotrexate?. RMD Open 6(2), 001282. https://doi.org/10.1136/rmdopen-2020-001282 (2020).
Koszarny, A., Majdan, M., Suszek, D., Wielosz, E. & Dryglewska, M. Relationship between rheumatoid arthritis activity and antithyroid antibodies. Pol. Arch. Med. Wewn. 123(7–8), 394–400. https://doi.org/10.20452/pamw.1829 (2013).
Zohaib, A. et al. Correlation of hypothyroidism with disease activity score-28 in patients of rheumatoid arthritis. Cureus 14(6), 26382. https://doi.org/10.7759/cureus.26382 (2022).
Chen, Y. L. et al. Joint damage is amplified in rheumatoid arthritis patients with positive thyroid autoantibodies. PeerJ 6, 4216. https://doi.org/10.7717/peerj.4216 (2018).
Atzeni, F. et al. Anti-thyroid antibodies and thyroid dysfunction in rheumatoid arthritis: Prevalence and clinical value. Autoimmunity 41(1), 111–115. https://doi.org/10.1080/08916930701620100 (2008).
Bianchi, G. et al. Thyroid involvement in chronic inflammatory rheumatological disorders. Clin. Rheumatol. 12(4), 479–484. https://doi.org/10.1007/BF02231775 (1993).
Doggui, R., El Ati-Hellal, M., Traissac, P., Lahmar, L. & El Ati, J. Adequacy assessment of a universal salt iodization program two decades after its implementation: A national cross-sectional study of iodine status among school-age children in Tunisia. Nutrients 9(1), 6. https://doi.org/10.3390/nu9010006 (2016).
Kay, J. & Upchurch, K. S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology 51(Suppl 6), vi5–vi9. https://doi.org/10.1093/rheumatology/kes279 (2012).
van Riel, P. L. & Renskers, L. The disease activity score (DAS) and the disease activity score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clin. Exp. Rheumatol. 34(5 Suppl 101), S40–S44 (2016).
Pan, X. F., Gu, J. Q. & Shan, Z. Y. Increased risk of thyroid autoimmunity in rheumatoid arthritis: A systematic review and meta-analysis. Endocrine 50(1), 79–86. https://doi.org/10.1007/s12020-015-0533-x (2015).
Liu, Y. J., Miao, H. B., Lin, S. & Chen, Z. Association between rheumatoid arthritis and thyroid dysfunction: A meta-analysis and systematic review. Front. Endocrinol. 13, 1015516. https://doi.org/10.3389/fendo.2022.1015516 (2022).
van der Helm-van Mil, A. H., Wesoly, J. Z. & Huizinga, T. W. Understanding the genetic contribution to rheumatoid arthritis. Curr. Opin. Rheumatol. 17(3), 299–304. https://doi.org/10.1097/01.bor.0000160780.13012.be (2005).
Ban, Y. et al. Arginine at position 74 of the HLA-DR beta1 chain is associated with Graves’ disease. Genes Immun. 5(3), 203–208. https://doi.org/10.1038/sj.gene.6364059 (2004).
Pörings, A. S., Lowin, T., Dufner, B., Grifka, J. & Straub, R. H. A thyroid hormone network exists in synovial fibroblasts of rheumatoid arthritis and osteoarthritis patients. Sci. Rep. 9(1), 13235. https://doi.org/10.1038/s41598-019-49743-4 (2019).
Conigliaro, P. et al. Autoimmune thyroid disorders and rheumatoid arthritis: A bidirectional interplay. Autoimmun. Rev. 19(6), 102529N. https://doi.org/10.1016/j.autrev.2020.102529 (2020).
Cárdenas Roldán, J. et al. Autoimmune thyroid disease in rheumatoid arthritis: A global perspective. Arthritis 2012, 864907. https://doi.org/10.1155/2012/864907 (2012).
El-Sherif, W. T., El Gendi, S. S., Ashmawy, M. M., Ahmed, H. M. & Salama, M. M. Thyroid disorders and autoantibodies in systemic lupus erythematosus and rheumatoid arthritis patients. Egypt. J. Immunol. 11(2), 81–90 (2004).
Nakamura, H. et al. Prevalence of interrelated autoantibodies in thyroid diseases and autoimmune disorders. J. Endocrinol. Invest. 31(10), 861–865. https://doi.org/10.1007/BF03346432 (2008).
Koszarny, A., Majdan, M., Dryglewska, M. & Tabarkiewicz, J. Prevalence of selected organ-specific autoantibodies in rheumatoid arthritis and primary Sjögren’s syndrome patients. Reumatologia 53(2), 61–68. https://doi.org/10.5114/reum.2015.51504 (2015).
Mahagna, H. et al. Rheumatoid arthritis and thyroid dysfunction: A cross-sectional study and a review of the literature. Best Pract. Res. Clin. Rheumatol. 32(5), 683–691. https://doi.org/10.1016/j.berh.2019.01.021 (2018).
Zhai, X. et al. An age-specific serum thyrotropin reference range for the diagnosis of thyroid diseases in older adults: A cross-sectional survey in China. Thyroid 28(12), 1571–1579. https://doi.org/10.1089/thy.2017.0715 (2018).
Huang, C. M. et al. Hypothyroidism risk associated with rheumatoid arthritis: A population-based retrospective cohort study. Medicine 101(1), 28487. https://doi.org/10.1097/MD.0000000000028487 (2022).
Tekaya, R. et al. Relationship between autoimmune thyroid disorders and rheumatoid arthritis. J. Adv. Med. Pharm. Sci. 7(1), 1–6. https://doi.org/10.9734/JAMPS/2016/24689 (2016).
Waldenlind, K., Saevarsdottir, S., Bengtsson, C. & Askling, J. Risk of thyroxine-treated autoimmune thyroid disease associated with disease onset in patients with rheumatoid arthritis. JAMA Netw. Open 1(6), 183567. https://doi.org/10.1001/jamanetworkopen.2018.3567 (2018).
Emamifar, A., Hangaard, J. & Jensen Hansen, I. M. Thyroid disorders in patients with newly diagnosed rheumatoid arthritis is associated with poor initial treatment response evaluated by disease activity score in 28 joints-C-reactive protein (DAS28-CRP): An observational cohort study. Medicine 96(43), 8357. https://doi.org/10.1097/MD.0000000000008357 (2017).
Hussein, S., Mansour, H., Hussein, M., Abdel Aziz, A. & Aziz, N. Thyroid autoantibodies in Egyptian patients with autoimmune rheumatic diseases: Relation to disease activity and functional impairment. Egypt. J Hosp. Med. 80(2), 936–942. https://doi.org/10.21608/ejhm.2020.103659 (2020).
Waseem, M. et al. Effect of thyroid dysfunction on disease activity of patients with rheumatoid arthritis. Int. J. Res. Med. Sci. 7(2), 491–495. https://doi.org/10.18203/2320-6012.ijrms20190360 (2019).
Posselt, R. T. et al. Prevalence of thyroid autoantibodies in patients with systematic autoimmune rheumatic diseases. Cross-sectional study. Sao Paulo Med. J. 135(6), 535–540. https://doi.org/10.1590/1516-3180.2017.0089110617 (2017).
Joshi, P., Agarwal, A., Vyas, S. & Kumar, R. Prevalence of hypothyroidism in rheumatoid arthritis and its correlation with disease activity. Trop. Doct. 47(1), 6–10. https://doi.org/10.1177/0049475515627235 (2017).
Nadeem, M., Khaliq, A., Bhat, M., Mustafa, F. & Mushtaqe, M. Spectrum of thyroid disorders in sero positive rheumatoid arthritis. J. Thyroid Disord. Ther. 06, 1–6 (2017).
Ghitany. Autoimmune thyroid disorders in seropositive versus seronegative rheumatoid arthritis (2015).
Karakiliç, G. D., Borman, P., Kocaoğlu, S., Büyük, F. & Bakirci, E. Ş. Hypothyroidism and autoimmune thyroid disorders in rheumatoıd arthritis: Relationship wıth disease activity. Rom. J. Int. 62(2), 160–167. https://doi.org/10.2478/rjim-2024-0002 (2024).
Elattar, E. A., Younes, T. B. & Mobasher, S. A. Hypothyroidism in patients with rheumatoid arthritis and its relation to disease activity. Egypt Rheumatol. Rehabil. 41, 58–65. https://doi.org/10.4103/1110-161X.132458 (2014).
Azeem, H. A., Alkabeer, A., Hashim, A. M., Rayan, M. M. & Ahmed, A. H. Study of prevalence of hypothyroidism in rheumatoid arthritis patients and its impact on disease severity. Int. J. Clin. Rheumatol. 14, 151 (2019).
Saqre, I. M., El-Bahnasawy, A. S., Farag, S.E.-D.M. & Bazeed, F. B. Autoimmune thyroid disease in Egyptian patients with rheumatoid arthritis. Egypt Rheumatol. 41, 167–177 (2019).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception of the work; D.M and E.B wrote the main manuscript. S.R, M.M, A.B and H.S were in charge of data collection and analysis. K.Z, S.R and S.B did the conception of the work. A.K and H.W reviewed the litterature. All authors revised the manuscript, approved the final version, and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and informed consent statements
The study was conducted according to the Declaration of Helsinki and the research protocol approved by the Ethics Committee of the Pasteur Institute of Tunis (No.2022/9/I).) and proved to be of no direct individual benefit. Helsinki Declaration has been followed for involving human subjects in this study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahmouni, S., Dhifallah, M., Mrad, M. et al. Prevalence and impact of thyroid dysfunction in patients with rheumatoid arthritis. Sci Rep 15, 34491 (2025). https://doi.org/10.1038/s41598-025-13650-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13650-8