Abstract
Degenerative scoliosis (DS) is a significant health concern, affecting approximately 32–68% of the Chinese population aged 65 and above. This study aims to investigate the correlation between multifidus muscle atrophy and the severity of spinal curvature in DS patients, thereby providing evidence-based recommendations for the clinical prevention and management of DS. After applying the inclusion and exclusion criteria, 231 patients with chronic low back pain admitted to the Department of Spinal Surgery, Zhongda Hospital affiliated with Southeast University between January 2023 and January 2024 were ultimately selected as the study population. Based on imaging diagnosis, chronic low back pain patients without DS were assigned to the control group (non-DS, n = 81), while patients with scoliosis were assigned to the observation group (DS, n = 150). The observation group was further subdivided into mild scoliosis (n = 72), moderate scoliosis (n = 56), and severe scoliosis (n = 22) groups based on the degree of curvature. ImageJ software was used to measure the cross-sectional area (CSA) of the multifidus muscle at the mid-level of L4 and L5 on T2-weighted magnetic resonance imaging (MRI) scans and calculate the degree of atrophy. The proportion of males and bone mineral density (BMD) were significantly higher in the non-DS group compared to the DS group (P < 0.05). The multifidus cross-sectional area (CSA) and functional cross-sectional area ratio (LCSA/GCSA) were significantly higher in the non-DS group than in the DS group (P < 0.05). Patients in the severe scoliosis group were significantly older than those in the mild and moderate groups, and had significantly lower BMD than the mild group (P < 0.05). The LCSA/GCSA was highest in the mild scoliosis group, lowest in the severe scoliosis group, and intermediate in the moderate group (P < 0.05). CSA was significantly higher in the mild scoliosis group than in the severe group (P < 0.05). In the mild and moderate scoliosis groups, the convex-side CSA and LCSA/GCSA were significantly greater than those on the concave side (P < 0.001). In the severe scoliosis group, no significant difference was found in convex-side versus concave-side CSA (P = 0.307), but convex-side LCSA/GCSA remained significantly greater than concave-side (P = 0.007). Pearson correlation and linear regression analysis showed no correlation between multifidus LCSA/GCSA and Cobb angle in non-DS patients (P > 0.05), but a significant negative correlation existed in DS patients (P < 0.05). The absolute value of the correlation coefficient increased with worsening scoliosis severity (severe group > moderate group > mild group). Multifidus muscle atrophy is closely associated with degenerative scoliosis. Multifidus LCSA/GCSA negatively correlates with scoliosis severity in DS patients, but not in non-DS patients. The convex side exhibits less atrophy compared to the concave side in DS patients. The difference in concave-convex sides is more pronounced in patients with mild to moderate conditions. Increasing age and reduced BMD may be associated with worsening scoliosis severity. When BMD < − 0.900 T-Score, LCSA/GCSA < 0.805, and the patient is female, the likelihood of developing DS is high.
Similar content being viewed by others
Introduction
With the acceleration of global population aging, degenerative scoliosis (DS), a common health concern among the elderly, is receiving increasing attention. As a degenerative condition, the incidence of DS rises with age. DS demonstrates a high prevalence within the ethnic Han Chinese population aged over 40 years in China, reaching 13.3%. This prevalence increases to 14.4% among individuals aged 50 years and older1. DS becomes substantially more common in the population aged 65 years and above, affecting between 32 and 68% of this group2,3,4. Multiple prior studies have consistently identified paraspinal muscle atrophy as a significant factor strongly associated with the development of DS5,6,7. Consequently, alterations in both the function and structure of the paraspinal muscles are frequently observed in patients with DS.
Among the paraspinal muscles, the multifidus muscle (MF) is a primary contributor to spinal stability8. Located in the innermost layer of the spine, it has the most extensive muscular attachment area, increasing segmental tension, reducing relative motion between segments, and supporting spinal stability9. Furthermore, the MF, recognized as a critical regulator of postural balance10,11, persistently counteracts spinal imbalance through compensatory mechanisms during the progression of spinal degeneration. This continuous high-load state consequently leads to pain, fatigue, and atrophic degeneration within the MF. Due to the structural and biomechanical characteristics of the spine, pathological changes in the paraspinal muscles of patients with lumbar degenerative disorders most frequently occur at the L4-5 spinal level12,13. Research by Wang G et al. further confirmed that MF exhibits more pronounced atrophy compared to the erector spinae (ES) in patients with DS14. The study by Shuang Liu et al.15 also provided biomechanical evidence for significant atrophy and fatty infiltration in the paraspinal muscles at the L4-5 level. Additionally, the majority of DS cases originate at the L4-5 segment16. Based on these findings, this study will focus on the multifidus muscle at the L4-5 level to investigate the association between the degree of MF atrophy and the severity of DS.
Currently, although existing studies suggest an association between DS and paraspinal muscle atrophy17,18, the exact causal relationship and underlying mechanisms remain unclear. Differences in paraspinal muscle degeneration between non-DS and DS patients, among patients with varying severities of DS, and between the concave and convex sides of the spine in DS patients have been scarcely investigated. Furthermore, while numerous studies have focused on factors associated with DS1,15, they often lack in-depth analysis of relevant risk factors and fail to explore rehabilitation interventions, thereby failing to provide practical guidance for the clinical prevention and management of DS. Therefore, this study aims to validate the correlation between the LCSA/GCSA ratio and Cobb angle in patients with degenerative scoliosis using radiographic (X-ray) and magnetic resonance imaging (MRI) measurements. The specific objectives are as follows: (1) To compare differences in paraspinal muscle degeneration between non-DS and DS patients; (2) To compare differences in paraspinal muscle degeneration among DS patients with varying disease severities; (3) To compare differences in paraspinal muscle degeneration between the concave and convex sides of the spine in DS patients; (4) To analyze the correlation between DS and paraspinal muscle degeneration. Ultimately, Logistic regression analysis will be employed to identify key risk factors for DS development, enabling early identification of high-risk patients prone to developing DS and facilitating timely interventions. This approach seeks to enhance clinical efficacy in managing DS.
Materials and methods
Study participants
This study utilized the hospital electronic medical record system to consecutively enroll 402 patients admitted to the Spine Surgery Department of Zhongda Hospital, Southeast University for chronic low back pain between January 2023 and January 2024. Following the application of inclusion and exclusion criteria, 231 patients hospitalized for symptoms including chronic low back pain were ultimately included as the study cohort19. Non-DS patients were assigned to the control group, while DS patients comprised the observation group. The observation group was further stratified into three subgroups based on curve severity: mild, moderate, and severe scoliosis. Demographic and clinical characteristics, including sex, age, body mass index (BMI), and curve direction, were documented for all groups.
Methods and assessments
Inclusion criteria
① Diagnosed with DS (Cobb angle ≥ 10°) 20 via X-ray or CT, with imaging findings consistent with symptoms and signs; ② Availability of lumbar MRI, lumbar CT, lumbar anteroposterior/lateral radiographs, dynamic radiographs, and other imaging data; ③ Age 45–80 years, any gender; ④ No significant clinical improvement after 3 months of conservative treatment; ⑤ Informed consent obtained from patients.
Exclusion Criteria
① Dysplastic, pathological, or traumatic scoliosis; ② History of previous lumbar surgery; ③ History of primary or metastatic lumbar tumors, lumbar fractures, ankylosing spondylitis, or rheumatoid arthritis; ④ Imaging findings inconsistent with clinical symptoms and signs. ⑤ Patients with incomplete imaging or clinical data.
Scoliosis severity assessment
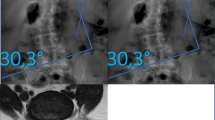
Cobb angle was used as the indicator of scoliosis severity21. A Cobb angle ≥ 10° defined degenerative scoliosis, while < 10° indicated no DS. Mild scoliosis: Cobb angle 10°-20°; Moderate scoliosis: Cobb angle 20°-40°; Severe scoliosis: Cobb angle > 40°19. A larger Cobb angle indicates greater severity. All patients underwent standard standing full-spine anteroposterior and lateral radiographs for Cobb angle measurement. The angle was measured between lines drawn along the superior endplate of the uppermost vertebra and the inferior endplate of the lowermost vertebra of the curve with the greatest angulation (See Figs. 1, 2). Inter-rater reliability among the three surgeons was assessed using the intraclass correlation coefficient (ICC) for Cobb angle measurement.
Multifidus atrophy assessment
This study employed a Siemens 3.0 T Vida MRI scanner (Siemens Healthcare GmbH, Erlangen, Germany) for routine lumbar imaging of all participants, including controls. Axial T2-weighted sequences were acquired at the mid-disc level of the L4/5 segment, with transverse images retrieved via the institutional Picture Archiving and Communication System (PACS). As depicted in Fig. 3, manual tracing of MF contours was performed on axial slices using irregular curves—a method validated for high intra- and inter-rater reliability22,23.
ImageJ software (NIH, Bethesda, MD, USA) was utilized to quantify: (1) Gross Cross-Sectional Area (GCSA): total bilateral MF area; (2) Lean Cross-Sectional Area (LCSA): functional muscle area excluding perimuscular fat, ligaments, and osseous structures24.
The LCSA/GCSA ratio served as the atrophy index, where higher values indicate milder atrophy and lower values reflect severe degeneration24,25.
Three blinded spine surgeons (5–10 years’ experience), trained in standardized protocols and unaware of clinical data, independently performed measurements. Final values represented the mean of triplicate assessments with established inter-rater reliability (ICC > 0.85).
Consistency assessment
Intraclass correlation coefficient (ICC) was used to assess the consistency of Cobb angle, MF CSA, and LCSA/GCSA measurements among the three spinal surgeons.
Statistical analysis
This study used SPSS 22.0 statistical software (IBM, Armonk, NY, USA) for data analysis.Continuous variables underwent normality testing. Normally distributed variables were analyzed using the independent samples t-test, with results presented as mean ± standard deviation (mean ± SD). Non-normally distributed variables were analyzed using the Wilcoxon rank-sum test, with results expressed as median (interquartile range) [Median (IQR)]. For quantitative data, it is described in the form of mean ± standard deviation. Qualitative data are expressed by frequency. In the comparison of variables between groups, the independent sample t-test was used for continuous variables, and the χ2 test was used for categorical variables. Through Pearson correlation analysis, the correlation between the severity of the disease and the degree of multifidus muscle atrophy in patients with DS was explored; Linear regression analysis was used to evaluate the influence of the indicators of multifidus atrophy degree (LCSA/GCSA) on the condition of DS. For the occurrence of scoliosis and the related risk factors of the degree of scoliosis, a logistic regression model was used for in-depth analysis. The variance inflation factor (VIF) was used to assess multicollinearity among variables. Variables with a VIF > 10, indicating severe collinearity, were excluded. The remaining significant variables were then entered into a multivariate logistic regression model to identify independent risk factors. Odds ratios (OR) with their corresponding 95% confidence intervals (CI) were calculated. Predictive models were ultimately constructed and their predictive performance was evaluated using receiver operating characteristic (ROC) curve analysis, calculating the area under the curve (AUC), the optimal cut-off value, sensitivity, and specificity. G*Power 3.1.9.7 was used to conduct a post-hoc power analysis for the primary outcome (the correlation between Cobb angle and multifidus LCSA/GCSA). The analysis employed a two-tailed Pearson correlation test with a power threshold of 0.80 and a significance level α of 0.05. In this study, the significance level α was set at 0.05, and P < 0.05 was taken as the criterion for judging that the difference was statistically significant.
Ethics statement
All methods were performed in accordance with the relevant guidelines and regulations stipulated by the Clinical Research Ethics Committee of the Zhongda Hospital, Southeast University and the Declaration of Helsinki.This study was approved by the Clinical Research Ethics Committee of the Zhongda Hospital, Southeast University(2022ZDSYLL406-P01). As a retrospective analysis, this study did not require registration and adhered to the STROBE guidelines for reporting retrospective research.
Results
Baseline data results
Comparison of baseline characteristics between non-DS group and DS group
This study conducted a comprehensive comparative analysis of the baseline data of patients in the DS group and the non-DS group. The results showed that there was no statistically significant difference between the two groups in terms of indicators such as age, body mass index (BMI), and the prevalence of hypertension and diabetes (P > 0.05). However, in terms of gender distribution and bone mineral density level, there were significant differences between the two groups (P < 0.05). For specific data, please refer to Table 1.
Comparison of baseline characteristics between DS severity group
The severity of DS was further grouped. The results showed that there were no statistically significant differences in BMI, hypertension, diabetes and the direction of scoliosis (P > 0.05). The age of patients with severe scoliosis was significantly higher than that of the mild (P = 0.001) and moderate (P = 0.026) groups, and the bone mineral density was significantly lower than that of the mild group (P = 0.005), as shown in Table 2.
Severity and characteristic analysis of multifidus muscle degeneration
CSA and LCSA/GCSA in non-ds group and DS-group
This section aims to clarify the impact of DS on multifidus muscle degeneration by comparing the morphological indicators of multifidus muscles in patients with non-scoliosis (Non-DS) and scoliosis (DS), which is also one of the core goals of exploring abnormal muscle function in patients with scoliosis. The severity of multifidus muscle degeneration was analyzed. The results showed that the cross-sectional area of the multifidus muscle (CSA) and the LCSA/GCSA index in the non-DS group were significantly higher than those in the DS group (P < 0.001). This result suggests that compared with non-scoliosis patients, DS patients have more obvious atrophy of the multifidus muscle. From a clinical perspective, as a core muscle group maintaining spinal stability, the reduction in the cross-sectional area of the multifidus muscle may further affect spinal balance function and has a potential association with the progression or aggravation of scoliosis symptoms, as shown in Table 3 and Fig. 4.
CSA and LCSA/GCSA in DS severity group
This section explores the impact of the severity of scoliosis on the degeneration differences of the multifidus muscles on both sides by analyzing the cross-sectional area (CSA) of the convex and concave sides and the cross-sectional area of the long side/transverse process (LCSA/GCSA) in patients with different degrees of scoliosis (DS), providing a basis for understanding the asymmetric degeneration patterns of the muscles in DS patients. In the mild and moderate scoliosis groups, the CSA of the convex side and LCSA/GCSA were significantly larger than those of the concave side (P < 0.001), indicating that compared with the concave side, the CSA of the convex side was larger, but the degree of atrophy was milder. There was no statistically significant difference in CSA between the convex side and the concave side in the severe scoliosis group (P = 0.307), but the LCSA/GCSA of the convex side was still significantly greater than that of the concave side (P = 0.007), indicating that the degree of atrophy of the convex side was less than that of the concave side. From a clinical perspective, this asymmetry of muscle degeneration that varies with the severity of scoliosis may be associated with the progression of spinal mechanical imbalance—significant differences in force on both sides at mild and moderate levels, leading to significant variations in muscle morphology and the degree of degeneration. In severe cases, the force state of the spine changes, which may reduce the morphological difference between the muscles on both sides, but the difference in the degree of degeneration is still partially retained. This provides a reference for the subsequent formulation of targeted muscle function intervention plans. See Table 4, Fig. 5.
Comparison of paraspinal muscle atrophy at the L4-5 level on MRI T2WI at the transverse lumbar vertebrae position in patients with different degrees of DS. Note (A) Mild DS (MF CSA = 922.15mm2, LCSA/GCSA = 0.82); (B) Moderate DS (MF CSA = 652.29mm2, LCSA/GCSA = 0.65); (C) Severe DS (MF CSA = 415.71mm2, LCSA/GCSA = 0.36).
This section further analyzes the differences in the long side cross-sectional area/transverse process cross-sectional area (LCSA/GCSA) and convex cross-sectional area (CSA) among groups with different severity levels of scoliosis (mild, moderate, and severe), in order to clarify the changing patterns of muscular-related indicators during the disease progression and provide a basis for a deeper understanding of the impact of scoliosis on muscle morphology.All pairwise comparisons of LCSA/GCSA between severity groups showed statistically significant differences (P < 0.01). For convex CSA, no statistically significant difference was found between the Mild and Moderate scoliosis groups (P > 0.05), whereas significant differences were observed between the Mild and Severe groups and between the Moderate and Severe groups (P < 0.001 for both comparisons). From a clinical perspective, the significant differences in LCSA/GCSA among all severity groups make it potentially a valuable indicator for assessing the severity of scoliosis and muscle degeneration. The feature that convex CSA shows no significant difference between the mild and moderate groups but has a significant difference from the severe group suggests that the obvious change in the cross-sectional area of the convex side muscles may mainly occur during the stage when scoliosis progresses from moderate to severe. This has certain reference significance for judging the disease progression and formulating intervention strategies, as shown in Table 5.
Correlation analysis
This section explores the correlation between the Cobb Angle (a core assessment indicator for the severity of scoliosis) and LCSA/GCSA (a key indicator reflecting the degree of muscle atrophy) through Pearson correlation analysis, in order to clarify the potential association pattern between the degree of skeletal deformity and the degree of muscle degeneration in the state of scoliosis. Pearson correlation showed that there was no correlation between the Cobb Angle and LCSA/GCSA in the non-DS group (r = 0.082, P = 0.467), while there was a significant negative correlation in the DS group (r = -0.533, P < 0.001). From a clinical perspective, the significant negative correlation between the Cobb Angle and LCSA/GCSA in the DS group further confirms the linkage between the severity of scoliosis and muscle degeneration—the aggravation of spinal deformity may exacerbate muscle atrophy through mechanisms such as changes in mechanical load. Conversely, the decline in spinal stability caused by muscle atrophy may also promote the progression of scoliosis. This discovery provides an important basis for understanding the pathophysiological process of scoliosis, and at the same time suggests that LCSA/GCSA may serve as a potential reference indicator for indirectly reflecting the risk of scoliosis progression, offering new ideas for clinical monitoring and intervention. See Table 6.
This section explores the association between the Cobb Angle (a core assessment indicator for the severity of scoliosis) and LCSA/GCSA (a key indicator reflecting the degree of muscle atrophy) through Pearson correlation analysis. At the same time, it further analyzes the differences in the association between the two in groups with different severity of scoliosis. To clarify the potential correlation rules and changing characteristics between the degree of skeletal deformity and the degree of muscle degeneration in the state of scoliosis. The P values of the three groups of patients were all < 0.05. There was a significant negative correlation between the Cobb Angle and LCSA/GCSA, and the absolute value of the correlation coefficient increased with the aggravation of scoliosis (severe group > moderate group > mild group). From a clinical perspective, the significant negative correlation between the Cobb Angle and LCSA/GCSA in the DS group, as well as the changing trends of the correlation coefficients in different severity subgroups, further confirm the linkage relationship between the severity of scoliosis and muscle degeneration—the aggravation of spinal deformity may exacerbate muscle atrophy through mechanisms such as changes in mechanical load; Conversely, the decline in spinal stability caused by muscle atrophy may also promote the progression of scoliosis, and this interaction is more prominent when the condition is more severe. This discovery provides an important basis for understanding the pathophysiological process of scoliosis. At the same time, it suggests that LCSA/GCSA may serve as a potential reference indicator for indirectly reflecting the risk of scoliosis progression, especially in patients with moderate to severe cases. The monitoring of this indicator may have greater clinical value and offer new ideas for clinical monitoring and intervention, as shown in Table 7.
Logistic regression analysis
This section explores the association between gender, bone mineral density, and LCSA/GCSA and the risk of diabetes through multivariate model analysis, aiming to clarify the extent to which these factors influence the onset of diabetes and provide a reference basis for the risk assessment and prevention of diabetes. The multivariate model indicated that the risk of DS in men was 0.52 times that in women (OR = 0.52, 95% CI: 0.30–0.91); For every 1-unit decrease in bone mineral density, the risk of DS increases by 82% (OR = 1.82, 95% CI: 1.17–2.83). For every 0.1 reduction in LCSA/GCSA, the risk of developing DS increases 25-fold(OR = 0.04, 95% CI: 0.01–0.12). Clinically speaking, the above results indicate that gender, bone mineral density and LCSA/GCSA score are all associated with the risk of diabetes. Among them, the LCSA/GCSA score has the most prominent impact on the risk of diabetes, which may be related to the muscle state reflected by LCSA/GCSA—abnormalities in muscle function and morphology may increase the risk of diabetes by affecting metabolism and other pathways. This discovery provides a new direction for the prevention and intervention of diabetes. In the future, it may be possible to identify and intervene in high-risk groups of diabetes at an early stage by monitoring indicators such as LCSA/GCSA and BMD. At the same time, for the female group, efforts should also be made to enhance the screening and prevention of diabetes (Table 8).
The model had a good goodness of fit (Hosmer–Lemeshow test P = 0.78), the AUC of the AUC-ROC curve was 0.85, and the predictive efficacy was relatively high. This further indicates that the multivariate model constructed based on gender, bone mineral density and LCSA/GCSA score has certain practical value in the risk assessment of diabetes onset. See Fig. 6.
Logistic regression analysis of BMD and LCSA/GCSA identified the following cutoff values: − 0.900 T-score for BMD and 0.805 for LCSA/GCSA.
Post-hoc power analysis
To demonstrate the reliability of the research findings, a statistical power analysis was conducted to assess the correlation between the primary outcome measure ( Cobb angle and LCSA/GCSA ). Detailed results are presented in Table 9.
Results of the consistency test
The consistency test results for multifidus muscle cross-sectional area data showed an ICC of 0.98 (> 0.90), the ICC for Cobb angle was 0.91 (> 0.90), while the ICC for multifidus muscle LCSA/GCSA data was 0.95 (> 0.90). All results passed the consistency test, indicating that the measurements obtained by the three physicians demonstrated high agreement and reliability, as shown in Table 10.
Raw data supporting the key findings of this study are available in the Supplementary Materials.
Discussion
Currently, the pathomechanisms of DS remain incompletely elucidated, with poorly defined interrelationships among imaging parameters and uncertainty regarding whether exceeding a specific threshold in one parameter inevitably triggers changes in others. This retrospective analysis of imaging data from DS patients aimed to investigate parameter correlations and elucidate underlying pathogenic mechanisms. While this study is limited by a relatively modest sample size, power analysis—supported by precedent studies26,27—confirms robust statistical reliability of the conclusions. Nevertheless, large-scale validation is warranted prior to clinical implementation to ensure robustness and clinical applicability.
MRI is the established gold standard for evaluating muscle atrophy and fatty infiltration28,29,30. Accordingly, this study acquired axial T2-weighted images at the L4/5 mid-disc level to maximize paraspinal muscle CSA measurements31. However, it should be noted that while degenerative changes in paraspinal muscles correlate across lumbar levels, CSA values from a single plane cannot represent the entire lumbar spine32—a limitation attributable to significant inter-level CSA variability33. Furthermore, studies demonstrate a craniocaudal gradient of multifidus fatty infiltration (L3/L4 < L4/L5 < L5/S1)34. Thus, although our focus on the pathology-prone L4-5 segment optimizes detection sensitivity, the single-level design inherently limits generalizability to global paraspinal degeneration. Future investigations should incorporate multi-level assessments to enhance accuracy and reliability.
This study employed the Cobb angle to quantify scoliosis severity and the LCSA/GCSA ratio to assess MF atrophy. Pearson correlation analysis and linear regression modeling revealed a significant negative correlation between the MF LCSA/GCSA ratio and Cobb angle in DS patients. Notably, the absolute value of the correlation coefficient progressively increased with worsening curve severity, indicating stronger associations in advanced deformity. These findings underscore the clinical relevance of monitoring MF changes in DS management. As a core stabilizer of spinal integrity, reductions in MF CSA and LCSA/GCSA may progressively impair spinal balance and demonstrate a potential link to symptomatic deterioration in degenerative scoliosis.
Logistic regression analysis identified lower BMD, reduced lumbar-to-global LCSA/GCSA, and female sex as independent risk factors for DS. Specifically, the probability of DS significantly increased when BMD fell below − 0.900 T-score, LCSA/GCSA ratio was < 0.805, and the patient was female. In linear regression modeling, the paralytic scoliosis cohort demonstrated an R2 value of 0.284, indicating that 28.4% of the variance in Cobb angle could be explained by LCSA/GCSA alone. Subsequent testing confirmed the model passed the F-test (P < 0.001), corroborating that LCSA/GCSA exerts a deterministic effect on Cobb angle magnitude. This suggests that progressive Cobb angle enlargement is accompanied by intensified multifidus muscle atrophy. While these findings align with select prior studies, the retrospective design precludes establishment of causality between Cobb angle progression and multifidus degeneration. Whether increased Cobb angles drive muscular atrophy or pre-existing atrophy precipitates curve progression remains indeterminate. However, a robust correlation between these parameters is unequivocally demonstrated.
As a degenerative condition, DS exhibits progressive worsening with advancing age. This progression may be associated with a significant age-related decrease in paraspinal muscle CSA35. In the present study, no significant difference in age was observed between the mild and moderate scoliosis groups. However, patients with severe scoliosis were significantly older than those in both the mild and moderate groups. Collectively, these findings suggest that age may be correlated with DS severity. Moreover, due to physiological differences in muscle mass, males typically exhibit a larger paraspinal muscle CSA than females36. Given that a higher paraspinal muscle CSA confers a protective role against disease progression in DS patients37, the incidence of DS is lower in males compared to females. Consistent with previous reports, the proportion of males in the non-DS group was higher than in the DS group in our cohort. This further supports the conclusion that male sex is a protective factor, while female sex represents a risk factor for DS. The absence of a significant age difference between the mild and moderate scoliosis groups may be attributable to their limited sample sizes.
Denervation and muscle disuse represent two primary mechanisms underlying paraspinal muscle atrophy38. As the largest muscle spanning the lumbosacral junction, the multifidus muscle is innervated by the dorsal rami of the lumbar spinal nerves39 and exhibits more intimate anatomical connections to the vertebral laminae and spinous processes compared to other paraspinal muscles40. This specific anatomy may explain the significantly reduced multifidus CSA observed in DS patients compared to non-DS patients in the present study. Yu-Leung Chan et al.41 found that abnormally high signal intensity within the paraspinal muscles was associated with the magnitude of the scoliotic curve, and this signal abnormality was exclusively localized to the multifidus muscle on the concave side. However, Chan et al. did not assign a definitive pathological diagnosis to this high signal intensity, nor could they determine whether it represented a primary etiological factor for the scoliosis or a secondary pathological change resulting from the curvature. They proposed that an increase in type II muscle fibers within the concave-side multifidus was the principal cause of the abnormal high signal. Consistent with this, our study demonstrated that multifidus CSA was largest in the mild curve group, intermediate in the moderate group, and smallest in the severe group. This progressive reduction in CSA with increasing curve severity suggests a corresponding decrease in type I fiber content and an increase in the proportion of type II fibers. Crucially, our findings extend beyond interpreting this solely as an MRI marker by providing concrete evidence at the CSA level. Notably, given that type I fiber content exhibits a significant inverse correlation with both mean low back pain intensity and Oswestry Disability Index (ODI) scores42, these results highlight the clinical importance of monitoring changes in multifidus CSA, particularly on the concave side. Thus, interventions such as targeted rehabilitation training, aimed at modifying these parameters, may potentially ameliorate low back pain and functional disability in these patients.
It is well-established that progressive worsening of DS leads to low back pain, lower extremity symptoms, and functional impairment, significantly impairing patients’ activities of daily living (ADL) and quality of life (QoL)43. Generally, conservative management is less effective than surgical intervention in improving functional outcomes and achieving sustained pain relief44,45. Consequently, the majority of patients ultimately require surgical treatment. Preoperative imaging parameters of the paraspinal muscles hold predictive value for surgical outcomes, with robust paraspinal musculature often predicting favorable clinical prognosis. Significantly, the study by Canvasser et al.46 further demonstrated that paraspinal muscle CSA not only significantly influences the prognosis following lumbar spine surgery but also serves as an important predictor of mortality across a range of major surgical procedures.
Collectively, these findings underscore the critical importance of restoring and improving paraspinal muscle function in the management of DS. Therefore, proactive implementation of interventions such as physical therapy and rehabilitation training to enhance paraspinal muscle strength, alleviate pain, and improve patients’ quality of life is strongly recommended. Cankurtaran D et al. postulated that physical activity can improve paraspinal muscle CSA35. Supporting this, studies have demonstrated a significant increase in multifidus CSA following an 8-week spinal stabilization exercise program compared to pre-exercise levels47. Motor control training (MCT) has been established as an effective exercise therapy modality for reducing pain and improving outcomes48, with its core principle focusing on enhancing the control and endurance of key paraspinal muscles49. Over the past three decades, clinical practice guidelines have increasingly emphasized patient self-management and the use of individualized exercise programs targeting functional improvement50, indicating a growing recognition of the importance of rehabilitative exercise. Specifically, Physiotherapeutic Scoliosis-Specific Exercises (PSSE) aim to strengthen weakened muscles and improve flexibility on the affected side, thereby promoting spinal balance and potentially mitigating the curvature. Consequently, PSSE is recommended by the International Society on Scoliosis Orthopaedic and Rehabilitation Treatment (SOSORT) guidelines as a routine intervention for scoliosis patients51. However, high-quality evidence supporting its efficacy remains limited, potentially due to a lack of specificity in the corrective maneuvers employed within PSSE programs. Therefore, for DS rehabilitation, targeted training protocols need to be developed based on the specific alterations in muscle strength between the convex and concave sides, differentiating exercises for concave versus convex sides and targeting superficial versus deep muscle layers. This tailored approach is essential to further enhance the specificity and efficacy of rehabilitation programs.
The musculoskeletal system plays a pivotal role in maintaining functional balance. As a closely interconnected and interdependent system, metabolic dysregulation within either component (muscle or bone) can adversely impact the other, leading to reciprocal pathological changes52. Studies indicate a strong association between reduced BMD and paraspinal muscle atrophy, with individuals exhibiting low BMD having a 5.7-fold higher risk of developing paraspinal muscle atrophy compared to those with normal BMD53. In the present study, no significant difference in BMD was observed between the mild and moderate curve groups. However, patients with severe scoliosis exhibited significantly lower BMD than those in the mild curve group, suggesting a potential association between decreasing BMD and increasing curve severity. Bjerke et al.54 recommend that preoperative anti-osteoporosis pharmacotherapy be proactively considered for patients with a T-score < − 1.0 to optimize outcomes following lumbar spine surgery. Building upon these findings, our study incorporated absolute BMD values as a continuous variable rather than solely the dichotomous diagnosis of osteoporosis. This analysis revealed that low BMD is an independent risk factor for DS, even in individuals whose BMD values fall above the diagnostic threshold for osteoporosis. This key finding underscores the need for a more nuanced clinical approach in managing patients with low BMD. Specifically, intervention should be initiated upon detecting BMD below a clinically relevant threshold value(BMD < -0.900 T-Score) , rather than delaying treatment until the formal diagnostic cutoff for osteoporosis is met.
Under physiological conditions, bilateral paraspinal muscles maintain symmetrical biomechanical loading. Scoliotic deformity disrupts this equilibrium, particularly during degenerative progression. To compensate for asymmetrical stress, the paraspinal musculature endures chronic overload, precipitating pain, fatigue, and degenerative atrophy. The convex side exhibits compensatory hypertrophy, whereas the concave side undergoes progressive atrophy, manifested by reduced CSA and increased fatty infiltration in concave paraspinal muscles55. Prior studies have demonstrated asymmetric paraspinal degeneration in DS, with concave-sided deterioration exceeding convex-sided changes, and bilateral deterioration severity correlating positively with Cobb angle magnitude56. Our findings corroborate these observations, providing additional evidence for the dose-dependent relationship between scoliotic severity and paraspinal muscle degeneration. We propose that multifidus CSA and the LCSA/GCSA ratio serve as predictive biomarkers for DS development, particularly when multifidus LCSA/GCSA falls below the critical threshold of 0.805, which warrants heightened clinical vigilance.
Current research predominantly focuses on paraspinal muscle alterations in patients with congenital and idiopathic scoliosis57, as well as in other lumbar degenerative conditions58. There has been relatively little attention paid to paraspinal muscle changes and concave-convex asymmetry in patients with DS. Even among studies examining asymmetry, the majority utilize the fat infiltration rate (FIR) as the metric for assessing paraspinal muscle atrophy59,60. Studies employing the lean cross-sectional area to gross cross-sectional area ratio (LCSA/GCSA) as an evaluation metric are scarce. Recent meta-analyses over the past five years have only addressed the strong correlation between muscle atrophy and spinal degeneration in general61, with none specifically focusing on multifidus muscle atrophy in degenerative scoliosis. Therefore, to enrich the discourse in this area, this study analyzed concave-convex asymmetry in DS patients with varying curve severities, utilizing the LCSA/GCSA ratio as an indicator of paraspinal muscle atrophy. Key findings revealed significant concave-convex differences in both CSA and the LCSA/GCSA ratio within the mild and moderate DS groups. However, in the severe DS group, while a significant difference in the LCSA/GCSA ratio persisted, the difference in CSA was no longer significant. The underlying reason for this observation in severe DS may be that the CSA of the multifidus muscle was significantly reduced bilaterally, thereby diminishing the absolute difference between sides to a level undetectable by statistical tests. Furthermore, CSA encompasses both functional (lean) and non-functional (fatty) tissues, whereas the LCSA/GCSA ratio, through its normalization process, provides a more accurate reflection of structural and functional asymmetry, particularly in detecting atrophy of muscle fibers. These findings suggest that in severe DS patients, bilateral muscle fiber atrophy occurs. Residual muscle fibers on the convex side may undergo compensatory hypertrophy, while atrophy on the concave side is likely more homogeneous, involving reductions in both Type I and Type II muscle fibers.
This study found that all CSA and LCSA/GCSA metrics in the severe group were significantly lower than those in the mild and moderate groups, indicating more pronounced atrophy, which aligns with previous research. Furthermore, the differences in these metrics between the mild and moderate groups were found to be less pronounced compared to the differences observed relative to the severe group. Based on these findings, we propose that compensatory mechanisms likely exist during the early stages of DS. These mechanisms—such as hypertrophy of residual muscle fibers and compensatory activation of other muscles—may mitigate significant declines in paraspinal muscle CSA between mild and moderate severity. However, as the curve progresses into severe deformity, a decompensation phase ensues, leading to marked paraspinal muscle atrophy in severe DS patients. Crawford et al.62 also noted that the increase in paraspinal muscle fat content does not follow a linear progression, and its rate of change varies across different age groups, accelerating markedly in later stages. Collectively, these findings indicate that paraspinal muscle degeneration is a complex process influenced by multiple factors and does not proceed at a constant rate. We hypothesize the existence of a critical threshold in the progression of paraspinal muscle atrophy. Once this threshold is exceeded, rapid muscle atrophy occurs, whereas changes remain relatively gradual before reaching this point, resulting in minimal differences in measured metrics. Therefore, the precise identification of this critical threshold through the integration of multiple evaluation metrics holds significant clinical value. Early intervention based on this threshold could prevent severe functional impairment in DS patients, warranting further investigation.
Limitations and future directions
Firstly, the relatively small sample size limited more detailed multivariable analysis and may affect generalizability and reliability; future multi-center studies with larger cohorts are needed. In particular, the mild and moderate groups were underpowered; therefore, the corresponding conclusions warrant validation in larger cohorts. Secondly, as a cross-sectional retrospective observational study, it lacks longitudinal follow-up data to establish causality; long-term follow-up studies are required.Thirdly, using only LCSA/GCSA to measure atrophy, without incorporating muscle density or fat infiltration metrics, introduces potential bias.Finally, although the severe group showed considerable CSA differences between convex and concave sides, these differences did not reach statistical significance due to the small sample size (n = 22) and high variability.
To address these gaps, our future research should prioritise:
-
1.
Extend the research duration to increase the sample size.
-
2.
Implement Long-term follow-up studies (≥ 3 years).
-
3.
Measure CSA and LCSA across multiple spinal levels.
-
4.
Integrate additional indicators such as HRQOL scales (e.g., SRS-22), standing full-spine radiographs to assess global alignment, and MRI-based 3D muscle volume analysis to comprehensively evaluate muscle atrophy.
Conclusion
In summary, this study demonstrates a significant correlation between paraspinal muscle atrophy and DS. Paraspinal muscle atrophy may play a crucial role in the pathogenesis and progression of DS. Lower bone mineral density, a reduced LCSA/GCSA ratio, and female sex were identified as significant risk factors for DS development. Paraspinal muscle atrophy worsened with DS progression, with the convex side exhibiting a larger CSA and less pronounced atrophy compared to the concave side. Based on these findings, the management of DS patients should prioritize the assessment of multifidus muscle function and acknowledge the concave-convex asymmetry in muscle strength. Targeted training protocols aimed at enhancing the strength of the back and core musculature should be implemented to provide improved spinal support, thereby mitigating disease progression and enhancing patient quality of life. Future research should focus on expanding the sample size and incorporating histopathological evidence to strengthen the validity and accuracy of these conclusions, ultimately providing practical clinical guidance.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
References
Xu, L. et al. Degenerative lumbar scoliosis in Chinese Han population: prevalence and relationship to age, gender, bone mineral density, and body mass index. Eur. Spine J. 22(6), 1326–1331 (2013).
Schwab, F. et al. Adult scoliosis: Prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine 30(9), 1082–1085 (2005).
Kebaish, K. M. et al. Scoliosis in adults aged forty years and older prevalence and relationship to age, race, and gender. Spine 36(9), 731–736 (2011).
Diebo, B. G. et al. Adult spinal deformity. Lancet 394(10193), 160–172 (2019).
Wang, Z. et al. Radiographic risk factors for degenerative lumbar spondylolisthesis: A comparison with healthy control subjects. Front. Surg. 9, 11 (2022).
Lee, E. T. et al. Association of lumbar paraspinal muscle morphometry with degenerative spondylolisthesis. Int. J. Environ. Res. Public Health 18(8), 9 (2021).
Wagner, S. C. et al. Severe lumbar disability is associated with decreased psoas cross-sectional area in degenerative spondylolisthesis. Glob. Spine J. 8(7), 716–721 (2018).
Wilke, H. J. et al. Stability increase of the lumbar spine with different muscle groups. A biomechanical in vitro study. Spine 20(2), 192–198 (1995).
Danneels, L. A. et al. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur. Spine J. 9(4), 266–272 (2000).
Penning, L. Psoas muscle and lumbar spine stability: a concept uniting existing controversies—Critical review and hypothesis. Eur. Spine J. 9(6), 577–585 (2000).
Ignasiak, D. et al. The effect of muscle ageing and sarcopenia on spinal segmental loads. Eur. Spine J. 27(10), 2650–2659 (2018).
Crawford, R. J. et al. Geography of lumbar paravertebral muscle fatty infiltration the influence of demographics, low back pain, and disability. Spine 44(18), 1294–1302 (2019).
Bao, T. et al. Relationship between paravertebral muscle degeneration and spinal-pelvic sagittal parameters in patients with lumbar disc herniation. Sci. Rep. 14(1), 192 (2024).
Wang, G. L. et al. Quantitative MRI and X-ray analysis of disc degeneration and paraspinal muscle changes in degenerative spondylolisthesis. J. Back Musculoskelet. Rehabil. 28(2), 277–285 (2015).
Liu, S. et al. Association between paraspinal muscle parameters and single-segment degenerative lumbar spondylolisthesis: Retrospective, cross-sectional cohort study. Spine 50(12), 841–848 (2025).
Lee, E. T., et al. Association of lumbar paraspinal muscle morphometry with degenerative spondylolisthesis. Int. J. Environ. Res. Public Health 18(8) (2021).
Ran, L. et al. Low Hounsfield units on computed tomography are associated with cage subsidence following oblique lumbar interbody fusion (OLIF). Spine J. 22(6), 957–964 (2022).
Wong, E. et al. Adult degenerative lumbar scoliosis. Orthopedics 40(6), E930–E939 (2017).
Ploumis, A., Transfledt, E. E. & Denis, F. Degenerative lumbar scoliosis associated with spinal stenosis. Spine J. 7(4), 428–436 (2007).
Aebi, M. The adult scoliosis. Eur. Spine J. 14(10), 925–948 (2005).
Silva, F. E. & Lenke, L. G. Adult degenerative scoliosis: Evaluation and management. Neurosurg. Focus 28(3) (2010).
Kalichman, L., Carmeli, E. & Been, E. The association between imaging parameters of the paraspinal muscles, spinal degeneration, and low back pain. Biomed. Res. Int. 2017, 2562957 (2017).
Solgaard Sorensen, J. et al. Low-field magnetic resonance imaging of the lumbar spine: Reliability of qualitative evaluation of disc and muscle parameters. Acta Radiol. 47(9), 947–953 (2006).
Fortin, M. et al. Paraspinal muscle morphology and composition: a 15-yr longitudinal magnetic resonance imaging study. Med. Sci. Sports Exerc. 46(5), 893–901 (2014).
Li, H. et al. Incidence and risk factors of posterior cage migration following decompression and instrumented fusion for degenerative lumbar disorders. Medicine (Baltimore) 96(33), e7804 (2017).
Tang, Y. et al. Assessment of the association between paraspinal muscle degeneration and quality of life in patients with degenerative lumbar scoliosis. Exp. Ther. Med. 20(1), 505–511 (2020).
Sun, X. Y. et al. Correlation between multifidus muscle atrophy, spinopelvic parameters, and severity of deformity in patients with adult degenerative scoliosis: The parallelogram effect of LMA on the diagonal through the apical vertebra. J. Orthop. Surg. Res. 14(1), 276 (2019).
Fortin, M. & Macedo, L. G. Multifidus and paraspinal muscle group cross-sectional areas of patients with low back pain and control patients: a systematic review with a focus on blinding. (1538–6724 (Electronic)).
Yanik, B., Keyik, B. & Conkbayir, I. Fatty degeneration of multifidus muscle in patients with chronic low back pain and in asymptomatic volunteers: Quantification with chemical shift magnetic resonance imaging. Skeletal Radiol. 42(6), 771–778 (2013).
Shafaq, N. et al. Asymmetric degeneration of paravertebral muscles in patients with degenerative lumbar scoliosis. Spine 37(16), 1398–1406 (2012).
Niemelainen, R., Briand, M. M. & Battie, M. C. Substantial asymmetry in paraspinal muscle cross-sectional area in healthy adults questions its value as a marker of low back pain and pathology. Spine 36(25), 2152–2157 (2011).
Huang, W. et al. The association between paraspinal muscle degeneration and osteoporotic vertebral compression fracture severity in postmenopausal women. J. Back Musculoskelet. Rehabil. 36(2), 323–329 (2023).
Urrutia, J. et al. Is a single-level measurement of paraspinal muscle fat infiltration and cross-sectional area representative of the entire lumbar spine?. Skeletal Radiol. 47(7), 939–945 (2018).
Ding, J. Z. et al. Different degeneration patterns of paraspinal muscles in degenerative lumbar diseases: A MRI analysis of 154 patients. Eur. Spine J. 31(3), 764–773 (2022).
Cankurtaran, D., Yigman, Z. A. & Umay, E. Factors associated with paravertebral muscle cross-sectional area in patients with chronic low back pain. Korean J. Pain 34(4), 454–462 (2021).
Kalichman, L., Carmeli, E. & Been, E. The association between imaging parameters of the paraspinal muscles, spinal degeneration, and low back pain. Biomed. Res. Int. 2017, 14 (2017).
Li, C. et al. Radiological changes of paraspinal muscles: A comparative study of patients with isthmic spondylolisthesis, patients with degenerative lumbar spondylolisthesis, and healthy subjects. J. Pain Res. 15, 3563–3573 (2022).
Wang, H. et al. Incidence and risk factors of persistent low back pain following posterior decompression and instrumented fusion for lumbar disk herniation. J. Pain Res. 10, 1019–1025 (2017).
Wang, K. et al. The role of multifidus in the biomechanics of lumbar spine: A musculoskeletal modeling study. Bioeng. Basel 10(1), 12 (2023).
Wang, Z. et al. Advances in research on fat infiltration and lumbar intervertebral disc degeneration. Front. Endocrinol. (Lausanne) 13, 1067373 (2022).
Chan, Y. L. et al. MRI evaluation of multifidus muscles in adolescent idiopathic scoliosis. Pediatr. Radiol. 29(5), 360–363 (1999).
Purushotham, S. et al. Microscopic changes in the multifidus muscle in people with low back pain associated with lumbar disc herniation. Sci. Rep. 14(1), 31927 (2024).
Ploumis, A. et al. Progression of idiopathic thoracic or thoracolumbar scoliosis and pelvic obliquity in adolescent patients with and without limb length discrepancy. Scoliosis Spinal Disord. 13, 18–18 (2018).
Lykissas, M. G. & Aichmair, A. Current concepts on spinal arthrodesis in degenerative disorders of the lumbar spine. World J. Clin. Cases 1(1), 4–12 (2013).
Smith, J. S. et al. Operative versus nonoperative treatment of leg pain in adults with scoliosis a retrospective review of a prospective multicenter database with two-year follow-up. Spine 34(16), 1693–1698 (2009).
Canvasser, L. D. et al. Paraspinous muscle as a predictor of surgical outcome. J. Surg. Res. 192(1), 76–81 (2014).
Kim, S., Kim, H. & Chung, J. Effects of spinal stabilization exercise on the cross-sectional areas of the lumbar multifidus and psoas major muscles, pain intensity, and lumbar muscle strength of patients with degenerative disc disease. J. Phys. Ther. Sci. 26(4), 579–582 (2014).
Searle, A. et al. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin. Rehabil. 29(12), 1155–1167 (2015).
Hides, J. A. et al. Effect of motor control training on muscle size and football games missed from injury. Med. Sci. Sports Exerc. 44(6), 1141–1149 (2012).
Foster, N. E. et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet 391(10137), 2368–2383 (2018).
Negrini, S. et al. 2016 SOSORT guidelines: Orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis Spinal Disord 13, 3 (2018).
Frontera, W. R. Physiologic changes of the musculoskeletal system with aging: A brief review. Phys. Med. Rehabil. Clin. N. Am. 28(4), 705–711 (2017).
Ekin, E. E. & Altunrende, M. E. The association of reduced bone density with paraspinal muscle atrophy and adipose tissue in geriatric patients: A cross-sectional CT study. Turk. J. Med. Sci. 49(2), 538–542 (2019).
Bjerke, B. T. et al. Incidence of osteoporosis-related complications following posterior lumbar fusion. Glob. Spine J. 8(6), 563–569 (2018).
Chen, M. et al. Quantitative analysis of paravertebral muscle asymmetry and its correlation with spinal deformity in patients with degenerative lumbar scoliosis: A retrospective case-control study. Quant. Imaging Med. Surg. 14(5), 3593–3605 (2024).
Xie, H. et al. Surface electromyography study on asymmetry in paravertebral muscle degeneration in patients with degenerative lumbar scoliosis. Sci. Rep. 12(1), 18675 (2022).
Zhang, J. et al. Can C2–6 cobb angle replace C2–7 cobb angle? An analysis of cervical kinetic magnetic resonance images and X-rays. Spine 44(4), 240–245 (2019).
Zhang, C. et al. Correlation and risk factor analysis of multifidus muscle atrophy in degenerative lumbar spondylolisthesis. Front. Med. (Lausanne) 12, 1609660 (2025).
Xie, H. et al. Degenerative characteristics of multifidus at different vertebral levels of scoliosis in patients with degenerative lumbar scoliosis and relationship of these degenerative characteristics with surface electromyography activity. BMC Musculoskelet. Disord. 23(1), 1074 (2022).
Wang, X. et al. Comparison of multifidus degeneration between scoliosis and lumbar disc herniation. BMC Musculoskelet. Disord. 23(1), 891 (2022).
Stevens, S. et al. Unilateral changes of the multifidus in persons with lumbar disc herniation: A systematic review and meta-analysis. Spine J. 20(10), 1573–1585 (2020).
Crawford, R. J. et al. Rate of lumbar paravertebral muscle fat infiltration versus spinal degeneration in asymptomatic populations: An age-aggregated cross-sectional simulation study. Scoliosis Spinal Disord. 11, 21 (2016).
Funding
The research was supported by grants from the National Natural Science Foundation of China (82202768), Zhongda Hospital Affiliated to Southeast University, Jiangsu Province High-Level Hospital Construction Funds (GSP-LCYJFH20), the Fundamental Research Funds for the Central Universities (2242020K40156).
Author information
Authors and Affiliations
Contributions
Jiangkai Yu and Cong Zhang contributed to the work equally. Jiangkai Yu: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Cong Zhang: Writing – review & editing. Yan Zhou: Resources, Supervision, Writing – review & editing. Jing Wang:Resources. Youheng Zhang:Software. Yuntao Wang: Data curation, Formal analysis, Project administration, Supervision, Writing – review & editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Ethical Committee of the Zhongda Hospital, Southeast University approved the study (2022ZDSYLL406-P01). Written informed consent was obtained from all patients.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, J., Zhang, C., Zhou, Y. et al. Correlation analysis between multifidus muscle atrophy and the severity of degenerative scoliosis retrospective, cross-sectional study. Sci Rep 15, 30133 (2025). https://doi.org/10.1038/s41598-025-15622-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15622-4