Abstract
To evaluate the clinical value of polyethylene glycolized recombinant human granulocyte-stimulating factor (PEG-rhG-CSF) for hematopoietic reconstitution after autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma (NDMM) patients. This study analyzed data from 70 NDMM patients undergoing ASCT, with 33 receiving PEG-rhG-CSF and 37 receiving rhG-CSF. The median time of neutrophil and platelet engraftment, median transfusions of blood products, treatment-related adverse reactions, long-term hematopoietic reconstitution and economic benefits were compared between the two groups. Both groups had similar median neutrophil engraftment, but PEG-rhG-CSF resulted in slower platelet engraftment (13.7 vs. 11.4 days, p < 0.001), more platelet transfusions (2.9 vs. 1.6 units, p < 0.001), higher incidence of grade ≥ 3 diarrhea (60.6 vs. 29.7%, p = 0.006), longer diarrhea duration (9.0 vs. 5.5 days, p = 0.004), and higher costs (RMB 77,126 vs. RMB 68,361, p = 0.027). PEG-rhG-CSF support post-ASCT in NDMM led to slower platelet engraftment, greater platelet transfusion requirements, increased incidence and duration of severe diarrhea, and greater costs, indicating its potential as an expensive treatment option.
Similar content being viewed by others
Introduction
The current standard treatment approach for newly diagnosed multiple myeloma (NDMM) eligible for transplantation involves induction therapy followed by sequential autologous stem cell transplantation (ASCT) consolidation1. Conditioning regimens preceding autologous stem cell transplantation frequently utilize high-dose chemotherapy, leading to significant neutropenia and thrombocytopenia that elevate the susceptibility to infections and hemorrhages, thereby contributing to early transplantation-related mortality2. The process of hematopoietic reconstitution following ASCT is a crucial component of therapeutic intervention in multiple myeloma (MM). This process is influenced by several factors, including the patient’s overall health status, the disease condition prior to transplantation, the number of infused CD34+ cells, and the specific type of granulocyte colony-stimulating factor (G-CSF) utilized3,4,5,6,7,8. Studies have shown that the use of recombinant human granulocyte stimulating factor (rhG-CSF) enhances neutrophil engraftment following ASCT in individuals with MM, leading to a decrease in the duration of granulocytopenia and a subsequent reduction in the occurrence of severe infections during hematopoietic recovery9,10,11,12. Consequently, rhG-CSF is commonly employed as adjunctive treatment to facilitate hematopoietic reconstitution post-ASCT.
Polyethylene glycolized recombinant human granulocyte stimulating factor (PEG-rhG-CSF) is a sustained-release formulation of rhG-CSF that has been modified through polyethylene glycolization. This modification reduces plasma clearance, extending the half-life of the drug while preserving its biological activity. PEG-rhG-CSF offers enhanced drug solubility, improved bioavailability, and increased stability of the formulation13. Clinical trials have demonstrated that the efficacy and safety of PEG-rhG-CSF in the prevention of febrile neutropenia (FN) following chemotherapy for lymphomas and solid tumors is comparable to that of rhG-CSF14,15,16. Due to its demonstrated efficacy, convenience, and cost-effectiveness, PEG-rhG-CSF has received approval for use in prophylaxis against COVID-19 infection and FN following chemotherapy in patients with solid tumors17,18,19.
Nevertheless, there is a scarcity of studies comparing the effectiveness of PEG-rhG-CSF and rhG-CSF for hematopoietic reconstitution following ASCT in patients with NDMM. Therefore, we carried out a retrospective study in a real-world setting to assess the effectiveness, safety, and cost-efficiency of PEG-rhG-CSF versus rhG-CSF for hematopoietic reconstitution after ASCT in NDMM.
Materials and methods
Patients
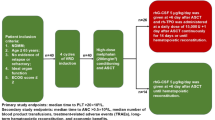
This study conducted a retrospective analysis of 70 patients with NDMM who underwent ASCT at the China-Japan Union Hospital, Jilin University, between January 2018 and June 2023. Prior to January 2021, rhG-CSF was the standard treatment protocol at our institution. However, since 2021, accumulating evidence from multiple randomized controlled trials and observational studies has highlighted the clinical benefits of PEG-rhG-CSF over rhG-CSF14,15,16,20. As a result, the majority of patients treated from January 2021 onward were transitioned to PEG-rhG-CSF. Patients were categorized based on the type of G-CSF administered post-ASCT, with 33 patients in the PEG-rhG-CSF cohort and 37 patients in the rhG-CSF cohort. Prior to ASCT, all patients underwent Durie–Salmon (DS) staging, International Staging System (ISS) staging, Revised International Staging System (R-ISS), mSMART 3.0 risk stratification, and assessment of disease response status21,22,23. Informed consent was obtained from all patients, permitting the use of their data for research purposes. The study received approval from the Ethics Committee of China-Japan Union Hospital of Jilin University. All research methods were performed in accordance with the relevant guidelines and regulations. Patient data was extracted from the hospital’s electronic record management system. Participants in the study were required to meet specific criteria, including being newly diagnosed with multiple myeloma, being aged 65 or younger, undergoing induction therapy with 4 cycles of bortezomib plus lenalidomide and dexamethasone (BRD) regimen to achieve complete remission (CR), very good partial response (VGPR), or partial response (PR) before transplantation, having no evidence of relapse or refractory disease, possessing appropriate organ function (defined as left ventricular ejection fraction ≥ 40%, exertional expiratory volume in 1 s or exertional lung volume in second ≥ 40% of predicted value, and creatinine clearance ≥ 40 ml/min), and having an ECOG score of ≤ 2 (Fig. 1). Lenalidomide was administered at a dosage of 25 mg daily from day 1 to day 14, with the treatment cycle repeating every 21 days24.
Processes for grouping NDMM patients suitable for ASCT who participated in our retrospective study. NDMM, newly diagnosed multiple myeloma; ASCT, autologous stem cell transplantation; BRD, bortezomib, lenalidomide and dexamethasone; PEG-rhG-CSF, polyethylene glycolized recombinant human granulocyte-stimulating factor; rhG-CSF, recombinant human granulocyte-stimulating factor.
Conditioning regimen
All patients were administered intravenous melphalan at a total dose of 200 mg/m2 on days − 3 and − 2 preceding autologous stem cell transplantation (ASCT).
Supportive care
All patients received a prophylactic regimen consisting of 50 mg of fluconazole daily for Candida infection prevention, a double-strength tablet (160/800 mg) of cotrimoxazole taken three times weekly on alternate days to prevent Pneumocystis jirovecii infection pneumonia, and 400 mg of acyclovir twice daily for herpesvirus prevention25,26. Additionally, hepatitis B virus-infected individuals were administered 0.5 mg of entecavir daily to prevent hepatitis B virus activation20,27,28. Kangfuxin liquid, a traditional Chinese medicine, was routinely administered during pretreatment and hematopoietic reconstitution to prevent oral mucosal ulcers. In cases of oral mucosal ulcers, the treatment regimen included a combination of oral gel and supplementation with multivitamins and micronutrients. In the PEG-rhG-CSF cohort, a subcutaneous dose of 100 μg/kg of PEG-rhG-CSF was administered 48 h subsequent to the transfusion of autologous hematopoietic stem cells29. In instances where FN manifested following ASCT reinfusion, supplementary rhG-CSF was administered at a dosage of 5 µg/kg/day until neutrophil engraftment was achieved. FN is defined as a single oral temperature of ≥ 38.3 °C or ≥ 38 °C for over an hour, with an absolute neutrophil count < 0.5 × 109/L or < 1 × 109/L expected to drop to 0.5 × 109/L within 48 h. The rhG-CSF group receiving rhG-CSF was administered a dosage of 5 μg/kg/day beginning on day 6 following autologous stem cell transfusion and continuing until neutrophil engraftment. A red blood cell suspension was administered when the hemoglobin level fell below 60 g/L, while platelets were transfused when the platelet count dropped below 20 × 109/L. Throughout the process of hematopoietic reconstitution, patients experiencing FN are prescribed anti-bacterial and anti-fungal medications on a personalized basis.
Response assessment criteria
The impact of PEG-rhG-CSF and rhG-CSF on short-term hematopoietic reconstitution (SHR), adverse reactions prior to SHR were evaluated as short-term response.
The SHR criteria is defined as neutrophil and platelet engraftment30. Neutrophil engraftment is achieved when the absolute neutrophil count (ANC) is ≥ 0.5 × 109/L for three consecutive days, while platelet engraftment is achieved when the platelet count is ≥ 20 × 109/L for three consecutive days without the need for platelet transfusion20.
ASCT-related adverse reactions were determined by referring to the Common Terminology Criteria for Adverse Events, version 5.031.
The impact of PEG-rhG-CSF and rhG-CSF on long-term hematopoietic and immune reconstitution was assessed at 6 months following ASCT as long-term response30. The assessment of immune reconstitution was conducted through the analysis of lymphocyte subsets and the quantification of immunoglobulin levels. Hematopoietic reconstitution was assessed by conducting a complete blood count analysis.
Economic benefits
To conduct a comparative analysis of the median duration of hospital stays and the median hospitalization costs (expressed in RMB) between the two cohorts.
Statistical analysis
Statistical analyses were conducted utilizing the Statistical Package for the Social Sciences (SPSS) software, version 29.0. For group comparisons adhering to a normal distribution, the Student’s t-test was employed. In cases where the data did not conform to a normal distribution, the Mann–Whitney U-test was applied. The chi-square test was utilized to assess differences in the incidence of specific variables between the two groups. All p-values were two-tailed, with a threshold of p < 0.05 set for statistical significance.
Results
Patient characteristics
Table 1 provides an overview of patient characteristics before ASCT. The median age of the cohort was 55.2 years, with an age range of 36 to 70 years. The gender distribution was 60% male and 40% female. Patients at DS stages II and III comprised 7.1% and 92.9% of the cohort, respectively. The distribution of patients across ISS stages I, II, and III was 31.4%, 32.9%, and 35.7%, respectively. According to the R-ISS, 27.1% of patients were classified as stage I, 45.7% as stage II, and 27.1% as stage III. In terms of the mSMART 3.0 classification, 54.3% of patients were identified as high-risk, while 45.7% were categorized as standard-risk. Prior to ASCT, 65.7% of patients achieved a complete response (CR), 30% achieved a very good partial response (VGPR), and 4.3% achieved a partial response (PR). There were no statistically significant differences observed in median age, sex ratio, proportion of initial DS staging, ISS staging, R-ISS staging, risk stratification, and pre-transplantation disease status between patients in the PEG-rhG-CSF group and rhG-CSF group (p > 0.05).
Short-term hematopoietic reconstitution
The median number of CD34+ cells transfused in the PEG-rhG-CSF group and the rhG-CSF group was 3.6 × 106/kg and 3.8 × 106/kg, respectively. This difference was not statistically significant (p = 0.793), as illustrated in Table 2 and Fig. 2A. Additionally, 22 cases (66.7%) in the PEG-rhG-CSF group received supplementary treatment with rhG-CSF during FN. The median duration of supplementary rhG-CSF administration in this group was 4 days, with a range of 2 to 11 days. In the PEG-rhG-CSF cohort, four patients necessitated the administration of red blood cell suspensions, whereas in the rhG-CSF cohort, two patients required such intervention. The median number of transfused red blood cell suspension in the PEG-rhG-CSF cohort was 0 units, with a range of 0 to 3.5 units. Similarly, the median number in the rhG-CSF cohort was also 0 units, with a range spanning from 0 to 4 units. Statistical analysis indicated that there was no statistically significant difference between the two groups (p = 0.397) (refer to Fig. 2B). In the PEG-rhG-CSF cohort, all 33 patients (100%) underwent platelet transfusion, whereas in the rhG-CSF cohort, 35 patients (94.6%) received platelet transfusions. The median number of platelet units transfused was 2.9 in the PEG-rhG-CSF group and 1.6 in the rhG-CSF group, with a statistically significant difference (p < 0.001) (Fig. 2C). The median time to neutrophil engraftment in the PEG-rhG-CSF and rhG-CSF groups was 11.3 and 11 days, respectively (p = 0.324) (Fig. 2D). The median time to platelet engraftment differed significantly between the two groups, with values of 13.7 and 11.4 days, respectively (p < 0.001) as shown in Fig. 2E. While the duration of neutropenia and antibiotic treatment was longer in the PEG-rhG-CSF group (7.3 and 6.8 days, p = 0.249; 10 and 8.8 days, p = 0.207, respectively), these differences were not statistically significant. The post-transplantation neutrophil and platelet change curves demonstrated a consistent pattern of recovery in both groups. Specifically, an increase in neutrophil count was observed on day +9, with the curve for the PEG-rhG-CSF group showing a slower rise compared to the rhG-CSF group (Fig. 2F). The increase in platelet levels was observed on day +11 in the PEG-rhG-CSF group, while in the rhG-CSF group, this increase was noted on day +9, with platelet counts surpassing those of the PEG-rhG-CSF group (Fig. 2G).
Hematopoietic reconstitution after ASCT in the PEG-rhG-CSF and rhG-CSF groups. (A) median number of CD34+ cells transfused in both groups; (B) median number of red cell suspensions transfused during hematopoietic reconstitution; (C) median number of platelets transfused during hematopoietic reconstitution; (D) median time to neutrophil engraftment; (E) median time to platelet engraftment; (F) curve plot of neutrophil counts after ASCT; (G) curve plot of platelet counts after ASCT.
ASCT related adverse reactions prior to SHR
The ASCT related adverse reactions prior to SHR exhibited comparable characteristics in both cohorts as illustrated in Table 3. The predominant hematologic adverse reactions observed were grade ≥ 3 neutropenia and thrombocytopenia, demonstrating a notable level of concordance in occurrence rates across the two study groups. The prevalence of anemia in the study population was predominantly mild to moderate, with no statistically significant variance observed between the comparison groups. The majority of non-hematologic adverse reactions were graded as ≤ 2 and encompassed symptoms such as nausea and vomiting (81.8 vs. 73%, p = 0.379), elevated transaminases (15.2 vs. 10.8%, p = 0.726), elevated bilirubin (24.2 vs. 27%, p = 0.790), elevated creatinine (6.1 vs. 10.8%, p = 0.677), and fever (48.6 vs. 63.6%, p = 0.157), with no statistically significant distinction between the two cohorts. The prevalence of diarrhea in the two cohorts was 90.9% and 91.9%, respectively, with grade 1–2 diarrhea observed in 30.3% (n = 10) of patients in the PEG-rhG-CSF group and 62.2% (n = 23) in the rhG-CSF group. Grade 3–4 diarrhea was reported in 60.6% (n = 20) of patients in the PEG-rhG-CSF group and 29.7% (n = 11) in the rhG-CSF group (p = 0.006). The median duration of diarrhea in the PEG-rhG-CSF group was significantly longer compared to the control group (9 days vs. 6 days, p = 0.004). There was no statistically significant difference observed between the two groups in terms of the median duration of nausea and vomiting (5 and 4 days, p = 0.141) and the median number of days receiving intravenous nutrition (7 and 6 days, p = 0.330). The primary adverse reaction linked to the administration of G-CSF was bone pain, which manifested at comparable frequencies in both cohorts (12.1% and 8.1%, p = 0.699).
Long-term hematopoietic reconstitution and immune reconstitution follow-up
There were 2 instances of loss to follow-up in each group. At 6 months post-ASCT, the median neutrophil counts of patients who remained in the study were 2.9 × 109/L in the PEG-rhG-CSF group and 3.1 × 109/L in the rhG-CSF group (p = 0.386) (Table 4). The median hemoglobin levels were 124.1 g/L and 124.7 g/L, respectively (p = 0.925). Additionally, the median platelet counts were 153.1 × 109/L in the PEG-rhG-CSF group and 141.9 × 109/L in the rhG-CSF group (p = 0.397), indicating no statistically significant differences between the two groups. The median lymphocyte counts of patients were 1082 /ul in the PEG-rhG-CSF group and 1162 /ul in the rhG-CSF group (p = 0.397). There was no statistically significant difference in the counts of CD4+ , CD8+ , CD19+ and CD3+ CD56+ lymphocytes between the two groups of patients. The median levels of immunoglobulin G, immunoglobulin A, and immunoglobulin M were comparable between the two patient groups, with no statistically significant differences observed.
Economic benefits
The median length of hospitalization did not significantly differ between the two groups, with durations of 27.1 days in the PEG-rhG-CSF group and 26.4 days in the rhG-CSF group (p = 0.499). The median hospitalization cost was RMB 77,126 in the PEG-rhG-CSF group and RMB 68,361 in the rhG-CSF group, showing a statistically significant variance between the groups (p = 0.027). The excess expenditure in the PEG-rhG-CSF group was a median of RMB 8765.
Discussion
The utilization of rhG-CSF following haematopoietic stem cell transplantation has been shown to decrease the duration of neutropenia and expedite neutrophil engraftment, ultimately mitigating the likelihood of severe infections and engraftment failure10. Previous studies have utilized PEG-rhG-CSF to prevent neutropenia following solid tumor chemotherapy, demonstrating a notable superiority over rhG-CSF32. Nevertheless, there remains a scarcity of clinical research on the utilization of PEG-rhG-CSF for hematopoietic reconstitution following ASCT. An analysis of the efficacy, safety, duration of hospitalization, and associated costs between PEG-rhG-CSF and conventional G-CSF in patients with NDMM holds considerable clinical importance and practical value. Such a comparison can offer valuable insights for healthcare providers in making informed clinical decisions.
The efficacy of PEG-rhG-CSF in preventing neutropenia following chemotherapy in patients with solid tumors has been validated through clinical trials. A retrospective study was conducted to investigate the effectiveness and safety of primary prophylactic use of PEG-rhG-CSF as supportive care for elderly patients with advanced non-small-cell lung cancer who were chemotherapy-naive and had been treated with docetaxel plus ramucirumab. The study found that only 1 case (1.9%) of FN was observed, and 3 cases (5.6%) experienced grade ≥ 3 neutropenia. These results confirm the high efficacy of PEG-rhG-CSF in preventing FN following chemotherapy for solid tumors33. A separate meta-analysis indicated a high level of quality and certainty in the evidence supporting the use of single-dose PEG-rhG-CSF for the primary prevention of febrile neutropenia following chemotherapy, as opposed to multiple-dose injections of non-PEG-rhG-CSF34. The prophylactic administration of PEG-rhG-CSF following chemotherapy for solid tumors offers several benefits, including a reduction in the frequency of injection, a decrease in the incidence of febrile neutropenia, a decrease in the need for antibiotics, an improvement in treatment adherence, and a reduction in the financial burden on patients.
Nevertheless, there is a scarcity of clinical studies on the use of PEG-rhG-CSF for hematopoietic reconstitution following ASCT. A retrospective study was conducted to assess the efficacy and safety of PEG-rhG-CSF compared to rhG-CSF for hematopoietic recovery following allogeneic hematopoietic stem cell transplantation (allo-HSCT)35. The median time to neutrophil engraftment in the PEG-rhG-CSF group was 13.5 days, while in the rhG-CSF group it was 13 days (p = 0.393). Similarly, the median time to platelet engraftment was 14 days in both the PEG-rhG-CSF and rhG-CSF groups (p = 0.094). The results indicate that there was no statistically significant variance in hematopoietic reconstitution between the use of PEG-rhG-CSF and rhG-CSF as supportive therapy following allo-HSCT, suggesting that PEG-rhG-CSF may serve as a viable alternative to rhG-CSF in this context. A multicenter retrospective study was conducted to assess the efficacy and ideal timing of PEG-rhG-CSF following ASCT in lymphoma patients36. The study found no statistically significant difference in the duration of neutrophil engraftment between the PEG-rhG-CSF and rhG-CSF cohorts. The duration for neutrophil engraftment was notably reduced when PEG-rhG-CSF was administered on either day 1 or day 3 following hematopoietic stem cell transplantation (9 and 9 days, respectively). This suggests that PEG-rhG-CSF may serve as a viable substitute for rhG-CSF in lymphoma patients undergoing autologous stem cell transplantation.
Our study found that there was no statistically significant disparity in neutrophil engraftment time between the two forms of G-CSF, suggesting that PEG-rhG-CSF is comparable to rhG-CSF in facilitating granulopoietic recovery post-ASCT, aligning with findings from prior research20,32,36,37,38. However, in the PEG-rhG-CSF cohort, approximately two-thirds of the patients required supplementary rhG-CSF as a salvage intervention during episodes of febrile neutropenia. Thus, it is not appropriate to assume that PEG-rhG-CSF can serve as a substitute for rhG-CSF in facilitating neutrophil recovery in patients with NDMM following high-dose melphalan conditioning and ASCT.
A statistically significant disparity was observed in the timing of platelet engraftment between the two groups, with the PEG-rhG-CSF group exhibiting a notably slower rate. Additionally, the PEG-rhG-CSF group required a significantly higher number of platelet transfusions compared to the rhG-CSF group, a consequence attributed to the delayed platelet engraftment. Thus, our hypothesis posits that PEG-rhG-CSF exerts a negative regulatory influence on platelet engraftment. Ringdén O et al. conducted a study indicating that G-CSF prolongs the time to platelet engraftment following allogeneic bone marrow transplantation39. Subsequent research has indicated that granulocyte colony-stimulating factor (G-CSF) may impede platelet engraftment following hematopoietic stem cell transplantation (HSCT) by obstructing the differentiation of common myeloid progenitors and megakaryocytic-erythroid progenitors into megakaryocytes and platelets40. Subsequent clinical studies have corroborated the findings of a retrospective analysis which investigated the impact of G-CSF support on hematopoietic reconstitution following allo-HSCT. The analysis revealed a shorter time to platelet engraftment in the control group, which did not receive G-CSF, compared to the experimental group that did receive G-CSF (14 days vs. 18 days, p = 0.015), indicating a potential inhibitory effect of G-CSF on platelet engraftment post-HSCT41. A separate investigation examined the impact of a PEG-rhG-CSF biosimilar on hematopoietic recovery following autologous stem cell transplantation in patients with multiple myeloma. Findings from this research indicated a statistically significant increase in the number of platelet transfusions required in the PEG-rhG-CSF biosimilar cohort compared to the rhG-CSF cohort (2 units vs. 1 unit, p < 0.01), suggesting a potential delay in platelet engraftment associated with PEG-rhG-CSF20. Our study has provided novel findings indicating that the use of PEG-rhG-CSF following ASCT in patients with NDMM results in prolonged platelet engraftment time and increased platelet transfusion requirements. These results have significant clinical implications and warrant further investigation. Our hypothesis posits that PEG-rhG-CSF exerts a more pronounced inhibitory influence on platelet engraftment compared to rhG-CSF, attributable to its distinct pharmacokinetic profile. Given the limited sample size in this study, additional validation is required through enlargement of the sample size in subsequent investigations. Concurrently, animal modeling studies will be conducted to delve deeper into the underlying mechanisms.
Grade ≥ 3 adverse reactions, primarily hematologic toxicities such as neutropenia and thrombocytopenia, were observed in both groups in this study, aligning with findings from prior research. Conversely, non-hematological adverse reactions, including mucositis, renal insufficiency, and transaminase elevation, were predominantly ≤ grade 2, indicating a manageable level of safety. The increased incidence and extended duration of grade 3–4 diarrhea observed in the PEG-rhG-CSF cohort in this study may be ascribed to several factors: the slower rate of granulocyte elevation, mucosal damage resulting from melphalan administration, and impairment of the intestinal mucosal barrier in the absence of neutrophilic cells, which may predispose patients to concurrent infections. Furthermore, PEG-rhG-CSF is known to have diarrhea as a potential adverse effect. Compared to PEG-rhG-CSF, rhG-CSF was associated with a reduction in the severity of diarrhea and a shorter duration of symptoms. This finding, which contradicts previous research, warrants further investigation through prospective studies with larger sample sizes to confirm its validity20.
While there was no significant statistical variance in the duration of hospital stays between the two cohorts, the median hospitalization expenses in the PEG-rhG-CSF group rose by RMB 8765. Initially, 66.7% of patients in the PEG-rhG-CSF group received additional rhG-CSF during FN. Subsequently, patients in this group exhibited an extended platelet engraftment period and a higher frequency of platelet transfusions. Additionally, the PEG-rhG-CSF cohort demonstrated a slightly elevated incidence of grade 3–4 diarrhea and prolonged duration of diarrhea, necessitating a greater number of intravenous nutritional interventions. Lastly, the PEG-rhG-CSF group experienced a marginally prolonged duration of neutropenia and increased utilization of antibiotics. These factors are probable contributors to the discrepancy in hospitalization costs noted between the two groups.
Our study has some limitations. Initially, the limited sample size and absence of random sampling may introduce selection bias in the findings. Furthermore, due to the diverse and unique characteristics of patients with multiple myeloma, efforts were made to enhance the uniformity of the study cohort by retrospectively selecting individuals eligible for transplantation who received BRD induction therapy for a duration of 4 cycles, while excluding those treated with alternative regimens such as bortezomib plus cyclophosphamide and dexamethasone (BCD), bortezomib plus thalidomide and dexamethasone (BTD), and daratumumab plus BRD (D-BRD). However, the reliability of results across various samples remains uncertain. Additionally, retrospective studies are inherently influenced by subjective factors, and the accuracy of medical records is not regulated by the study’s design, leading to potential confounding variables and bias. Therefore, future research should focus on conducting prospective clinical studies with larger sample sizes.
Conclusion
No advantage was found for hematopoietic reconstitution after ASCT in the PEG-rhG-CSF group compared to the control group supported by rhG-CSF. Notably, the administration of PEG-rhG-CSF has been associated with an extended duration for platelet engraftment and an increased frequency of platelet transfusions. Furthermore, individuals receiving PEG-rhG-CSF support exhibited a higher incidence and longer duration of severe diarrhea, as well as increased median hospitalization costs. Therefore, it appears that PEG-rhG-CSF may be a more expensive choice for supportive treatment following ASCT in NDMM.
Data availability
The deidentified data generated in this study are available upon appropriate request to the corresponding author at wenxu@jlu.edu.cn.
References
Bazarbachi, A. H. et al. Induction therapy prior to autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma: An update. Blood Cancer J. 12, 47. https://doi.org/10.1038/s41408-022-00645-1 (2022).
Jantunen, E. et al. Early treatment-related mortality in adult autologous stem cell transplant recipients: A nation-wide survey of 1482 transplanted patients. Eur. J. Haematol. 76, 245–250. https://doi.org/10.1111/j.1600-0609.2005.00605.x (2006).
Hassan, M. N. et al. Autologous peripheral blood stem cell transplantation among lymphoproliferative disease patients: Factors influencing engraftment. Oman Med. J. 34, 34–43. https://doi.org/10.5001/omj.2019.06 (2019).
Aladağ Karakulak, E. et al. CD34+ hematopoietic progenitor cell dose as a predictor of engraftment and survival in multiple myeloma patients undergoing autologous stem cell transplantation. Turk. J. Med. Sci. 50, 1851–1856. https://doi.org/10.3906/sag-2001-173 (2020).
Serin, I. et al. Plerixafor in autologous stem cell transplantation: Does it affect engraftment kinetics?. Transfus. Apher. Sci. Off. J. World Apher. Assoc. Off. J. Eur. Soc. Haemapher. 62, 103809. https://doi.org/10.1016/j.transci.2023.103809 (2023).
Pham, T. et al. Comparison of biosimilar filgrastim with originator filgrastim for peripheral blood stem cell mobilization and engraftment in patients with multiple myeloma undergoing autologous stem cell transplantation. Transfusion 55, 2709–2713. https://doi.org/10.1111/trf.13233 (2015).
Bassi, S. et al. Safety and efficacy of granulocyte colony-stimulating factor biosimilars in engraftment after autologous stem cell transplantation for haematological malignancies: A 4-year, single institute experience with different conditioning regimens. Blood Transfus. Trasfusione del sangue 13, 478–483. https://doi.org/10.2450/2015.0198-14 (2015).
Serin, I. et al. Plerixafor in autologous stem cell transplantation: Does it affect engraftment kinetics?. Transfus. Apher. Sci. 62, 103809. https://doi.org/10.1016/j.transci.2023.103809 (2023).
Klein, E. M. et al. Antibiotic prophylaxis or granulocyte-colony stimulating factor support in multiple myeloma patients undergoing autologous stem cell transplantation. Cancers 13, 3439. https://doi.org/10.3390/cancers13143439 (2021).
Singh, V. et al. G-CSF use post peripheral blood stem cell transplant is associated with faster neutrophil engraftment, shorter hospital stay and increased incidence of chronic GVHD. Leuk. Lymphoma 62, 446–453. https://doi.org/10.1080/10428194.2020.1827244 (2021).
Cioch, M. et al. Biosimilar granulocyte colony-stimulating factor is effective in reducing the duration of neutropenia after autologous peripheral blood stem cell transplantation. Transpl. Proc. 46, 2882–2884. https://doi.org/10.1016/j.transproceed.2014.09.070 (2014).
Singh, A. D. et al. Granulocyte colony-stimulating factor use after autologous peripheral blood stem cell transplantation: Comparison of two practices. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 24, 288–293. https://doi.org/10.1016/j.bbmt.2017.10.026 (2018).
Molineux, G. Pegfilgrastim: Using pegylation technology to improve neutropenia support in cancer patients. Anticancer Drugs 14, 259–264. https://doi.org/10.1097/00001813-200304000-00002 (2003).
Roder, L., Konrardy, K., Grauer, D. & Hoffmann, M. Effects of filgrastim versus pegfilgrastim on outcomes of DA-R-EPOCH for non-Hodgkin’s lymphoma. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 29, 5075–5082. https://doi.org/10.1007/s00520-021-06045-8 (2021).
Gebremariam, G. T., Fentie, A. M., Beyene, K., Sander, B. & Gebretekle, G. B. Cost-effectiveness of pegfilgrastim versus filgrastim for prevention of chemotherapy-induced febrile neutropenia in patients with lymphoma: A systematic review. BMC Health Serv. Res. 22, 1600. https://doi.org/10.1186/s12913-022-08933-z (2022).
Zhu, X. et al. Pegfilgrastim on febrile neutropenia in pediatric and adolescent cancer patients: A systematic review and meta-analysis. Hematology 28, 2172292. https://doi.org/10.1080/16078454.2023.2172292 (2023).
Gwak, H. et al. COVID-19 prevention guidance and the incidence of febrile neutropenia in patients with breast cancer receiving TAC chemotherapy with prophylactic pegfilgrastim. J. Clin. Med. https://doi.org/10.3390/jcm11237053 (2022).
Kurogochi, T. et al. Efficacy and cost-effectiveness of pegfilgrastim for preventing febrile neutropenia during docetaxel, cisplatin, and 5-fluorouracil therapy for esophageal cancer. Anticancer Res. 43, 2293–2298. https://doi.org/10.21873/anticanres.16393 (2023).
Moon, Y. W. et al. Eflapegrastim versus pegfilgrastim for chemotherapy-induced neutropenia in Korean and Asian patients with early breast cancer: Results from the two phase iii advance and recover studies. Cancer Res. Treat. 55, 766–777. https://doi.org/10.4143/crt.2022.987 (2023).
Martino, M. et al. Effectiveness of biosimilar pegfilgrastim in patients with multiple myeloma after high-dose melphalan and autologous stem cell transplantation. Ann. Hematol. 102, 1915–1925. https://doi.org/10.1007/s00277-023-05228-z (2023).
Gonsalves, W. I. et al. Utilization of hematopoietic stem cell transplantation for the treatment of multiple myeloma: A mayo stratification of myeloma and risk-adapted therapy (mSMART) consensus statement. Bone Marrow Transplant. 54, 353–367. https://doi.org/10.1038/s41409-018-0264-8 (2019).
Greipp, P. R. et al. International staging system for multiple myeloma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 23, 3412–3420. https://doi.org/10.1200/jco.2005.04.242 (2005).
Palumbo, A. et al. Revised international staging system for multiple myeloma: A report from international myeloma working group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 33, 2863–2869. https://doi.org/10.1200/jco.2015.61.2267 (2015).
Cavallo, F. et al. Stem cell mobilization in patients with newly diagnosed multiple myeloma after lenalidomide induction therapy. Leukemia 25, 1627–1631. https://doi.org/10.1038/leu.2011.131 (2011).
Huijgens, P. C. et al. Fluconazole versus itraconazole for the prevention of fungal infections in haemato-oncology. J. Clin. Pathol. 52, 376–380. https://doi.org/10.1136/jcp.52.5.376 (1999).
Gkirkas, K. et al. Low-dose cotrimoxazole administered in hematopoietic stem cell transplant recipients as prophylaxis for Pneumocystis jirovecii pneumonia is effective in prevention of infection due to nocardia. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 25, e298–e299. https://doi.org/10.1016/j.bbmt.2019.07.018 (2019).
Wang, X., Ren, J., Liang, X. & He, P. Efficacy and cost of G-CSF derivatives for prophylaxis of febrile neutropenia in lymphoma and multiple myeloma patients underwent autologous hematopoietic stem cell transplantation. Hematology 26, 950–955. https://doi.org/10.1080/16078454.2021.2003071 (2021).
Portuguese, A. J. et al. Revisiting the utility of granulocyte colony-stimulating factor post-autologous hematopoietic stem cell transplantation for outpatient-based transplantations. Transplant. Cell Ther. 29, 696.e691-696.e697. https://doi.org/10.1016/j.jtct.2023.08.021 (2023).
Qin, Y. et al. A phase I study of different doses and frequencies of pegylated recombinant human granulocyte-colony stimulating factor (PEG rhG-CSF) in patients with standard-dose chemotherapy-induced neutropenia. Chin. J. Cancer Res. 29, 402–410. https://doi.org/10.21147/j.issn.1000-9604.2017.05.04 (2017).
Zubair, A. C. et al. CD34(+) CD38(−) and CD34(+) HLA-DR(−) cells in BM stem cell grafts correlate with short-term engraftment but have no influence on long-term hematopoietic reconstitution after autologous transplantation. Cytotherapy 8, 399–407. https://doi.org/10.1080/14653240600847241 (2006).
Goldsmith, S. R. et al. A phase Ib trial of isatuximab, bendamustine, and prednisone in relapsed/refractory multiple myeloma. Ann. Hematol. 103, 4557–4565. https://doi.org/10.1007/s00277-024-05975-7 (2024).
Li, X. et al. Is PEGylated G-CSF superior to G-CSF in patients with breast cancer receiving chemotherapy? A systematic review and meta-analysis. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 28, 5085–5097. https://doi.org/10.1007/s00520-020-05603-w (2020).
Tachihara, M. et al. Docetaxel plus ramucirumab with primary prophylactic pegylated granulocyte-colony stimulating factor support for elderly patients with advanced NSCLC: A multicenter prospective single arm phase 2 trial: Dragon study (WJOG9416L). JTO Clin. Res. Rep. 4, 100569. https://doi.org/10.1016/j.jtocrr.2023.100569 (2023).
Yoshinami, T. et al. Comparison between a single dose of PEG G-CSF and multiple doses of non-PEG G-CSF: A systematic review and meta-analysis from clinical practice guidelines for the use of G-CSF 2022. Int. J. Clin. Oncol. 29, 681–688. https://doi.org/10.1007/s10147-024-02504-4 (2024).
Yang, F. et al. [Comparative study on the efficacy and safety between pegfilgrastim (PEG-rhG-CSF) and recombinant human granulocyte colony-stimulating factor in promoting hematopoietic recovery after allogeneic hematopoietic stem cell transplantation after hematological malignancy]. Zhonghua xue ye xue za zhi Zhonghua xueyexue zazhi 38, 831–836. https://doi.org/10.3760/cma.j.issn.0253-2727.2017.10.002 (2017).
Li, S. et al. The effectiveness and optimal timing of PEG-rhG-CSF after autologous peripheral blood stem cell transplantation: A multicenter experience. Indian J. Hematol. Blood Transfus. Off. J. Indian Soc. of Hematol. Blood Transfus. 40, 190–195. https://doi.org/10.1007/s12288-023-01704-8 (2024).
Castagna, L. et al. Pegfilgrastim versus filgrastim after high-dose chemotherapy and autologous peripheral blood stem cell support. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 21, 1482–1485. https://doi.org/10.1093/annonc/mdp576 (2010).
Vanstraelen, G. et al. Pegfilgrastim compared with filgrastim after autologous hematopoietic peripheral blood stem cell transplantation. Exp. Hematol. 34, 382–388. https://doi.org/10.1016/j.exphem.2005.11.013 (2006).
Ringdén, O. et al. Treatment with granulocyte colony-stimulating factor after allogeneic bone marrow transplantation for acute leukemia increases the risk of graft-versus-host disease and death: A study from the acute leukemia working party of the European group for blood and marrow transplantation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 22, 416–423. https://doi.org/10.1200/jco.2004.06.102 (2004).
Li, Y. et al. G-CSF administration results in thrombocytopenia by inhibiting the differentiation of hematopoietic progenitors into megakaryocytes. Biochem. Pharmacol. 169, 113624. https://doi.org/10.1016/j.bcp.2019.113624 (2019).
Sarıcı, A. et al. The effect of G-CSF used after allogeneic hematopoietic stem cell transplantation on engraftment times and platelet suspension replacement numbers. Transfus. Apher. Sci. Off. J. World Apher. Assoc. Off. J. Eur. Soc. Haemapher. 61, 103482. https://doi.org/10.1016/j.transci.2022.103482 (2022).
Acknowledgements
The research was conducted by a multidisciplinary team of clinicians affiliated with the Department of Oncology and Hematology, the Central Research Laboratory, and the Medical Laboratory Center of China-Japan Union Hospital at Jilin University.
Funding
This work was supported by grants from Science and Technology Development Project of Jilin Province (YDZJ202201ZYTS092 to WZ) and Natural Science Foundation of Jilin Province (20210401174YY to WZ). The source of funding had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
W.Z. concepted and designed the study. Z.Z., D. C., Y. B., W. Z. provide study materials or patients. R.W. and H. T. collected the data wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, R., Tian, H., Zhu, Z. et al. The hidden costs of PEG-rhG-CSF in autologous stem cell transplantation for newly diagnosed multiple myeloma. Sci Rep 15, 30992 (2025). https://doi.org/10.1038/s41598-025-15360-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15360-7