Abstract
Methylene blue (MB) dye is highly stable and non-degradable under natural conditions, posing significant threats to both ecosystems and human health. Conventional adsorbents, such as metal oxides, zeolites, bioadsorbents, and activated carbon, suffer from limited porosity, which restricts their adsorption efficiency. Moreover, their high cost results in low economic viability. In this study, a novel eco-friendly crosslinked diatomite–chitosan/calcium alginate (DM-CS@CA) composite powder was synthesized using diatomite (DM), chitosan (CS), and sodium alginate (SA) as raw materials. The composite was prepared without the use of any chemical crosslinking agents, employing encapsulation techniques and drying processes. The adsorbent was characterized and analyzed using a range of techniques, including Fourier-transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), Brunauer–Emmett–Teller (BET) surface area analysis, X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), and atomic force microscopy (AFM). Analysis of the experimental data revealed that the adsorption isotherm fits well with the Langmuir model, and the adsorption kinetics are consistent with the pseudo-second-order model. Under optimal conditions of pH 8, temperature 25 °C, and an adsorbent dosage of 1 g/L, the maximum adsorption capacity reached 549.74 mg/g.
Similar content being viewed by others
Introduction
Water is the most important part of an organism to maintain life, and human water should be safe, adequate and pollution-free1. At present, the world dye output is estimated to exceed 800,000 tons/year2. Dye pollution not only pollutes the water environment, but also involves ecosystems3,4,5 and human health6,7. Organic dyes are regarded as a kind of serious pollutants in surface water because of their wide application in textile, leather, paper and printing industries8,9. Among many organic dye wastewater, methylene blue (MB) is the representative dye in this kind of dye wastewater10,11. The dye is an azo dye wastewater, which will form organic “cationic” quaternary amine salt ion groups in aqueous solution, so the molecular structure is difficult to be broken and the chromaticity is high12. MB has a variety of negative effects on humans and animals, causing regurgitation, vomiting, cyanosis, jaundice, etc. Its complex aromatic structure is resistant to biodegradation13, and dye derivatives may be mutagenic and carcinogenic, causing atopic dermatitis, skin irritation, and organ dysfunction such as the kidney, liver, brain, and reproductive system14. Therefore, MB removal is an important research direction, with a focus on identifying effective treatment techniques that do not leave toxic residues or produce problematic by-products.
At present, the main methods to remove dye wastewater are physical method15,16,17, chemical method18,19,20, biological method21,22 and membrane separation technology23,24. The adsorption method in the physical method has the advantages of high efficiency, simplicity and low cost, which has aroused widespread concern in the field of wastewater treatment25. Zhao et al.26 synthesized hydrogels using organic montmorillonite (OMMT), sodium carboxymethylcellulose (CMC-Na) and polyacrylic acid (PAA), and the synergistic effect of CMC-Na and OMMT and the formation of hydrogen bonds between CMC-Na and PAA changed the structure of ACM nanocomposite hydrogels, resulting in excellent pore structure, swelling rate and adsorption capacity to various cationic dyes. Maryam et al.27 integrated calcium oxide (CaO) derived from eggshell waste with graphene oxide (GO) to form a novel multifunctional adsorbent and catalyst CaO@GO. It has the advantage of large adsorption capacity. However, these studies are used in industrial production in terms of high cost and toxicity, which is not conducive to streamlined production and application.
In order to find more efficient and environmentally friendly dye adsorbents, people continue to carry out research. The yield of diatomite is rich in China, which is mainly distributed in East China, West China and Northeast China28. Diatomite is a kind of siliceous rock, which is mainly composed of diatoms29. Its main chemical composition is SiO2, and it also contains a small amount of metal oxides such as CaO30. Physical properties such as large specific surface area make diatomite itself have good physical adsorption31. In addition, the surface is also rich in silicon hydroxyl functional groups, which can also play a major role in adsorption. Therefore, it is widely used in environmental protection, chemical industry, building materials and other fields. Chitosan is one of the most widely used natural polymer adsorption materials32. It is a deacetylated derivative of chitin and has special properties such as non-toxic, hydrophilic and biodegradable33. Chitosan has rich amino and hydroxyl groups, so it has been widely used in the field of sewage treatment by many researchers. When chitosan is mixed into diatomite, it can increase the pore diameter and mechanical strength of diatomite, which is more conducive to the adsorption of dyes34. However, the adsorption of MB by single modification is not satisfactory, so it needs to be modified. Sodium alginate is a cheap and easily available natural polysaccharide polymer with good biocompatibility, non-toxicity and degradability35. The hydroxyl and carboxyl groups rich in alginate molecules can be cross-linked with calcium chloride to form gel spheres with coated structure. Therefore, sodium alginate is widely used in sewage treatment, food industry, pharmaceutical industry and so on36.
Based on the above analysis of materials and previous studies, sodium alginate was used to coat diatomite and chitosan, and the prepared DM-CS@CA composites were characterized, and then methylene blue solution was removed by adsorption. The preparation conditions of the samples were studied in detail; the effects of temperature, initial adsorbent dosage, initial MB concentration, adsorption time and initial pH on the adsorption were explored; and the repeatability and application potential of the materials were evaluated.
Materials and experiments
Materials
Diatomite (CP) is purchased from Xilong Science Co., Ltd. (China). Chitosan (medium viscosity, 200–400 mPa s) Purchased from Shanghai Mairell biochemical Technology Co., Ltd. CH3COOH (99.5%, AR) is purchased from Taicang Shanghai Test Reagent Co., Ltd. Sodium alginate (90%, AR) and calcium chloride (AR ≥ 96%) were purchased from Shanghai McLean biochemical Technology Co., Ltd. Methylene blue (≥ 98.5%, AR) was purchased from Tianjin Chemical Reagent Research Institute Co., Ltd. All the above chemical reagents are used as they are.
Preparation of DM-CS@CA powder
At 333 K, CS was dissolved in acetic acid (2wt%) solution and stirred (1500r/min) in a magnetic agitator for 1 h to become transparent and viscous. Then add DM to it, keep the temperature, and continue to stir 30 min to form a uniform solution. After the solution was transferred to a centrifuge and 5 min was centrifuged at the speed of 4000r/min, the precipitate was put into the oven for drying (353 K for 3 h), ground and screened (100mesh). The intermediate product was recorded as DM-CS.
DM-CS was added to deionized water at room temperature (298k), and SA was slowly added to it, stirring for 1 h until homogenized. The uniform solution was dropped into calcium chloride (2wt%) solution to form beads, solidified at room temperature for 24 h, dried (323 K for 6 h), ground and screened (50 mesh). The sample was marked as DM-CS@CA Fig. 1).
Adsorption test
In order to systematically study the adsorption of MB by DM-CS@CA, the methylene blue solution of 1000 mg/L was prepared, through the concentration of diluted original solution and adding different mass of DM-CS@CA, the effects of initial MB concentration and initial adsorbent mass on the adsorption were studied. At the same time, by adding 0.05 M HCl and 0.05 M NaOH to change the pH value (3–11) and temperature (25 ℃, 35 ℃, 45 ℃) of the solution, the effects of pH value and temperature on the adsorption were studied. In all these experiments, the light absorption of MB at 666 nm was measured using a 722G spectrophotometer to calculate the amount of MB adsorbed. The adsorption capacity (Qe) and removal efficiency (R) are calculated using the following formulas (1) and (2), respectively.
Where C0 and Ce represent the initial concentration of MB and the concentration at adsorption equilibrium (mg/L), m represents the mass of the adsorbent used (mg), and V represents the volume of MB. In addition, Qe is the adsorption capacity (mg/g) of the adsorbent, and R is the removal efficiency of the adsorbent.
Characterization method
To determine the crystalline structure and phase composition of the composite adsorbent material, X-ray diffraction (XRD) measurements were conducted using a Bruker D2 Phaser diffractometer (Germany) with a scanning range of 10° to 80° (2θ) at a scan rate of 2°/min. Fourier Transform Infrared (FTIR) spectroscopy was performed on a Thermo Fisher Scientific Nicolet iS20 spectrometer (USA) to identify functional groups and investigate their structural evolution in the composite material, with a resolution of 4 cm⁻¹ and a wavenumber range of 400–4000 cm⁻¹. The microstructure of the composite adsorbent was characterized by scanning electron microscopy (SEM) using a Hitachi SU8010 system (Japan). The Brunauer-Emmett-Teller (BET) method, implemented via a Quantachrome Autosorb NOVA 2200e analyzer (USA), was employed to evaluate variations in pore size distribution and specific surface area of diatomite before and after modification, as well as to monitor pore structural changes in the adsorbent during pre- and post-adsorption states. X-ray photoelectron spectroscopy (XPS) analysis was carried out using a Thermo Scientific K-Alpha spectrometer (USA) to probe the elemental composition and chemical states of the adsorbent. Consistent with previous characterization protocols, the morphological features and particle size distribution of the adsorbent were analyzed by transmission electron microscopy (TEM) on an FEI Talos F200X G2 instrument (USA). Complementary atomic force microscopy (AFM) studies were executed with a Bruker Dimension Icon system (Germany) to quantify surface roughness and nanoscale textural heterogeneities of the adsorbent.
Regeneration experiment
After adding the adsorbent to the HCl solution (0.1 M) at 25 ℃ for 1 h, the regenerated adsorbent was removed and washed with a large amount of deionized water, repeated at least 3 times until neutral, and then dried in the oven. Then the adsorption-desorption-reabsorption process was evaluated in methylene blue solution to study its reusability.
Results and discussion
Characterization and analysis
FTIR
Figure 2 (a) records the FTIR spectra of DM, CS, DM-CS and DM-CS@CA. For DM, the strong absorption peak on 464 cm−1 is the bending vibration of Si-O, the absorption peak on 798 cm−1 is the symmetrical stretching vibration of Si-O-Si, which is an important feature of amorphous silica in diatomite, and the absorption peak of 1092 cm−1 is the asymmetric stretching vibration of Si-O-Si, which reflects the structure of silicon-oxygen network. There is a broad absorption peak near 3439 cm−1, indicating the stretching vibration of Si-OH on the surface, indicating that the surface of diatomite is rich in hydroxyl groups37. For CS, the stretching vibrations in 1080 cm−1 C-OH show the existence of hydroxyl groups in chitosan molecules, the peak in 1595 cm−1 is the vibration of -NH2 chains in chitosan molecules, and the broad peaks near 3421 cm−1 are the stretching vibrations of -OH and -NH2 molecules. The three characteristic peaks of DM-CS, DM are located in 463 cm−1, 795 cm−1 and 1083 cm−1, respectively, and are retained successfully. There is an absorption peak at 2874 cm−1, which is the stretching vibration of C-H of chitosan, indicating that CS has been successfully loaded onto the surface of DM. For DM-CS@CA, the broad absorption peak of 3429 cm−1 is that the -NH2 and -OH of CS interact with the Si-OH bond of DM to promote the binding of polar groups38. The absorption peaks at 1631 cm−1 and 1440 cm−1 are the asymmetric stretching vibration and the symmetrical stretching vibration of C = O in the -COOH, respectively, which characterize the existence of carboxylates.
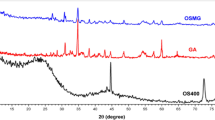
XRD
Figure 2 (b) shows the XRD spectra of DM, DM-CS and DM-CS@CA, respectively. It is found that there are prominent diffraction angles of diatomite at 21.9 °, 26.8 °, 36.8 °and 50.2 °. The diffraction peak at 26.8 °is sharp and has high peak intensity, indicating that the diatomite crystal has a high degree of order. These characteristic peaks also appear in DM-CS and DM-CS@CA, mainly due to the retention of the crystal structure of DM, which is consistent with the discussion of 3.1.1. When the final product is formed, the diffraction peak of DM decreases. The reason for this phenomenon may be that DM is an inorganic hydrophilic compound, dispersed in chitosan solution32 and loaded in calcium alginate, thus forming a favorable interaction and increasing the adsorption capacity of MB.
SEM 、TEM and AFM
The micro-morphology of DM and DM-CS@CA was obtained by SEM analysis. The surface of DM has many natural nano-pores symmetrically distributed, just like a ceramic disk, and the surface is smooth, as shown in Fig. 3 (a)-(b). This random porous structure increases the surface area and the internal space of the channel39. In Fig. 3 (c)-(d), the surface of the adsorbent becomes rougher, and the diatomite skeleton pores with small round pores and the larger interpolymeric pores of the adsorbent materials can be seen obviously, so that the hydroxyl groups are exposed on the surface of the adsorbents, thus increasing the adsorption of MB.In addition to SEM (Scanning Electron Microscopy) analysis, TEM (Transmission Electron Microscopy) characterization was also conducted. Comparative TEM imaging (Fig. 3(e)-(f)) between diatomite and the adsorbent revealed that the adsorbent exhibited a rough and heterogeneous surface texture, which further confirms the successful incorporation of diatomite and chitosan within the calcium alginate matrix40.The AFM 3D image (Fig. 3(g)-(h)) distinctly reveals two characteristic regions on the adsorbent surface: bright and dark-gray zones. The dark-gray regions correspond to porous structures within the adsorbent material41, where color intensity inversely correlates with pore depth (darker hues indicating greater depth) and areal dimensions directly reflect pore diameter (larger areas signifying expanded pore sizes). Comparative analysis with diatomite’s AFM topography demonstrates a marked enlargement in pore dimensions, confirming the structural evolution of the adsorbent system.
BET
Table 1 shows the surface area (SBET), micropore volume (Vµp) and average pore diameter (D) of DM, DM-CS@CA and DM-CS@CA-MB. Although the micropore volume of the modified adsorbent decreased from 0.007 cm3/g to 0.002 cm3/g, the average pore size increases, which is consistent with the conclusion of 3.1.1 above. The increased pore size is interpolymerization pore, which makes it easier for MB to enter into the pore diameter. Before and after the adsorption of MB by the adsorbent, the micropore volume almost did not change, but the surface area and the average pore size increased, which indicated that MB occupied all the sites of the adsorbent and adsorbed uniformly on the outer surface and the inner surface of the pore.
XPS
The XPS spectra of the modified materials before and after adsorption were further analyzed. Figure 4 (a) is a complete XPS spectrum. It is obvious that there is more S after adsorption, which confirms that MB is adsorbed. And the contents of C and N are also increasing, and there are no new peaks, which further proves that MB is electrostatically adsorbed on the surface of the adsorbent. In the C1s spectrogram (Fig. 4 (b)), it is observed that the binding energies of O-C = O, C-O-C and C-C bonds are 284.14 eV, 285.96 eV and 287.72 eV, respectively. After the adsorption of MB, these characteristic peaks moved to 283.90 eV, 285.48 eV and 287.20 eV, respectively. The decrease of binding energy and the increase of peak intensity further prove that these bonds are involved in the process of adsorption of MB. In the high resolution O1s spectrogram (Fig. 4 (c)), the intensity of O-H peak increases significantly, and the binding energy increases from 531.89 eV to 531.99 eV, at the same time, the binding energy of O-C-O peak also increases significantly, indicating that oxygen-containing functional groups such as hydroxyl and carboxyl groups in the adsorbent strongly interact with MB42, which promotes electron transfer. In the N1s spectrum of Fig. 4 (d), the binding energy of C-N and C-NH2 bond decreases from 398.79 eV and 401.54 eV to 398.60 eV and 401.24 eV, which further confirms that MB is adsorbed on the adsorbent43.
Factors affecting adsorption performance
Effect of adsorbent dosage on adsorption performance
Different amounts of adsorbents play an important role in maximizing economic benefits in the process of dye adsorption. In order to study the optimal amount of adsorbents needed for adsorbing MB, experiments were carried out to change the amount of adsorbents only while other variables were constant. As shown in Fig. 5 (a), when the DM-CS@CA of 0.5 g/L is added, the removal rate of MB is 83.78%, and the removal rate increases gradually with the increase of the amount of adsorbent. This is because the high adsorption dose provides many active sites for dye adsorption44, and the effective surface area increases accordingly. However, when the dosage of adsorbent is 1.0 g/L, the removal rate almost tends to balance. This may be due to the agglomeration of the adsorption sites45, resulting in the decrease of the adsorption sites and the adsorption capacity. According to this study, the best dosage is 1 g/L.
Effect of pH on adsorption performance
The change of pH value will change the surface properties of the adsorbate and have a great influence on the adsorption capacity of the adsorbent. It can be seen from Fig. 5 (b) that during pH = 8, DM-CS@CA has the maximum adsorption capacity and removal rate of MB. At this time, the electrostatic force between the dye and the adsorbent makes the dye enter the interior and surface of the adsorbent channel. The surface of the adsorbent contains a large amount of OH- and a small amount of NH3+46, which has excellent adsorption capacity for MB in theory. In the process of pH from 3 to 8, the adsorption amount of MB by the adsorbent is low. This is mainly because MB is a typical cationic dye. In the acidic environment, the concentration of H+ is too high, and the dye molecules compete with H+ for the OH−, on the surface of the adsorbent, which leads to the gradual decrease of the adsorption capacity. However, with the increase of the value of pH, the concentration of H+ in the solution decreased, and the competition between H + and dye molecules decreased, so the adsorption capacity increased gradually. In the process of pH from 8 to 11, the adsorption capacity is also decreasing. One of the reasons for this phenomenon is that there is a competitive attraction to dye molecules under excessive OH+ concentration, resulting in a decrease in adsorption capacity. Another reason is that the NH3+ in the dye occupies the dominant position in the adsorption process, which makes the adsorbent reduce the adsorption of MB. However, when pH = 11 was found, the adsorption capacity increased slightly. This is due to the fact that in an alkaline environment, MB will be ionized in aqueous solution47 and the color will become lighter.
Effect of initial concentration of 3 MB on adsorption performance
As shown in Fig. 5 (c), when other conditions remain the same, the increase of the initial concentration will obviously lead to the improvement of the adsorption capacity of the adsorbent for MB. When the initial concentration increases from 200 mg/L to 800 mg/L, the adsorption capacity increases due to the increase of concentration gradient driving force, but the removal efficiency increases at first and then decreases. When the initial concentration reaches 1000 mg/L, the adsorption reaches saturation state due to the limited active site of the adsorbent. Considering the best economic efficiency, at the initial concentration of 400 mg/L, the surface of the adsorbent was in contact with the dye most thoroughly, and the removal efficiency was the highest, which was 93.72%.
Adsorption isotherm
The adsorption isotherm reflects the balance between the dye concentration in the solution and the dye concentration on the surface of the adsorbent under specific conditions48. The shape of the adsorption isotherm reflects the interaction between dyes and adsorbents. In this study, two most widely used models are selected for analysis: Langmuir and Freundlich isotherm model49.
The Langmuir isotherm assumes that adsorption occurs on the homogeneous and equipotential surface of the adsorbent as a monolayer cover50. The Langmuir model assumes that the surface of the adsorbent is uniform and the energy is equal, and the adsorption process takes place at a specific site. As long as the dye molecule occupies one site, it does not interact with any other ions or molecules in the adjacent region. Its numerical equation is expressed as follows:
Where Qm is the maximum adsorption capacity (mg/g) of MB, Qe is the equilibrium adsorption capacity for MB (mg/g), C0 and Ce are the initial and equilibrium MB concentration (mg/L), KL is the Langmuir isotherm constant (L/mg), and RL is the separation factor.
The Freundlich isotherm model is based on heterogeneous surface adsorption and is based on the assumption that the dye concentration on the adsorbent surface will increase with the increase of dye concentration51. Its numerical equation can be expressed as follows:
Where KF is the Freundlich isotherm constant and n is the heterogeneity factor.
The Temkin model addresses adsorbate-adsorbent interactions by continuously reducing the heat of adsorption of all dye molecules covered by the adsorbent surface52,53. Its numerical equation can be expressed as:
Where K represents the adsorption heat constant (J/mol), and A represents the Timkin equilibrium association constant (L/g).
In this study, the adsorption isotherm of MB on the adsorbent was obtained by changing the dye concentration in the range of 200-1000 mg/L. It can be seen from Table 2; Fig. 6 (d) that the adsorption efficiency decreases with the increase of temperature, which may be due to the acceleration of the movement speed of dye particles at high temperature and the corresponding increase of the probability of collision with the adsorbent. as a result, more dye particles can escape and cannot enter the adsorbent pore, thus reducing the adsorption capacity. It shows that the lower temperature is beneficial to the adsorption.
It can be seen from Fig. 6 (a), Fig. 6 (b) and Fig. 6 (c) as well as Table 2 that the correlation coefficients of the Langmuir model fit are higher than those of the Freundlich and Temkin simulation fits in the nonlinear fit and linear fit of the Langmuir, Freundlich and Temkin isotherms. For Langmuir isotherms, the linear fitting correlation coefficient (R2 = 0.9957) is much larger than the nonlinear fitting correlation coefficient (0.7462). It is obvious and easy to know that the adsorption occurs on the surface of the adsorbent and is monolayer adsorption37. This means that chitosan and sodium alginate can effectively modify diatomite and greatly increase the adsorption of MB. The MB particles absorbed by the adsorbent are completely independent and will no longer be adsorbed after reaching the saturation value. Generally speaking, the value of n is an index to evaluate whether the adsorption process is excellent or not. when n >1, the adsorption process is easier to occur, but when n is between 0 ~ 1, the adsorption is more difficult to occur. At these three temperatures, the values of n are all greater than 1, and the adsorption process is spontaneous and easy to occur.
Adsorption thermodynamics
In order to determine whether the adsorption of MB by DM-CS@CA is spontaneous or non-spontaneous, its thermodynamic properties are studied at 25 ℃, 35 ℃ and 45 ℃, and the changes of Gibbs free energy (Δ G), enthalpy (Δ H) and entropy (Δ S) are calculated54. The equation is as follows:
Where Kf is the thermodynamic equilibrium constant, Qe is the adsorption capacity of MB at equilibrium (mg/g), Ce is the concentration of MB at equilibrium (mg/L), R = 8.314 is the gas constant (J/(mol k)), and T is the absolute temperature (K).
It can be seen from Fig. 6 (e) and Table 3 that ΔG is negative, indicating that the process is feasible and a spontaneous process. At the same time, with the increase of temperature, ΔG increases from − 6.70 kJ/mol to −4.00 kJ/mol, indicating that the adsorption of DM-CS@CA to MB decreases at higher temperature, it is preferred at lower temperature55. The high negative value of ΔG also reflects the good adsorbability. At the same time, ΔH is also negative, that is, the reaction is exothermic, and the adsorption capacity decreases with the increase of temperature35,56. Similar results have also been reported in the literature. The conclusions of ΔG and ΔH are also consistent. ΔS is negative, indicating that the randomness of the adsorbent interface decreases during the adsorption process.
Adsorption kinetics
Adsorption kinetics is mainly used to describe the dynamic process of adsorbate concentration and equilibrium adsorption capacity with time in the process of reaction adsorption57, which is helpful to understand the adsorption performance of the adsorbent and the adsorption mechanism of the adsorption process. For the kinetic study, 0.1 g adsorbent was added to different concentrations of MB of 100mL and stirred at 25 °C for 120 min at the natural pH value (pH = 8) of the solution. The kinetic mechanism of MB adsorption on DM-CS@CA materials was studied by using quasi-first-order kinetic model, quasi-second-order kinetic model and Weber-Morris particle diffusion model58. The integral forms of quasi-first-order equation, quasi-second-order equation and intra-particle diffusion model are expressed as follows:
Where k1 is the quasi-first-order rate constant (1/min), k2 is the quasi-second-order rate constant (g/(mg min)), Qe is the equilibrium adsorption capacity of MB (mg/g), Qt is the MB adsorption capacity at t time (mg/g), Ki is the rate constant (mg/g min-0.5), and C is the boundary layer thickness constant (mg/g).
The Elovich model describes the heterogeneous adsorption of analytes by chemisorption, assuming that there are no interactions between adsorbent species59. Its numerical equation can be expressed as:
where Qt is the MB adsorption capacity (mg/g) at time t; α is the initial adsorption rate (mg/g·min); β is a constant (mg/g).
Figure 7 (a) shows the effects of time and initial concentration on the adsorption of MB, and studies the adsorption kinetics of 200, 400, 600 and 800 mg/L with MB concentration. In the early stage of adsorption, the adsorption capacity increased rapidly, mainly because the adsorbent had enough adsorption sites, and the dyes were rapidly adsorbed to the pore and surface of the adsorbent by electrostatic adsorption. At all concentrations, the dye adsorption reached equilibrium very quickly, and then increased slowly. This is due to the fact that after a large number of active sites are occupied, the adsorbent sites reach saturation.
According to the correlation coefficient (R2), and the theoretical adsorption capacity is close to the actual adsorption capacity60, which kinetic model can better characterize the adsorption process of MB from aqueous solution. As can be seen from Fig. 7 (a-d) and Table 4, Whether linear fitting or nonlinear fitting, The R2 of the pseudo second order kinetic model is superior to the pseudo first order kinetic model and the Elovich kinetic model. In the pseudo second order kinetic model, although the R2 of nonlinear fitting is more than 0.9990, the R2 of linear fitting is 0.9999. Moreover, the Qe calculated by pseudo second order linear fitting is closest to the actual Qe and is effective. As can be seen in Fig. 7 (e), the line of the intra-particle diffusion model does not pass through the origin, which does not accord with the reality, which indicates that the intra-particle diffusion is not the main factor leading to the particle velocity limit in the kinetic process. Therefore, the quasi-second-order kinetic model is more suitable for the adsorption of MB by DM-CS@CA. It shows that there is electron exchange between the two adsorbents in the process of adsorbing MB, which is discussed with 3.1.5.
Regeneration research
The research on the recovery and utilization performance of adsorbents is essential, which is helpful to improve the utilization rate of resources and is of great significance to practical application. The regeneration experiment is the use of desorption cycle method, the reuse of adsorbents for adsorption. The adsorption experiments of DM-CS@CA were carried out for five times in succession. It can be seen from Fig. 8 that the adsorbent shows remarkable stability and efficient adsorption capacity during the experiment. After five cycles, the adsorption rate of the adsorbent for MB decreased from 93.72 to 74.93%, and the continuous cycle still had a high adsorption rate. The reason for the decrease in adsorption rate may be that MB still occupies a certain position in the pores of the adsorbent during the resolution process61,62. To sum up, as an environment-friendly adsorbent, DM-CA@CA has high economic benefits.
Mechanism analysis
The adsorbent adsorbs the dye on the surface of the adsorbent and in the three-dimensional network structure, the adsorbent has a larger specific surface area than other adsorbent materials, so that the adsorption site of the adsorbent is exposed to the dye, thus improving the adsorption efficiency. As we all know, MB is a cationic dye, and according to pHpzc, between ph3 ~ 10.5, MB is always positively charged in aqueous solution. The network structure of sorbents has certain functional groups, including hydroxyl groups (-OH), amines (-NH2), carboxyl groups (-COOH), and amides (-CONH2)63. The adsorption efficiency is further improved. These two or more mechanisms work together to achieve effective dye adsorption.
Conclusion
In this study, chitosan and sodium alginate were used to modify diatomite. After a number of full characterization of the modified material and evaluation of all related parameters, the efficient and excellent adsorption rate of DM-CS@CA to methylene blue was determined. When the temperature is 25 ℃, pH = 8 and the amount of adsorbent is 1 g/L, the best adsorption performance of MB is observed. Under the adsorption time of 1 h, the maximum adsorption efficiency can be 93.72%, and the maximum adsorption capacity can reach 549.74 mg/g. The adsorption kinetics was studied by quasi-first-order kinetic model and quasi-second-order kinetic model, and the adsorption kinetics was fitted linearly and non-linearly by Langmuir isotherm model and Freundlich isotherm model. The final results show that quasi-second-order kinetic model and Langmuir isotherm model are more consistent with the adsorption process. Finally, the regeneration experiment of the adsorbent was carried out, and the adsorption rate could reach 74.93% after five cycles. It shows that the adsorbent is green and efficient, and can be used in large-scale industrial production. In addition, due to equipment and other reasons, the change of surface charge when the adsorbent adsorbs methylene blue is difficult to detect continuously during the experiment, and the effect of interfering ions on the adsorption process may have a certain impact on the adsorption. In-depth discussions will continue in subsequent research.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Mchich, Z. Engineering of highly bio-adsorbent zinc oxide/cellana Tramoserica seashells for efficient removal of anionic dye: BBD optimization, density functional theory study, and adsorption mechanism[J]. Sep. Purif. Technol. 363, 132015 (2025).
Shahla, A. et al. Bionanocomposite MIL-100(Fe)/Cellulose as a high-performance adsorbent for the adsorption of methylene blue[J]. Sci. Rep. 14, 14497 (2024).
Gao, Y. F. et al. Chitosan-polyvinyl alcohol-diatomite hydrogel removes methylene blue from water[J]. Int. J. Biol. Macromolecules: Struct. Function Interact. 254,127886 (2024).
Dai, X. J. et al. 2D-3D magnetic NiFe layered double hydroxide decorated diatomite as multi-function material for anionic, cationic dyes, arsenate, and arsenite adsorption[J]. Appl. Clay Sci. 229, 107–118 (2022).
Jiang, F. Remarkable synergy between sawdust Biochar and attapulgite/diatomite after co-ball milling to adsorb methylene blue[J]. RSC Adv. 13, 14384–14392 (2023).
Dominguez, M., Mendoza, J. & Figueroa, K. Adsorption of methylene blue dye using common walnut shell (Juglans regia) like biosorbent: implications for wastewater treatment[J]. Green Chem. Lett. Rev. 17, 2362267(2024).
Elkholy, A. S. et al. Synthesis of activated carbon composited with Egyptian black sand for enhanced adsorption performance toward methylene blue dye[J]. Sci. Rep. 13, 4209 (2023).
Wei, S. et al. Preparation and application of green chitosan/ploy (vinyl alcohol) porous microspheres for the removal of hexavalent chromium[J]. Mater. Sci. Eng. B 284,115922 (2022).
Maia, L. A. et al. Activated carbon from palm fibres used as an adsorbent for methylene blue Removal[J]. J. Polym. Environ. 29, 1162–1175 (2021).
Ghazala et al. Removal of methylene blue (MB) dye from water and wastewater using acid-activated chicken bone in a batch adsorption process[J]. Sci. Rep. 15, 23098 (2025).
Merah, M. et al. High biosorption of cationic dye onto a novel material based on paper mill sludge[J]. Sci. Rep. 13, 15926 (2023).
Cao, V. et al. Characterizing the impact of MnO2 addition on the efficiency of Fe0/H2O systems[J]. Sci. Rep. 11, 9814 (2021).
Jinze et al. Synthesis and characterization of zwitterionic hydrogels with Ag@g-C3N4 for adsorption-photocatalytic removal of Methylene blue and Methyl orange dyes[J]. Adsorption 31, 1–13 (2025).
Avinash, G. P. et al. Sustainable Adsorption of Methylene Blue by Biocompatible Agro Waste Biomass: Optimization, Kinetic Isotherms, and Ecotoxicity evaluation[J] (Biomass Conversion and Biorefinery, 2024).
Saad, E. M., Wagdy, M. & Orabi, A. S. Advanced nano modification of ecofriendly glauconite clay for high efficiency methylene blue dye adsorption[J]. Sci. Rep. 14, 23614 (2024).
Blachnio, M., Zienkiewicz-Strzalka, M. & Derylo-Marczewska, A. Synthesis of composite sorbents with Chitosan and varied silica phases for the adsorption of anionic Dyes[J]. Molecules (Basel Switzerland). 29, 2087 (2024).
Dai, N. et al. Cetyltrimethylammonium Bromide-Modified laponite@diatomite composites for enhanced adsorption performance of organic Pollutants[J]. Langmuir: ACS J. Surf. Colloids. 40, 8427–8439 (2024).
Rezig, W., Elaziouti, A., Laouedj, N. & Hadjel, M. Synthesis and characterization of novel calcined ferrihydrite-modified diatomite (FMD3X6) and its UVA light-assisted heterogeneous photodegradation of VG3 dye[J]. Desalination Water Treat. 271, 254–271 (2022).
Pramanik, D. D., Garg, P., Ganesan, D. & Pramanik, A. Removal of malachite green from an aqueous environment using chitosan-xanthan gum coagulation system: A response surface methodology approach[J]. J. Water Process. Eng. 65, 105896 (2024).
Salam, N. F. A., Alhosary, S., Abadir, M. & Zabochnicka, M. The using of response surface methodology to optimize Methyl orange removal from textile dyeing wastewater[J]. Desalination Water Treat. 305, 182–191 (2023).
Parmar, N. D. & Shukla, S. R. Decolourization of dye wastewater by microbial methods- A review(Article)[J]. Indian J. Chem. Technol. 25, 315–323 (2018).
Chatha, S. A. S. et al. Biological color stripping: A novel technology for removal of dye from cellulose fibers[J]. Carbohydr. Polym. 87, 1476–1481 (2012).
Ahmed, A. E., Majewska, N. K. & Grzegorzek, M. Removal of reactive dyes from aqueous solutions using ultrafiltration membranes[J]. Environ. Prot. Eng. 47, 109–120 (2021).
Oyarce, E. et al. Removal of dyes by Polymer-Enhanced ultrafiltration: an Overview[J]. Polymers 13, 3450 (2021).
Samara, J. et al. Reusable Porous SiO2 for Methylene Blue Removal: A Study on Adsorption and Regeneration Cycles[J]. Water, Air, & Soil Pollution. 235, (2024).
Yuanhang et al. Carboxymethyl cellulose sodium modified poly(acrylic acid) and organic montmorillonite nanocomposite hydrogels with excellent purification ability for cationic dye wastewater[J]. Int. J. Biol. Macromol. 308, 142521 (2025).
Maryam et al. Multi-functional cao@go catalyst and adsorbent derived from eggshell waste for removal of environmental pollutants[J]. Fuel 396, 135308 (2025).
Zhang, W. et al. Modification of diatomite and its application in the field of environment[J]. Guangdong Chem. Ind. 46, 49 (2019).
Antosik, A. K., Mozelewska, K., Musik, M. & Miądlicki, P. Influence of diatomite and its base modifications on the self-adhesive properties of silicone pressure-sensitive adhesives[J]. Sci. Rep. 13, 13691 (2023).
Hao, L. et al. Functionalized diatomite/oyster shell powder doped electrospun polyacrylonitrile submicron fiber as a high-efficiency adsorbent for removing methylene blue from aqueous solution: thermodynamics, kinetics and isotherms[J]. J. Mol. Liq. 298, 112022 (2020).
Zhou, H. F. et al. Preparation of cellulose/diatomite composite material and its adsorption characteristics and Kinetics[J]. Bioresources 15, 8728–8743 (2020).
Wu, X. J., Wang, J. D. & Cao, L. Q. Characterization and adsorption performance of chitosan/diatomite membranes for orange G removal[J]. E-POLYMERS 16, 99–109 (2016).
Marguerite, R. Chitin and chitosan: properties and applications[J]. Prog. Polym. Sci. 31, 601–632 (2006).
Nady, A. et al. Strengthening the surface and adsorption properties of diatomite for removal of Cr(VI) and methylene blue dye[J]. Arab. J. Geosci. 15, 10 (2022).
Zhao, Y. T. & Li, B. G. Preparation and Superstrong Adsorption of a Novel La(III)-Crosslinked Alginate/Modified Diatomite Macroparticle Composite for Anionic Dyes Removal from Aqueous Solutions[J]. Gels (Basel, Switzerland). 8, 810 (2022).
Shi, T. Z. et al. Highly efficient adsorption of heavy metals and cationic dyes by smart functionalized sodium alginate Hydrogels[J]. Gels (Basel, Switzerland). 8, 343(2020).
Liu, G. H. et al. Insight into the photocatalytic properties of diatomite@ni/nio composite for effective photo-degradation of malachite green dye and photo-reduction of cr (VI) under visible light[J]. J. Environ. Manage. 254, 109799 (2020).
Song, X. R. et al. Preparation and characterization of magnetic chitosan-modified diatomite for the removal of Gallic acid and caffeic acid from sugar solution[J]. Carbohydr. Polym. 219, 316–327 (2019).
Han, G. Y. et al. Eco-friendly polycaprolactone-bound diatomite filter for the removal of metal ions and micro/nanoplastics from water[J]. Sci. Total Environ. 905, 166956 (2023).
Aljeboree, M. A., Jasim, K. K. & Sabah, A. N. High-Capacity Dye Adsorption by Low-Cost Combo Hydrogel: Kinetic, Thermodynamic, and Regeneration Study[J]. Journal of Inorganic and Organometallic Polymers and Materials, (prepublish). 1–24, (2025).
Sharabiani et al. Modification of polyvinylidene fluoride and polysulfone flat sheet membraneusing perovskite nanoparticles for treatment of humic acid in a submerged membrane system[J]. Desalination Water Treat. 297, 17–25 (2013).
Yu, K. J. et al. A novel chitosan-based hydrogel microspheres for efficient heavy metal-ion adsorption[J]. Mater. Today Commun. 41, 110488 (2024).
Li, X. P. et al. Precisely controlled electrostatically sprayed sodium alginate/carboxymethyl Chitosan hydrogel microbeads as super-adsorbent for adsorption of cationic dye[J]. Int. J. Biol. Macromol. 283, 137989 (2024).
Selim, A. Q. et al. Cr (VI) Uptake by a Composite of Processed Diatomite with MCM-41: Isotherm, Kinetic and Thermodynamic studies[J]26084–92 (MICROPOROUS AND MESOPOROUS MATERIALS, 2018).
Song, X. L. et al. Effective and selective adsorption of As(III) via imprinted magnetic Fe3O4/HTCC composite nanoparticles[J]. J. Environ. Chem. Eng. 5, 16–25 (2017).
Zhao, D. J. & Shen, X. Z. Preparation of Chitosan-Diatomite/Calcium alginate composite hydrogel beads for the adsorption of congo red Dye[J]. Water 15, 2254 (2023).
Ma, T., Wu, Y. H., Liu, N. N. & Wu, Y. Y. Hydrolyzed polyacrylamide modified diatomite waste as a novel adsorbent for organic dye removal: adsorption performance and mechanism studies[J]. Polyhedron 175, 114227 (2020).
Li, Y. et al. Absorbents based on maleic anhydride-modified cellulose fibers/diatomite for dye removal(Conference Paper) [J]. Journal of Materials Science. 49, 6696–6704 (2014).
Dai, H. J. & Et Green and facile fabrication of pineapple Peel cellulose/magnetic diatomite hydrogels in ionic liquid for methylene blue adsorption[J]. CELLULOSE 26, 3825–3844 (2019).
Li, W. Y. et al. Tunable Adsorption Properties of Bentonite/carboxymethyl cellulose-g-poly(2-(dimethylamino) Ethyl methacrylate) Composites Toward Anionic dyes[J]124260–270 (CHEMICAL ENGINEERING RESEARCH & DESIGN, 2017).
Şen, N. E. & Şenol, Z. M. Effective removal of allura red food dye from water using cross-linked chitosan-diatomite composite beads[J]. Int. J. Biol. Macromol. 253, 126632 (2023).
Hayder, O. et al. Adsorption of Rhodamine B dye from solution using 3-((1-(4-((1H-benzo[d]imidazol-2-yl) amino) phenyl) ethylidene) amino) phenol (BIAPEHB)/ P(AA-co-AM) composite[J]. Desalination and Water Treatment. 321, 101019 (2025).
Hayder Jihad Majeed. Synthesis and application of novel sodium carboxy Methyl cellulose-g-poly acrylic acid carbon Dots hydrogel nanocomposite (NaCMC-g-PAAc/ CDs) for adsorptive removal of malachite green dye[J]. Desalination Water Treat. 320, 100822 (2024).
Hou, M. et al. Facile synthesis of Diatomite/β-Cyclodextrin composite and application for the adsorption of diphenolic acid from Wastewater[J]. Materials 15, 4588 (2022).
Radjai, M. et al. Adsorptive removal of cationic and anionic dyes on a novel mesoporous adsorbent prepared from diatomite and anionic cellulose nanofibrils: experimental and theoretical investigations[J]. J. Mol. Liq. 361, 119670 (2022).
Li, B. G. & Zhao, Y. T. Facile synthesis and ultrastrong adsorption of a novel polyacrylamide-modified diatomite/cerium alginate hybrid aerogel for anionic dyes from aqueous environment[J]. Int. J. Biol. Macromol. 253, 127114 (2023).
Sun, Z. M. et al. Carboxyl-rich carbon nanocomposite based on natural diatomite as adsorbent for efficient removal of cr (VI)[J]. J. Mater. Res. Technol. 9, 948–959 (2020).
Mittal, H., Ballav, N. & Shivani, B. Gum Ghatti and Fe3O4 magnetic nanoparticles based nanocomposites for the effective adsorption of methylene blue from aqueous solution[J]. J. Ind. Eng. Chem. 20, 2184–2192 (2014).
Gabriela Tubon-Usca. Enhanced adsorption of methylene blue in wastewater using natural zeolite impregnated with graphene Oxide[J]. Appl. Sci. 15, 2824 (2025).
Muntean, S. G., Nistor, M. A., Nastas, R. & Petuhov, O. Dyes and heavy metals removal from aqueous solutions using Raw and modified Diatomite[J]. Processes 11, 2245 (2023).
Kriti, K. et al. Synthesis of thermally stable psyllium Husk/4,4´-Diphenylmethane Diisocyanate-Based polyurethanes for effective and selective removal of cationic Dyes[J]. Water, Air, & Soil Pollution. 236, 277 (2025).
Li, H. et al. Electrostatically spun fabrication of Fe3O4@SiO2/PVA membranes for efficient Methyl orange (MO) adsorption and response surface optimization of the Processes[J]. Langmuir: ACS J. Surf. Colloids. 40, 19631–19643 (2024).
Rui, Z. et al. Research progress on the application of hydrogel adsorbent materials in wastewater treatment: A review[J]. Chem. Eng. Res. Des. 216, 1–24 (2025).
Author information
Authors and Affiliations
Contributions
Z. Y. B.: Conceptualization, Methodology, writing-original draft, software, Formal analysis, Validation; Y. F.: Conceptualization, Methodology, writing-review and editing; H. J. C.: Validation, writing-review and editing; M. Z.: Validation, writing-review and editing; F. L. W.: writing-review, Validation, software.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bu, Z., Fang, Y., Chen, H. et al. Study on the adsorption properties of organically modified diatomite for methylene blue. Sci Rep 15, 27561 (2025). https://doi.org/10.1038/s41598-025-13768-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13768-9