Abstract
Preeclampsia involves placental dysfunction, leading to significant gross and histological changes, including reduced placental weight and size, infarction, and calcification. Pathological placenta analysis predicts pregnancy outcomes, aids subsequent pregnancies, and illuminates the pathophysiology of poor initial pregnancy outcomes. The main aim of this study was to assess the histomorphometry variations of the placenta and its correlation with birth outcome among preeclamptic and normotensive mothers attending the labor ward, at Arba Minch General Hospital Southern Ethiopia from October to December 2023. The study included 119 participants, with 30 diagnosed with preeclampsia and 89 with normal blood pressure. After delivery, both placentas and babies weights were meticulously measured, and a detailed microscopic examination of placental specimens was conducted. Statistical analysis, using SPSS version 21, was carried out to explore the correlation between histopathological changes and birth weight, considering a significant p-value to be less than 0.05. In our study, we found that 5.6% of babies in normotensive group and 53.3% of babies born to preeclamptic women were underweight. We observed histopathological alterations at a higher rate in the preeclamptic group (p = 0.001). Additionally, we found a significant association between birth weight and histopathologic alterations in the placenta (p = 0.001). Preeclampsia causes placental tissue alteration and affects birth outcomes. Comprehensive obstetric care and targeted interventions are crucial for managing preeclampsia and minimizing risks.
Similar content being viewed by others
Introduction
The placenta, a round organ, reflects prenatal events and weighs about 470 grams with a diameter of 22 centimeters and a thickness of 2.5 centimeters. It’ is essential for fetal growth, providing nutrition, oxygen, and waste removal. During pregnancy, it facilitates maternal-fetal communication, hormone synthesis, and immunological tolerance1. Preeclampsia is a serious condition typically arising after 20 weeks of gestation, identified by sudden high blood pressure, often with protein in the urine. If a woman experiences two instances of elevated blood pressure within four hours, she should seek immediate medical attention2.
Preeclampsia is characterized by placental dysfunction, resulting in diminished blood flow and uteroplacental insufficiency, which in turn impacts fetal development and poses potential complications3. The compromised placenta induces endothelial dysfunction and distinct symptoms. The condition advances through two stages due to abnormal placentation and insufficient cytotrophoblast invasion, disrupting normal fetal development. Consequently, placental growth retardation and functional derangement impair fetal growth, leading to significant health consequences in newborns and later in life4.
Previous research has demonstrated that histological changes, such as fibrinoid necrosis, syncytial knots, calcifications, and endothelial proliferation, are notably elevated in the hypertensive cohort5. These alterations signify vascular damage within the placenta, which may compromise its ability to adequately facilitate fetal oxygenation and nourishment. Furthermore, the presence of these histological aberrations may serve as prognostic indicators for potential maternal and neonatal health complications6. These findings underscore the critical importance of vigilant blood pressure monitoring throughout gestation and the timely and effective management of preeclampsia to mitigate the possibility of adverse outcomes for both the mother and the neonate7.
The experience of motherhood is a profound one, and it is imperative to address the health challenges that many women encounter during this transformative period. Previous research into placental changes and their correlation with preeclampsia has predominantly centered on participants from Western nations. However, it is essential to acknowledge the multifaceted nature of these conditions, taking into consideration genetic, immunological, environmental, social, and cultural factors. Consequently, further research is indispensable to yield conclusive evidence.
The present study aims to contrast placental histomorphometry in preeclampsia and normal cases, to acquire a comprehensive understanding of the underlying pathological mechanisms. The primary goal of this project is to build a foundational body of knowledge in our nation, educate medical professionals about the effects of preeclampsia on placental macro and microarchitecture, and advocate for early identification and treatment to improve delivery outcomes. This research has the potential to furnish invaluable insights into the determinants of preeclampsia and related disorders, ultimately contributing to the development of more efficacious treatment and management options.
Materials and methods
Study settings, design and population
An institutional based comparative cross sectional study design was conducted at Arba Minch General Hospital, located in Arba Minch town, Gamo zone, Southern Ethiopia. Before Arba Minch University Comprehensive and Teaching Specialized Hospital was established, Arba Minch General Hospital served as the only referral hospital in Gamo Zone, Southern Ethiopia.
The hospital gives services in internal medicine, obstetrics/gynecology, surgery, and pediatrics, and serving 113,000 patients a year. Its clinical staff consists of 114 BSc nurses, 45 general practitioners, 52 midwives, 16 specialists, 6 integrated emergency surgical officers, 45 general practitioners, 30 health officers,1 ophthalmologist, 4 optometrists, 3 ophthalmic nurses,, 12 anesthetists, 3 HMIS, 28 laboratory technicians, 28 laboratory technologists, 34 pharmacists, 8 radiologists and 3 psychiatry nurses.
Eligibility criteria
Inclusion criteria
Pregnant women who were diagnosed to have preeclampsia and those pregnant women without preeclampsia were included.
Exclusion criteria
Maternal conditions that results in intrauterine growth restriction, change in the histology and gross morphology of the placenta, such as anemia, malaria, preexisting diabetes mellitus, multiple pregnancy, diagnosed single umbilical artery, and multiple pregnancy were excluded.
Sample size determination and sampling procedure
The sample size was calculated using Epi info version 7.2.5.0, based on the following assumptions; power = 80%, ratio of exposed to non-exposed = 1:3, percentage of outcome in unexposed = 53.4%, percentage of outcome in exposed = 83.3%.
The total sample size calculated was 119 which includes 30 (number of cases) and 89(number of controls)8.
As Arba Minch General Hospital has a pathology laboratory for pathological examination, it was purposively selected. Cases were chosen sequentially in order of arrival, and three consecutive controls were selected for each case using simple random sampling technique.
Study variables
Dependent variables
Pathological changes of placenta9, Gross morphology of the placenta, and Birth outcomes.
Independent variables
Socio-demographic variables, Obstetric data of the mothers, Gravidity and mode of delivery.
Operational definitions
Pathological changes of placenta
The Histo-pathological changes are characterized by one or more of the following features; syncytial Knots, Cytotrophoblastic proliferation, Cellular proliferation, Fibrinoid, Necrosis, Endothelial Proliferation Calcification, and Fibrosis.
Cases
Full-term pregnant women diagnosed with preeclampsia.
Controls
Full-term pregnant women without preeclampsia, experiencing uncomplicated pregnancies.
Preeclampsia diagnosis criteria
Preeclampsia is diagnosed as new-onset hypertension (systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg) occurring after 20 weeks gestation with or without proteinuria in accordance with clinical guidelines10.
Complete term pregnancy
Examined as deliveries occurring between 37 + 0 and 40 + 6 weeks of gestation. This strict reference was set in order to ensure homogeneity in the analysis of placental morphology11.
Birth outcomes
Primary outcome was measured as weight (grams) of the neonate immediately post birth which provides a quantifiable measure of fetal growth.
Data collection tools and procedures
The data were collected using a structured interviewer administered questionnaire combined with observational checklist. The questionnaire was prepared or adopted using different literatures3,12,13. It was divided into sub-sections covering to Socio-demographic characteristics and obstetric history. The observational checklist contains birth outcome related items and assessment of placenta including gross and microscopic findings.
The midwife collected placentas immediately following delivery in a sterile tray. The placentas were then subjected to thorough washing under running tap water and subsequently weighed using a traditional weighing machine. Following weighing, the placentas were immersed in a 10% formalin solution, then carefully placed in a formalin-filled jar and labeled with corresponding codes. The specimens were subsequently transported to the pathology department of Arba Minch General Hospital for gross and microscopic examination by the primary investigator and the pathologist. Detailed assessments, including measurements of the diameter and thickness of each placental tissue piece, evaluation of cord insertion, fetal membrane condition, cord vasculature, and placental cotyledons, as well as determination of the presence of retroplacental hemorrhage, extensive infarction, and fibrosis, were conducted. Additionally, calcification, fibrosis, necrosis, and hemorrhage were assessed on the cut surface of the placenta.
A representative sample of placental tissue, encompassing placental samples from the fetal and maternal surfaces, one cord sample, and a rolled fetal membrane sample, was collected and securely packaged in a single cassette labeled with a specific code. Cassettes containing placental tissue from cases and controls were stored in separate marked containers to prevent formalin leakage. Subsequently, these tissue cassettes were transported to the Pathology Laboratory of Alatyon General Hospital, Hawassa, Ethiopia for tissue processing and staining. The tissue underwent processing using a semi-automated tissue processor to remove formalin, followed by embedding in paraffin wax employing a tissue embedding system. The embedded tissue blocks were then sectioned at a thickness of 3–5 μm using a microtome, after which they were prepared for staining.
Hematoxylin and Eosin staining was performed using an automated tissue stainer, and the stained and labeled tissue slides were assessed by a pathologist at the Histology Laboratory of Arba Minch University. The primary investigator conducted an examination of the slides alongside the pathologist using a multi-headed microscope and documented histopathological findings in structured checklists based on the corresponding codes. Each tissue slide was methodically evaluated for the presence of syncytial knots, cytotrophoblastic cellular proliferation, fibroid necrosis, endothelial proliferation, calcification, hyalinized villi, and fibrosis. Comprehensive gross and microscopic findings were recorded for both each cases and controls according to the specific codes.
Data processing and analysis
After ensuring the completeness and consistency of the data, they were entered into Excel and exported to SPSS version 21 for analysis. Descriptive statistics, such as frequency, mean, and standard deviation, were calculated and presented using tables and graphs to characterize the study variable. The chi-square test was employed to assess placental histology in preeclampsia mothers compared to normotensive mothers. Fisher’s exact test was used for histological changes with expected cell counts of less than 5. Furthermore, an independent t-test was utilized to compare the mean placental measurement and mean birth weight between different groups. Additionally, a point biserial correlation analysis, a special type of Pearson correlation, was conducted to evaluate the relationship between birth weight and histopathologic changes. Statistical significance was attributed to results with a p-value of less than 0.05.
Data quality assurance
Data cleaning assessed completeness, consistency, outliers, and missing values. Data collectors and supervisors received two days of training on the study purpose, datacollection tools, techniques, and ethical procedures. A pretest was conducted on 5% of the sample outside the study area, and necessary revisions were made. To ensure consistency, 5% of the collected data were randomly selected and crosschecked; any discrepancies were corrected by supervisors and investigators. During the datacollection period, the principal investigator reviewed 5% of the forms daily for completeness.
Results
Socio-demographic characteristics
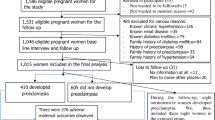
Among 119 mothers who participated in this study, 30 were preeclampsia and 89 were normotensive. More than half of controls, 46 (51.7%), were in the 26–32 age group, while three-fifths of the cases were in the 18–25 age group. Regarding the educational status,5(16.7%) of cases and 3(3.4%) of controls were illiterate (Table 1).
Obstetric and birth outcome characteristics
Among the participants 63.3% of cases and 44.9% of controls were primigravidae. In the case group 63.3% delivered via spontaneous vaginal delivery, compared to 61.8% in the comparative group. Cesarean section was utilized by 38.2% and 36.7% of participants in the comparison and case groups, respectively (Fig. 1).
In this study, among the babies delivered from the case and comparison groups, 5.6% of the babies in the comparative groups and 53.3% of the babies delivered from preeclampsia mothers were underweight. For the case and comparative groups, the mean birth weight was 2.5 kg ± 0.59 and 3.3 kg ± 0.51, (P = 0.001), respectively. It was observed that NICU admissions to hospitals in the case group was higher than controls, 11 (36.7%), 5 (5.6%), respectively (Table 2).
Using point Biserial correlation the correlation between birth weight and histopathological changes was determined and found that it was significantly correlated(correlation coefficient = 0.343,P < 0.001).
Histomorphometric findings of the placenta among cases and controls
The morphometric changes of the placenta were observed. The mean diameter of the placenta among cases was lower than controls 14.16 ± 1.29, 17.97 ± 1.84, respectively (P < 0.001).Similarly the weight of the placenta among cases was significantly lower than controls, measuring 463.33 ± 64.23grm and 649.4 ± 135.36grm, respectively, P = 0.001).
Among cases, 10% of the participants exhibited gross calcification compared to 2% in the control group. Retroplacental clotting was observed in 25.8% of the controls and 90% of the cases. Concerning cord insertion morphology, central cord insertion was detected in 23.3% of the cases and 49 (l.4%) of the controls. The eccentric type of cord insertion was found in 36.7% of the cases and 44.95% of the controls, while marginal cord insertions were observed in 90% of the cases and 5.6% of the controls.
On the other hand histopatholgical changes of the placenta were also recorded (Fig. 2). Cytotrophoblastic cellular proliferation was lower in controls than cases, rating 12.4% and 50%, respectively, P = 0.001. Fibrosis necrosis was observed higher in case group than control (40% vs. 11.2%, P = 0.001). Hyalinized villi and microscopical calcification seen in 16 (18%) of the comparative groups and 18 (60.0%) of the case groups(P = 0.001) (Table 3).
Discussion
The objective of this study was to conduct a comparative analysis of placental histomorphometry between mothers affected by preeclampsia and those with normotensive pregnancies. The research entailed an examination of the gross morphological and histological characteristics of placentas procured from preeclamptic and normotensive pregnant women admitted to Arba Minch General Hospital. The findings revealed distinct differences, with placentas from the preeclamptic cohort displaying reduced average diameter, thinner walls, and diminished overall size in comparison to placentas from normotensive counterparts.
The outcome is consistent with research conducted in Addis Ababa, Ethiopia, which discovered a significant distinction between the case and comparison groups in terms of average birth weight and placental features. In particular, the case group showed lower average birth weight and thinner placenta compared to the comparison group, as demonstrated by the statistical analyses. Likewise, results from a study in Ghana indicated a marked difference in mean placental weight between the case and comparison groups, which aligns with our findings14,15.
It is further supported by a study conducted in Nepal, there was a significant difference in mean placental weight and size between the control and case groups. The case group had a smaller mean placental diameter and thickness compared to the control group7.
The placenta’s lower weight, thickness, and size in women with preeclampsia may be due to poor blood flow, which results in insufficient nutrition and oxygen supply to the placenta, compromising its growth, capacity to grow and expand appropriately, and function. As a result, placental tissue may be thinner, smaller, and lighter than in a typical pregnancy.
In this study, the comparison group had a lower rate of syncytial knot formation, cytotrophoblastic cellular proliferation, and fibrinoid necrosis in terms of placental histological alterations compared to the case group. The findings are consistent with a study conducted in Pakistan, which discovered that syncytial knots were present in 62% of case groups and 22% of comparison groups, as well as fibrinoid necrosis and cytotrophoblastic cellular development in 66% and 68% of cases, respectively. The comparison groups’ results showed that 14% of instances exhibited cytotrophoblastic cellular growth, 18% had fibrinoid necrosis, and 22% had syncytial knots. An additional study conducted in Iraq found that the case group had higher histological alterations than the comparator group16,17.
Additionally, compared to mothers with normal blood pressure, preeclamptic women had a greater prevalence of histological abnormalities in numerous Indian investigations. The results of two studies, one conducted at Muhimbli National Hospital and the other showing a similar result support our findings that histological alterations are more common in preeclamptic mothers than in normotensive mothers12,18.
The study found that 53.3% of babies born to preeclamptic mothers and 5.6% of children in comparison groups were underweight. The case group had a mean birth weight of 2.5 kg ± 0.59, while the comparison group had a mean of 3.07 kg ± 0.55. This aligns with a Ghanaian study indicating significantly lower birth weights in the case group15.
Strength and limitation of the study
As strength it offers a unique placental tissue histomorphometric analysis and provides a better evaluation of preeclampsia-related alterations than a gross examination and improves comprehension of the pathophysiology of disease by using microscopic analysis.
The limitation of the study may include: causal inferences are not possible with a cross-sectional design and incapable of evaluating long-term impacts or temporal relationships.
Conclusion
The study found that preeclamptic women had more placental histopathology changes than normotensive women, with lighter and smaller placentas. Birth weight positively correlates with these alterations. Therefore, mothers with preeclampsia need appropriate obstetric care to minimize risks. This research will give insight to conduct future research on similar topic with large sample size.
Ethical considerations
The Institutional Review Board (IRB) of Arba Minch University granted ethical approval with protocol number IRB/23,092/2023. To obtain consent for data gathering, a formal letter of cooperation and ethical approval was given to the obstetrics and pathology department of Arba Minch General Hospital. Written informed consent was obtained from all participants. To protect patient privacy, the patient’s medical chart was coded during data collection. Every checklist has a code as well. The acquired result was disclosed anonymously, and no personal connection was made to the patient’s medical history. This study was conducted in accordance with the Declaration of Helsinki.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Huppertz, B. The anatomy of the normal placenta 2008.
Bulletins—Obstetrics ACoaGCoP ACOG PRACTICE BULLETIN.
M A. Study of placental changes in pregnancy induced hypertension. (2013).
Susan, J. & Fisher, P. D. Why is placentation abnormal in preeclampsia. (2015).
Mangal, S., Samaddar, A., Roy, U. & Saha, I. Histopathological changes in placenta in pregnancy-induced hypertensive patients and correlation with fetal outcome – a tertiary care hospital study. Biomedical Biotechnol. Res. J. (BBRJ) 5(4), 440–445 (2021).
Vaithy, K. S., Samal, A., Devi, R., U, K. R. & Sowmya Histopathological study of placental changes associated with pre-eclampsia and eclampsia in a tertiary care centre of puducherry. IP Archives Cytol. Histopathology Res. 4 (1), 57–60 (2019).
Jyoti Mishal, C. & Bhushan Jha, Y. A. 4 Archana chaudhary,5, Shailaja chhetri Shrestha NM. Gross and micro anatomical study of placenta from normal and hypertensive pregnancies. Nepal. J. Health Sci. 2(1), 7–11(2022).
Chhatwal, J., Chaudhary, D. N. & Chauhan, N. Placental changes in hypertensive pregnancy: a comparison with normotensive pregnancy. Int. J. Reprod. Contracept. Obstet. Gynecol. 7(9), 3808–3813 (2018).
Gordijn, T. Y. K. E. E. M. P. G. J. N. T. K. M. S. J. PATHOLOGY OF PLACENTA2018.
Chappell, L. C., Cluver, C. A., Kingdom, J. & Tong, S. Pre-eclampsia. Lancet 398 (10297), 341–354 (2021).
Spong, C. Y. Definition of Term Pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. 309(23), 2445–2446 (2013)
Furaha, A. & Russa, D. Comparison of gross morphological and histological features of placenta between hypertensive and normotensive pregnant women attending muhimbili National hospital. Tanzan. J. Health Res. 23 (2), 1–9 (2022).
Ranga, M. K., SS, Thankom, T. F. A., Mallika, M. C. V. & Indira, M. V. Morphological and histological variations of human placenta in hypertensive disorders of pregnancy. Int. J. Anat. Res. 5 (1.3), 3591–3598 (2017).
Wubale, Y. Gross morphological study of placenta in Ethiopia. Anat. J. Afr. 6 (2), 977–981 (2017).
Awuah, S. P., Okai, I., Ntim, E. A. & Bedu-Addo, K. Prevalence, placenta development, and perinatal outcomes of women with hypertensive disorders of pregnancy at Komfo Anokye teaching hospital. PLoS One. 15 (10), e0233817 (2020).
Ban Amer Mousa SFFMAJ. Study of placental shape and histopathological changes in pregnant, ladies with pre-eclampsia. Iraq Med. J. 3 (2), 41–46 (2019).
Talpur, R. A. S. S., Khowaja, S., Noor, N., Baloch, M. S. & Qazi, M. M. Histomorphometric variations of placenta in normal and hypertensive pregnancies. Islamabad Med. Dent. Coll. 9 (4), 242–248 (2020).
Siva Sree Ranga. M.K ATTF, M. C. & Vasantha Mallika, M. V. Indira morphological and histological variations of human placenta in hypertensive disorders of pregnancy. Int. J. Anat. Res. 5 (1), 3591–3598 (2017).
Acknowledgements
Firstly, we would like to thank Arba Minch University, College of Medicine and Health Sciences for funding this research. Secondly, Arba Minch General Hospital for giving a permission and facilitating the data collection. Finally, our appreciation also goes to the data collectors and study participants who devoted their time to provide genuine information for this research.
Funding
There is no funding to report.
Author information
Authors and Affiliations
Contributions
BA, TG, and HS involved in conceptualization, study design, execution, data gathering, analysis, and interpretation. FG, BA, and KN participated to the article’s drafting, revision, or critical evaluation and all authors gave the document final approval before submission to the journal.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Abebe, B., Nagarchi, K., Sime, H. et al. Histomorphometry variations of placenta and birth outcomes in preeclamptic and normotensive mothers at Arba minch general hospital Southern Ethiopia. Sci Rep 15, 27600 (2025). https://doi.org/10.1038/s41598-025-13666-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13666-0