Abstract
Lasers and electricity are commonly used energy sources in urological surgery. They all have both advantages and disadvantages in tissue removal efficiency and hemostasis, and it is difficult for them to replace one another in clinical practice. This study aimed to develop a special surgical tool which can simultaneously transmit high-power laser energy and conduct electricity for urologic surgical procedures. Since this tool integrates the function of conducting laser and electrical energy through a laser fiber, we call it integrated fiber. As a product of this study, the integrated fiber can connect with a thulium laser and a bipolar plasma generator simultaneously. Using this fiber, an ex vivo simulation of transurethral vaporization of porcine kidney tissues was conducted. In terms of tissue removal efficiency, the laser-plasma and the plasma group showed the highest and the lowest, respectively. For thermal damage, there was no significant difference between the laser-plasma and laser group. Though a prototype, with this integrated fiber, the combined laser-plasma energy can vaporize kidney tissue more efficiently without additional thermal damage. This integrated dual-functional laser-electric optical fiber shows good application prospects in urological surgery.
Similar content being viewed by others
Introduction
Lasers and electricity are commonly used energy sources in urological surgery. Each has its own advantages and disadvantages. Transurethral resection of the prostate (TURP) remains the historical benchmark surgical treatment for benign prostatic hyperplasia (BPH)1. TURP with bipolar plasma offers better durability but higher complication rates for elderly patients with multiple comorbidities compared to laser prostatectomy2. Laser prostatectomy, while advantageous in terms of hemostasis and shorter hospital stays, is less efficient and less cost-effective than TURP3. Consequently, it is challenging for either method to supplant the other.
Some urologists have successfully combined the use of a thulium laser with bipolar TURP for the endoscopic treatment of BPH4. However, these methods are applied sequentially rather than truly integrated, making the procedure time-consuming and labor-intensive.
To address this issue, we hypothesized that integrating laser and electrical energy into a single device could be a viable solution. Therefore, we attempted to incorporate electrical elements into the optical laser fiber to create a surgical tool capable of simultaneous both laser and electrical vaporization/resection for urologic procedures.
Materials and methods
Preparation of the integrated laser-electric fiber
A commercially available high-energy medical optical fiber (Raykeen, Shanghai, China), with a core diameter of 550 µm, and with a stripped outer layer at the distal end, was prepared for integration with the electrical components.
The electrical components included a fine metal wire with a diameter of 0.2 mm and a tiny stainless-steel tube with an outer diameter of 1.0 mm, which were attached to the bare optical fiber and insulated with a plastic tube. This is a heat shrink tube with an outer diameter of 1.5 mm before shrinking. Half (2.0 mm) of the small stainless-steel tube was exposed near its distal end. The proximal end of the fine metal wire was connected to the output of a bipolar plasma generator. During the surgery, only the frontmost tiny stainless-steel tube that comes into contact with the bio-tissue and acts on electrovaporization. Once self-ablated, it will be cut off on demand, and the second tiny stainless-steel tube will be pushed out to the front, and the insulated tube wrapped around the latter will also be stripped 2 mm to contact the tissue and exert electrovaporization. This process is repeated so that the integrated fiber can continue to function.
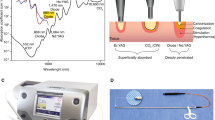
A standard transurethral laser resectoscope (F26, Raykeen, Shanghai, China) was used in this experiment. The outer sheath of the resectoscope was connected to a plasma generator to serve as part of the electric circuit. The knob lock of the resectoscope served as the access point for the circuit. The integrated fiber was passed through the fiber channel of the resectoscope and connected both to a thulium laser machine and a bipolar plasma generator. The final form of this new system is illustrated in the schematic diagram in Fig. 1.
Schematic diagram of the integrated dual-functional laser-electric optical fiber system. The integrated laser-electric fiber is adapted to the resectoscope and connected to the laser and plasma generator separately. The contents of the two circles are magnified images of the fiber tip and the plasma current access site, respectively.
Verification of integrated laser-electric fiber
To evaluate the functionality of the integrated fiber, a series of simulated transurethral vaporization tests were conducted. Isotonic saline was maintained at room temperature and served as the irrigation fluid. Commercial fresh porcine kidney tissue was utilized as the vaporization target. A continuous-wave thulium fiber laser [SRM-T120F (Youlu ®), Raykeen, Shanghai, China] and a bipolar plasma generator (Olympus, Tokyo, Japan) were employed in the tests, with energy outputs set at 50 W for the thulium laser and 100 W/50W (cutting/coagulation mode) for the plasma. The tests were categorized into three groups: laser–plasma, laser, and plasma group. In the laser–plasma group, simultaneous activation of the laser and plasma was achieved by depressing both foot switches concurrently. The distal end of the integrated fiber, the head end of the laser fiber and the exposed part of the tiny stainless-steel tube are used to vaporize the biological tissue. And the exposed tiny stainless-steel tube can also coagulate and stop bleeding by directly contacting the surface of the biological tissue.
All surgical procedures were performed by a single urologist to ensure consistency. Tissue specimens were collected post-vaporization and fixed in 4% formaldehyde solution. Hematoxylin and Eosin staining was subsequently performed on 5-μm-thick sections for histopathological analysis. The extent of thermal damage in the kidney tissue was examined and quantified.
Tissue removal efficiency was assessed using a weighing method. The kidney tissue was weighed before and after vaporization, and the duration of each vaporization procedure was recorded. Tissue removal efficiency (g/min) was calculated by dividing the change in tissue mass (grams) by the vaporization time (minutes).
Statistical analysis
Online software SPSS Pro (available at https://www.spsspro.com) was utilized for statistical analyses. Data was presented as mean ± SD. Results were analyzed using one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test. A p value of ≤ 0.05 was considered statistically significant.
Results
Product of the integrated dual-functional laser-electric fiber
An integrated dual-functional laser-electric optical fiber was successfully developed as shown in Fig. 2. An electrically conductive wire was securely attached to the fiber and connected to the built-in metal wire inside the integrated fiber, enabling direct connection with the output of the plasma generator. During surgery, any distal segment of the fiber, including the tiny stainless-steel tube, could be cut off if necessary.
Usability and safety
During all experiments, especially when the bipolar plasma generator was excited, the body of the resectoscope and electric circuit were inspected with a voltage tester, and no instances of electric leakage were detected.
The integrated fiber was manipulated similarly to an ordinary optical fiber in transurethral laser operations. When required, the tiny metal tube was easily maneuvered to a virtual bleeding point for hemostasis in coagulation mode. The surgical view remained clear and was not impaired by any part of the integrated fiber, including the stainless-steel tube at the tip of the fiber. The number of air bubbles generated during vaporization was not significant, and the surgical field visibility was not affected.
The tiny stainless-steel tube at the integrated fiber tip remained securely in place. When continuously excited under the laser-plasma mode, the stainless-steel tube maintained normal functionality for at least 5 min [(5.07 ± 0.34) min]. If substantial self-ablation occurred, the damaged stainless-steel tube could be easily cut off, and the adjacent segment was available for continued use.
The vaporization procedures could be seen in the supplemental video (Supplemental video 1).
Tissue removal efficiency
Macroscopically, after only one pass of vaporization with the integrated fiber, the vaporized defect caused by the laser-plasma energy was the deepest among the three groups.
The tissue removal efficiency of the laser-plasma group was significantly greater than that of the plasma group. The plasma group demonstrated the lowest efficiency. No significant difference was observed between the laser group and the plasma group (Fig. 3).
Thermal damage to the kidney tissue
For thermal damage to porcine kidney tissue, the carbonized, coagulated, and denatured layer was easily recognized under the microscope (Fig. 4).
Histopathological observation of thermal damage to the porcine kidney tissue by laser-plasma, laser, and plasma; HE stains. (A) 10 × , a large pathological section of porcine kidney tissue after vaporization by the laser-plasma, laser, and plasma energy with the integrated fiber. (B, C, D) 400 × , enlarged images of kidney tissue after laser-plasma, laser, and plasma vaporization.
The degree of thermal damage in the plasma group was lower than that in the laser-plasma and laser groups. No statistically significant difference was identified between the laser-plasma and laser groups (p > 0.05) (Fig. 5).
Comparison of thermal damage depth in the three groups. A significant difference was observed among the total groups (p < 0.001). The LSD test demonstrated statistically significant differences between the laser-plasma and plasma groups (p < 0.001) and between the laser and plasma groups (p < 0.001).
Discussion
Lasers and TUR are the two most used surgical devices in urology. At present, most of the medical institutions that have established urology departments are equipped with these two types of equipment. However, in clinical practice, these two devices are used independently, and they need to be equipped with their special accessories, laser fiber or resecting loop respectively, and both accessories are relatively high-value consumables. If it is possible to make an integrated tool that can conduct both laser and electrical energy, it can be used separately or simultaneously in compatible devices. In this way, the use of this integrated fiber has obvious cost-effective advantages. To date, there are no published reports addressing this integration.
In this study, the effectiveness of the integrated laser-electric optical fiber had been verified. This integrated fiber fitted the resectoscope well and could be manipulated as that of a popular medical optical fiber. Therefore, this lays the foundation for the further application of this integrated fiber to the urology clinic. In the future, with its double energy supplies, this integrated fiber could be expected to be applied to the transurethral resection or ablation of prostate or bladder tumor. Then, undoubtedly, the tissue removal efficiency would be improved greatly, and the unexpected bleeding would also be well controlled, even the obturator nerve reflex could also be effectively avoided.
Developing the electric circuit was challenging in this experiment. The outer sheath of the laser resectoscope was used as part of the electric circuit and the access point was strategically placed on the knob lock of the resectoscope. This approach was aligned with the circuit design of the Olympus resectoscope5. In vitro observations confirmed the safety of this setup. Off cause, additional in vivo verification remains essential.
In this experiment, vaporization, rather than resection or vaporesection, was employed to evaluate the biological effectiveness of the integrated fiber on porcine kidneys. It is well recognized that during resection or vaporesection, maintaining precise consistency in the volume of excised tissue is challenging, and this variability can significantly affect the assessment of tissue removal efficiency. In contrast, vaporization is not influenced by this limitation.
In this study, tissue removal efficiency approximately three to four times that of laser or plasma alone had been achieved. This result suggests that the simultaneous application of laser and plasma can help improve the tissue clearance rate. With the back-and-forth movement of the integrated fiber, the laser and the plasma energy are applied to the kidney tissue within a very short space–time interval. If this interval is shortened indefinitely, a mixed energy field of the laser and plasma will be formed, and these two superimposed energies will undoubtedly significantly improve the efficiency of tissue clearance. To further illustrate this superimposing process, we have created a demonstration animation, which can be found in the attached video (Supplemental videos 2 and 3). In the supplemental video 3, we removed the tiny stainless-steel tube so that the conductive tungsten wire head and the laser fiber head were flush and exposed to the insulating plastic sleeve. In this way, laser and plasma energy can be released and used to vaporize biological tissues at the same time, achieving complete synchronization of the two energies in time and space.
Regarding thermal damage to kidney tissue, no significant difference was observed between the laser-plasma group and the laser-only group. This finding aligns with previous studies6. It is commonly assumed that the integration of laser energy with plasma results in deeper thermal damage in kidney tissue. However, this assumption was challenged in the present study. Since the plasma-induced thermal damage is minimal and shallow, whereas the laser-induced damage is deeper, the laser emerges as the primary determinant of thermal damage depth. Consequently, the difference in thermal damage observed between the laser-plasma group and the laser group was not statistically significant.
It is important to note that the “plasma” used in this study is not the “plasma” in the real physical sense. The so-called “plasma” in bipolar plasma cutting surgery is essentially a partially ionized gaseous medium, primarily composed of ionized particles (such as ions and free electrons) and neutral molecules produced when high-frequency current passes through saline solution. We cannot hope that this laser-plasma interaction will be like a super-pulsed high-energy laser which can even induce plasma and proton acceleration for tumor treatment7,8. However, the introduction of high-energy lasers into bipolar plasma resection can theoretically bring the local medium closer to a complete plasma by increasing the ionization degree and temperature9. If the parameters are properly optimized, this composite energy system may provide a new solution with higher precision and lower thermal damage for minimally invasive surgery. Of course, its realization still needs further experimental verification and engineering innovation.
This study had some limitations. For a small stainless-steel tube, this was the last resort under our real conditions. After all, we are clinicians, not engineers. Under the current experimental energy level and continuous vaporization mode, the tube could last for up to approximately 5 min before self-ablation. Fortunately, the string-like arrangement of the tubes made it easy to cut them off when necessary, and the next tube will be pushed forward, and together with the optical laser fiber, act as that of the first one. And so on until the last tube. If the tube were made of high-temperature-resistant materials, such as tungsten or titanium alloys, the situation would be greatly improved. Additionally, it is necessary to continue to search for insulating materials with good biocompatibility and strong high-temperature resistance to replace the plastic tubes in this study. As a prototype, the tip of this integrated fiber is still lack of the flexibility and versatility of the TUR (Transurethral resection, TUR) loop, and this is exactly what we need to do in future research.
Conclusions
In summary, a prototype of an integrated dual-functional laser-electric optical fiber was successfully developed. Utilizing this integrated fiber, laser-plasma energy facilitates the vaporization of biological tissues with higher tissue removal efficiency and lower tissue thermal damage. This innovative fiber enables urologists to maximize the benefits of both laser and plasma energy in future urologic surgical procedures.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Porto, J. G. et al. Evaluating transurethral resection of the prostate over twenty years: A systematic review and meta-analysis of randomized clinical trials. World J Urol. 42, 639. https://doi.org/10.1007/s00345-024-05332-3 (2024).
Bouhadana, D. et al. Safety and efficacy of TURP vs. laser prostatectomy for the treatment of benign prostatic hyperplasia in multi-morbid and elderly individuals aged ≥ 75. World J Urol. 39, 4405–4412. https://doi.org/10.1007/s00345-021-03779-2 (2021).
Worthington, J. et al. Thulium laser transurethral vaporesection versus transurethral resection of the prostate for benign prostatic obstruction: The UNBLOCS RCT. Health Technol Assess. 24, 1–96. https://doi.org/10.3310/hta24410 (2020).
Coman, R. A. et al. Long-term results of multimodal treatment of the prostate using the Thulium Laser. Med. Pharm. Rep. 97, 338–346. https://doi.org/10.15386/mpr-2760 (2024).
Zeng, X. T. et al. Clinical practice guideline for transurethral plasmakinetic resection of prostate for benign prostatic hyperplasia (2021 Edition). Mil Med Res. 9, 14. https://doi.org/10.1186/s40779-022-00371-6 (2021).
Żywicka, B. et al. Usefulness of thulium-doped fiber laser and diode laser in zero ischemia kidney surgery-comparative study in pig model. Materials. 14, 2000. https://doi.org/10.3390/ma14082000 (2021).
Kroll, F. et al. Tumour irradiation in mice with a laser-accelerated proton beam. Nat. Phys. 18, 316–322. https://doi.org/10.1038/s41567-022-01520-3 (2022).
Labate, L. et al. Toward an effective use of laser-driven very high energy electrons for radiotherapy: Feasibility assessment of multi-field and intensity modulation irradiation schemes. Sci Rep. 1, 17307. https://doi.org/10.1038/s41598-020-74256-w (2020).
Jasiński, M. Advances in plasma and laser engineering. Materials. 17, 1768. https://doi.org/10.3390/ma17081768 (2024).
Acknowledgements
Thanks to Dr. Mingxia CHEN from the Department of Pathology of our hospital for her careful guidance in the histopathological observations involved in this study.
Generative AI in scientific writing
The authors did not use generative AI or AI-assisted technologies in the development of this manuscript.
Funding
This work was supported by the Science and Technology Innovation Program of Yantai Science and Technology Bureau. Policy-guided projects number: 2023YD062.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Dongchong SUN], [Yutao ZHANG], [Likun GAI], [Chenglin ZHANG], [Yanna WANG], [Hao ZHANG] and [Yalin SUN]. The first draft of the manuscript was written by [Dongchong SUN] and [Yalin SUN]. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All procedures performed in this study involving animal ex vivo organs were in accordance with the ethical standards of the Institutional Animal Ethics Committee of our hospital (approval no.qsap202305) and national research committee [Laboratory animal—Guideline for ethical review of animal welfare (GB/T 35892–2018) ].
Consent for publication
We confirm that the manuscript has been read and approved by all named authors. We confirm that the order of authors listed in the manuscript has been approved by all of us. All authors unanimously agreed to submit this manuscript to Current Urology.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary Material 2
Supplementary Material 3
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
SUN, D., ZHANG, Y., GAI, L. et al. Integrated optoelectronic fiber for transurethral surgery with simultaneous laser and electrovaporization. Sci Rep 15, 31290 (2025). https://doi.org/10.1038/s41598-025-15454-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15454-2